Abstract
The p53 tumor suppressor induces cell growth arrest and apoptosis in response to DNA damage. Because these functions are achieved largely by the transcriptional properties of p53, nuclear localization of the protein is essential. Indeed, the tumors with aberrant cytoplasmic localization of wild-type p53 often exhibit an impaired response to DNA damage. In this study, we report that Thr-55 phosphorylation induces the association of p53 with the nuclear export factor CRM1, leading to p53 nuclear export. We further show that MDM2 also promotes the CRM1-p53 association and Thr-55 phosphorylation is required for this process. Interestingly, inhibition of Thr-55 phosphorylation by a dietary flavonoid, apigenin, specifically blocks the CRM1-p53 association, restores p53 nuclear localization, and sensitizes tumor cells with cytoplasm localized wild-type p53 to DNA damage. These data provide insights into the regulation of p53 nuclear localization by post-translational modification and suggest an avenue for targeted therapy for cancers caused by aberrant cytoplasm localization of wild-type p53.
Keywords: apigenin, CRM1, MDM2, nuclear export
Wild-type p53 plays a key suppressor role in cell growth and tumor formation (1, 2). Although mutation is the most common mechanism to inactivate p53 function, significant fractions of wild-type p53 protein are found in the cytoplasm of some tumors (such as undifferentiated neuroblastoma and inflammatory breast carcinoma), which suggests that cytoplasmic localization of wild-type p53 also represents an important mechanism for abrogating p53 function (3, 4). The p53 tumor suppressor has been shown to be a nucleocytoplasmic shuttling protein (5). In normal cells, p53 is generally kept at a low level and is diffusely distributed throughout the cell. Upon DNA damage, the p53 protein is localized in the nucleus and induces cell growth arrest and apoptosis. Because subcellular localization of the p53 protein is a dynamic process, cellular mechanisms that tightly control nucleocytoplasmic shuttling of p53 under normal growth and DNA damage conditions must exist. Consequently, a thorough understanding of these mechanisms is important for treatment of cancers with aberrant cytoplasm localization of wild-type p53.
The activity of p53 is largely regulated by posttranslational modifications (6, 7). Previous studies in our laboratory showed that p53 is phosphorylated at Thr-55 by TAF1 under cell growth condition (8). TAF1 is the largest subunit of transcription factor TFIID and plays an important role in cell cycle regulation (9–11). The biochemical activity of TAF1 required for its cell growth function relies on its intrinsic protein kinase activity (12). Consistent with this view, we have shown that phosphorylation of p53 by TAF1 leads to p53 protein degradation and cell G1 progression (8). Because nucleocytoplasmic shuttling is essential for p53 degradation (13, 14), we tested whether Thr-55 phosphorylation could affect this process in this study. Our data suggest that Thr-55 phosphorylation is required for p53 nuclear export. p53 contains two leucine-rich nuclear export signals (NES), one in the C terminus (15) and the other in the N terminus (16). p53 nuclear export is mediated through the nuclear export factor CRM1 (15). To elucidate the molecular mechanism by which Thr-55 phosphorylation leads to p53 nuclear export, we show that Thr-55 phosphorylation promotes the CRM1-p53 interaction. MDM2 also promotes the CRM1-p53 interaction, and Thr-55 phosphorylation is required for this process. Importantly, inhibition of Thr-55 phosphorylation by a dietary flavonoid, apigenin, specifically blocks the CRM1-p53 association, restores p53 nuclear localization and sensitizes tumor cells with cytoplasm localized wild-type p53 to DNA damage. These data provide mechanistic insight into the regulation of p53 nuclear localization, and suggest an avenue for the development of therapies targeting cancers caused by abnormal cytoplasm localization of wild-type p53.
Results
Thr-55 Phosphorylation Leads to p53 Cytoplasmic Localization.
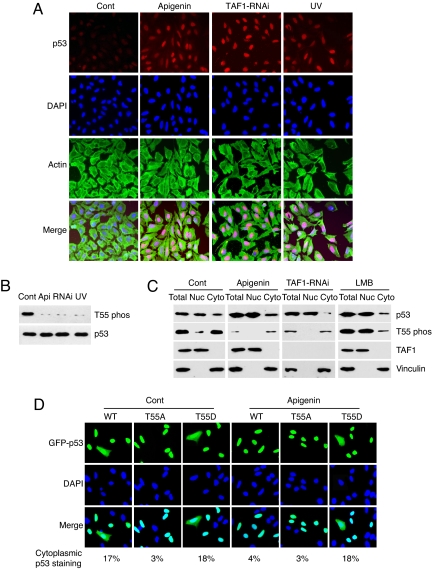
To test whether Thr-55 phosphorylation could affect p53 nucleocytoplasmic shuttling, we treated U2OS cells with apigenin that inhibits the TAF1 kinase activity, thus decreasing the Thr-55 phosphorylation level of endogenous p53 (Fig. 1B) (8). The assay shows that p53 is localized in the nucleus upon inhibition of Thr-55 phosphorylation (Fig. 1A). To provide specificity of the treatment, we show that apigenin does not affect p53 phosphorylation on Ser-15, Ser-20, and Ser-46, and acetylation on Lys-373 in U2OS cells (supporting information (SI) Fig. S1). It has been reported that apigenin inhibits casein kinase II (CKII) (17, 18) that can phosphorylate p53 at Ser-392 (19) (Fig. S1). To exclude the possibility that Ser-392 phosphoylation might affect p53 nuclear localization, we inhibited Thr-55 phosphorylation with TAF1-specific RNA oligonucleotides (TAF1-RNAi) (Fig. 1B), which does not affect Ser-392 phosphoylation (Fig. S1). The assay shows again that p53 is localized in the nucleus upon inhibition of Thr-55 phosphorylation (Fig. 1A). To further correlate Thr-55 phosphorylation to p53 nucleocytoplasmic shuttling, we show that UV treatment that reduced Thr-55 phosphorylation (Fig. 1B) (8) leads to p53 nuclear localization (Fig. 1A).
Fig. 1.
Thr-55 phosphorylation leads to p53 cytoplasmic localization. (A) U2OS cells were either untreated (Cont), or treated with apigenin, TAF1-RNAi or UV and processed for immunofluorescence with anti-p53 and anti-actin antibodies. (B) The level of Thr-55-phosphorylation in U2OS cells after apigenin (Api), TAF1-RNAi (RNAi) or UV treatment was detected with anti-phospho-Thr-55 antibody. (C) U2OS cells were untreated (Cont) or treated with apigenin, TAF1-RNAi, or LMB and fractionated. The levels of total p53, Thr-55-phosphorylated p53, TAF1, and vinculin in the cytoplasmic (Cyto) and nuclear (Nuc) fractions were examined. (D) Cellular localization of GFP-WT, GFP-T55A, and GFP-T55D are detected in U2OS cells in the absence (Cont) or presence of apigenin. The same cells were also stained with DAPI to visualize nuclei. Five hundred GFP-positive cells were counted from each transfection. The appearance of p53 staining in both cytoplasm and nucleus was scored and shown as the percentage of total transfected cells.
Next, we investigated the subcellular distribution of endogenous p53 that is phosphorylated at Thr-55. Because the Thr-55 phospho-specific antibody (Ab202) was unable to detect signals in immunostaining, we fractionated U2OS cells to cytoplasmic and nuclear fractions and detected Thr-55 phosphorylation by immunoblotting. The assay shows that Thr-55 phosphorylated p53 was mainly localized in the cytoplasmic fraction, although total p53 was distributed in both the nucleus and cytoplasm (Fig. 1C). Furthermore, we show that inhibition of TAF1 by either apigenin or TAF1-RNAi leads to nuclear localization of endogenous p53 in U2OS cells (Fig. 1C). To exclude the possibility that different p53 protein levels might affect p53 cellular localization, we pretreated cells with MG132 and obtained the same results (Fig. S2). The integrity of the cellular fractionation was verified by using immunoblotting with antivinculin (cytoplasm) and anti-TAF1 antibodies (nucleus). Together, these data suggest that TAF1-mediated Thr-55 phosphorylation promotes p53 cytoplasmic localization.
To confirm that Thr-55 phosphorylation directly affects p53 cytoplasmic localization, we carried out immunostaining by using wild-type p53 and Thr-55 phosphorylation mutants (T55A or T55D) that were linked to the green fluorescent protein (GFP) and expressed in U2OS cells. After examining GFP positive cells, we observed that ≈17% of the cells expressing GFP-p53 showed fluorescence in both nucleus and cytoplasm, although fluorescence in the remaining 83% was largely nuclear. This result is consistent with a published result (20). Similarly, 18% of the cells expressing GFP-T55D exhibited fluorescence in both the nucleus and cytoplasm. By comparison, only 3% of the cells expressing GFP-T55A exhibit fluorescence in both nucleus and cytoplasm (Fig. 1D). Importantly, apigenin treatment results in less GFP-p53 expressed cells showing GFP in the cytoplasm (4% compared with 17%) within 2 h, whereas GFP-T55A and GFP-T55D transfected cells are unaffected upon the treatment (Fig. 1D and Fig. S3). To exclude the possibility that Ser-392 phosphoylation might affect p53 nuclear localization, we show that apigenin alters GFP-S392A cytoplasmic localization to the same extent as wild-type p53 (Fig. S3). Together, these results demonstrate that Thr-55 phosphorylation plays a direct role in p53 subcellular distribution.
Because the TAF1 kinase is mainly localized in the nucleus, we considered the possibility that TAF1 phosphorylates p53 in the nucleus, which results in p53 nuclear export. p53 nuclear export is mediated through the nuclear export factor CRM1 (15), which can be specifically blocked by leptomycin B (LMB) (21). We therefore treated U2OS cells with LMB to block p53 nuclear export and assayed subcellular localization of Thr-55-phosphorylated p53. As shown in Fig. 1C (LMB), LMB treatment indeed restores localization of Thr-55-phosphorylated p53 in the nucleus, suggesting that TAF1-mediated Thr-55 phosphorylation is likely to lead to p53 nuclear export through the CRM1 pathway.
Thr-55 Phosphorylation Is Required for MDM2-induced p53-CRM1 Interaction and p53 Nuclear Export.
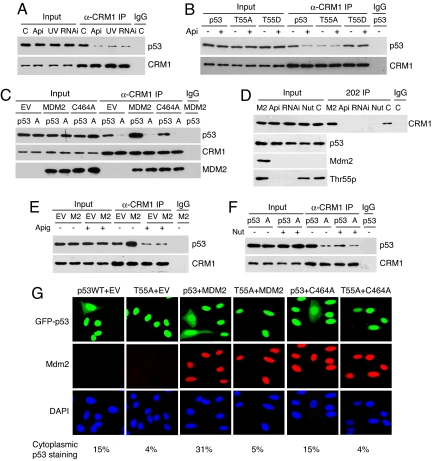
The above data raised the question of whether Thr-55 phosphorylation could affect the interaction of p53 with CRM1. To test this, we first treated U2OS cells with apigenin, TAF1-RNAi, or UV to block Thr-55 phosphorylation and assayed its effect on the endogenous p53-CRM1 association. As shown in Fig. 2A, p53 interacts with CRM1 under our assay conditions, and more importantly, treating cells with apigenin, TAF1-RANi, UV, or LMB (Fig. S4) significantly reduced the interaction. To ensure that Thr-55 phosphorylated p53 indeed interacts with CRM1, we verified the interaction by using Thr-55 phospho-specific antibody (Fig. 2D). Furthermore, we show that wild-type p53 and T55D interact with CRM1 more effectively than T55A in H1299 cells (Fig. 2B) and apigenin inhibits the association of CRM1 with wild-type p53 but not T55D (Fig. 2B). These data suggest that Thr-55 phosphorylation promotes the p53-CRM1 interaction, which leads to p53 nuclear export.
Fig. 2.
Thr-55 phosphorylation of p53 is required for MDM2-induced p53-CRM1 interaction and p53 nuclear export. (A–C, E, and F) The association of p53 with CRM1 was detected by immunoprecipitation with anti-CRM1 antibody and immunoblotting with anti-p53 antibody. (A) U2OS cells were either untreated (C) or treated with apigenin (Api), TAF1-RNAi (RNAi), or UV in the presence of MG132. (B) H1299 cells were transfected with either wild-type p53, T55A, or T55D in the presence or absence of apigenin. (C) Mdm2−/−;p53−/− MEF cells were transfected with either wild-type p53 or T55A (A) and MDM2 or C464A in the presence of MG132. (D) U2OS cells were treated with apigenin, TAF1-RNAi, or nutlin-3 (Nut) or transfected with MDM2 (M2) in the presence of MG132. The association of Thr-55 phosphorylated p53 with CRM1 was detected by immunoprecipitation with Thr-55 phospho-specific antibody (202 IP) and immunoblotting with anti-CRM1 antibody. (E) U2OS cells were transfected with either an empty vector (EV) or MDM2, and treated with or without apigenin in the presence of MG132. (F) H1299 cells were transfected with either wild-type p53 or T55A and treated or untreated with nutlin-3. (G) Cellular localization of GFP-p53WT or GFP-T55A was detected in the presence or absence of MDM2 or C464A in U2OS cells. Five hundred GFP- and MDM2-positive cells were counted from each transfection. The appearance of p53 staining in both cytoplasm and nucleus was scored and shown as the percentage of total transfected cells.
Because MDM2 has been reported to regulate nuclear export of p53 (20, 22, 23), we investigated the role of Thr-55 phosphorylation in this process. Overexpression of MDM2 significantly enhances the interaction of CRM1 with wild-type p53 but not T55A in Mdm2−/−;p53−/− MEF (Fig. 2C). In contrast, a RING-finger-domain mutant of MDM2, C464A, that was unable to induce p53 nuclear export (20) fails to increase the CRM1-p53 interaction (Fig. 2C). Further, we show that MDM2 enhances the binding of CRM1 to Thr-55 phosphorylated p53 (Fig. 2D). These results suggest that MDM2 enhances the p53-CRM1 interaction in a RING-finger domain dependent manner, and more importantly, Thr-55 phosphorylation is required for this process. Interestingly, with normalized p53 protein levels, wild-type p53, but not T55A was able to interact with CRM1 weakly in the absence of MDM2, suggesting that Thr-55 phosphorylation also regulates the p53-CRM1 interaction in an MDM2-independent manner (Fig. 2C).
To confirm the result that Thr-55 phosphorylation is required for MDM2-induced p53-CRM1 interaction, we show that block of Thr-55 phosphorylation by apigenin completely abolishes MDM2-induced p53-CRM1 interaction in U2OS cells (Fig. 2E). To ensure that MDM2 promotes the p53-CRM1 interaction through interacting with p53, we treated cells with nutlin-3 that specifically blocks the p53-MDM2 interaction (24). The assay shows that nutlin-3 treatment significantly reduces the interaction of CRM1 with p53 (Fig. 2F) and with Thr-55 phosphorylated p53 (Fig. 2D). Collectively, these results suggest that Thr-55 phosphorylation is required for MDM2-induced p53-CRM1 interaction.
To test whether Thr-55 phosphorylation of p53 is required for MDM2-induced p53 nuclear export, we overexpressed MDM2 and C464A in U2OS cells and examined the subcellular localization of GFP-p53 or GFP-T55A. The assay shows that MDM2 enhances wild-type p53 but not T55A cytoplasmic localization (Fig. 2G). By comparison, the C464A mutant was unable to alter p53 localization. Collectively, these results suggest that MDM2 induces the CRM1-p53 interaction and subsequently promotes p53 nuclear export, and significantly, Thr-55 phosphorylation of p53 plays a critical role in this process.
Inhibition of Thr-55 Phosphorylation by Apigenin Restores p53 Nuclear Localization in Neuroblastoma Cells.
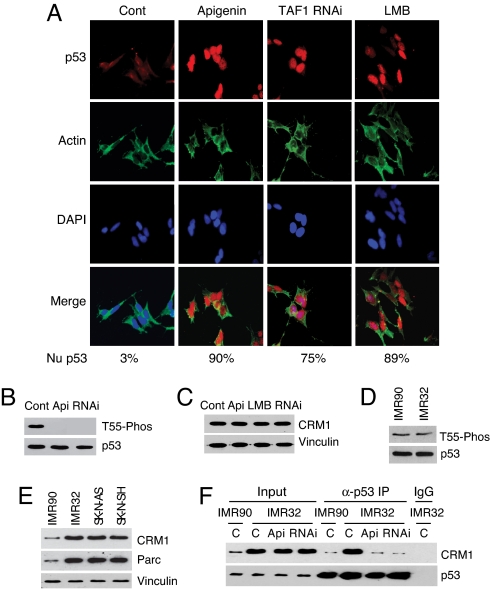
Several types of human tumors, such as undifferentiated neuroblastoma and inflammatory breast carcinoma, harbor inactive wild-type p53 because of its abnormal cytoplasmic localization (3, 4). The mechanisms by which p53 is localized in the cytoplasm of these cells are not completely understood. Our observation that Thr-55 phosphorylation enhances p53 nuclear export prompted us to examine whether Thr-55 phosphorylation may play a role in the abnormal localization of p53 and, more importantly, whether inhibition of Thr-55 phosphorylation may restore p53 nuclear localization in these cancer cells. As illustrated in Fig. 3, apigenin treatment inhibits Thr-55 phosphorylation (Fig. 3B) and results in more p53 in the nucleus (Fig. 3A), suggesting that apigenin restores p53 nuclear localization in neuroblastoma IMR32 cells. To confirm this result, we show that GFP-p53 and GFP-T55D are more nuclear localized than GFP-T55A in IMR32 cells, and apigenin treatment leads to GFP-p53 but not GFP-T55D nuclear localization (Fig. S5). Furthermore, we show that inhibition of TAF1 by specific RNAi oligos also restores p53 nuclear localization in neuroblastoma IMR32 cells (Fig. 3A). As expected, treating cells with LMB leads to p53 nuclear localization (Fig. 3A). To provide evidence for the specificity of apigenin treatment, we show that apigenin does not affect p53 phosphorylation on Ser-15, Ser-20, and Ser-46, and acetylation on Lys-373 in neuroblastoma IMR32 cells (Fig. S1). To exclude any effect that apigenin might have on the CRM1 pathway in neuroblastoma IMR32 cells, we show that apigenin was unable to restore a cytoplasmic localized PTEN construct (NES-PTEN that was tagged with a CRM1 nuclear export signal) (25) and endogenous STAT3 into the nucleus, whereas LMB does (Fig. S6). In addition, the CRM1 protein levels were not affected under our experimental conditions (Fig. 3C). These data suggest that apigenin specifically restores p53 nuclear localization through inhibition of Thr-55 phosphorylation in neuroblastoma cells.
Fig. 3.
Inhibition of Thr-55 phosphorylation restores p53 nuclear localization in neuroblastoma cells. (A) Neuroblastoma IMR32 cells were either untreated (Cont) or treated with apigenin, TAF1 RNAi, or LMB and processed for immunostaining. The same cells were also stained with DAPI. (B) The level of Thr-55 phosphorylated p53 was detected in IMR32 cells with or without apigenin or TAF1 RNAi treatment. (C) The CRM1 levels were examined by immunoblotting in IMR32 cells treated or untreated with apigenin, LMB, and TAF1 RNAi. (D) The levels of Thr-55 phosphorylated p53 were detected in IMR90 and IMR32 cells. (E) The levels of CRM1 and Parc were detected in IMR90, IMR32, SK-N-AS, and SK-N-SH cells. (F) The association of p53 with CRM1 was detected in IMR90 and IMR32 cells in the absence (C) or presence of apigenin or TAF1 RNAi treatment.
Because inhibition of Thr-55 phosphorylation led to p53 nuclear localization in IMR32 cells, we speculated that neuroblastoma cells might have higher Thr-55 phosphorylation levels. Therefore, we compared Thr-55 phosphorylation levels in IMR32 cells with those in a normal human cell line, IMR90. To our surprise, we found comparable levels of Thr-55 phosphorylation in both cell lines (Fig. 3D). Interestingly, however, the CRM1 protein is highly expressed in IMR32 and another 2 neuroblastoma cell lines, SK-N-AS and SK-N-SH examined (Fig. 3E). Importantly, the elevated CRM1 protein level leads to an increased p53-CRM1 interaction in IMR32 cells, which can be blocked by apigenin and TAF1-RNAi (Fig. 3F). These results suggest that inhibition of Thr-55 phosphorylation blocks the p53-CRM1 interaction and subsequently restores p53 nuclear localization in neuroblastoma cells. As reported, the p53 cytoplasmic anchor protein Parc is also highly expressed in these neuroblastoma cells (Fig. 3E) (26). Our data suggest that, in addition to Parc, the elevated CRM1 protein level and resultant p53-CRM1 interaction also contributes to p53 cytoplasmic localization in neuroblastoma cells.
Apigenin Sensitizes Neuroblastoma Cells to the Anticancer Drug Etoposide.
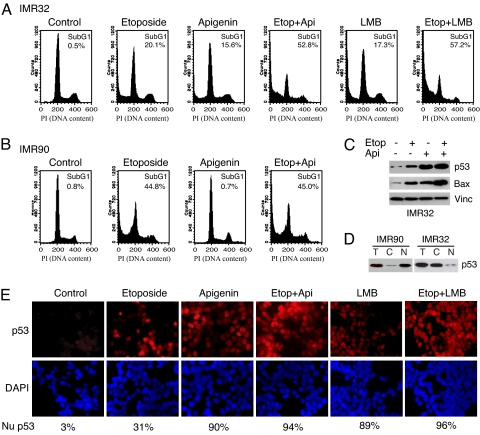
Neuroblastoma cells often exhibit an impaired response to low levels of DNA damage drug treatment (27). Because Thr-55 phosphorylation enhances p53 nuclear export via CRM1, we explore whether inhibition of Thr-55 by apigenin could sensitize neuroblastoma cells to DNA damage. Consistent with a previous report (26), IMR32 neuroblastoma cells respond poorly to low doses of the genotoxic drug etoposide: Only 20% of the cells became apoptotic (Fig. 4A). However, if cells were pretreated with 40 μM apigenin, significantly more cells (53%) became apoptotic in response to the same dose of etoposide (Fig. 4A). Treating cells with apigenin alone only had a moderate effect (15%) on apoptosis (Fig. 4A). To confirm this result, we show that apigenin treatment also increases expression of the p53 target gene Bax in response to the low dose of etoposide in IMR32 cells (Fig. 4C). Furthermore, LMB treatment in combination with the same dose of etoposide also leads to an increase in cell apoptotic response (57%), whereas LMB alone only had a moderate effect (17%) on apoptosis (Fig. 4A). Restoration of p53 nuclear localization by apigenin or LMB in IMR32 cells was confirmed by immunostaining (Fig. 4E). These results suggest that apigenin sensitizes neuroblastoma cells to DNA damage drug by restoring p53 nuclear localization. To verify this result, we show that IMR90 cells where p53 was mainly distributed in the nucleus (Fig. 4D) undergo apoptosis more effectively in response to the low dose of etoposide (45%), and more relevantly, apigenin at 40 μM was unable to sensitize these cells to DNA damage (Fig. 4B). Collectively, these results suggest that apigenin sensitizes neuroblastoma cells to DNA damage drug through restoring p53 nuclear localization.
Fig. 4.
Apigenin sensitizes neuroblastoma cells to the anticancer drug etoposide. (A) IMR32 cells were treated with 10 μM etoposide in the presence or absence of apigenin or LMB and processed for FACS analysis for apoptotic cells (subG1) according to DNA content (PI staining). (B) IMR90 cells were treated with 10 μM etoposide in the presence or absence of apigenin and processed for FACS analysis for apoptotic cells. (C) The levels of p53 and Bax in IMR32 and IMP90 cells that were either untreated or treated with apigenin and etoposide as indicated on the top. (D) IMR32 or IMR90 cells were fractionated and the p53 protein levels were detected in the cytoplasmic (C) and nuclear (N) fractions. (E) IMR32 cells were immunostained with anti-p53 antibody in the presence or absence of apigenin, LMB, or etoposide treatment. The appearance of p53 staining in the nucleus (Nu p53) was scored and shown as the percentage of total cells.
Discussion
p53 is diffusely distributed in normal cells and, upon DNA damage, p53 translocates to the nucleus and functions to activate target genes. In view of the importance of nuclear localization of p53 in tumor suppression, it is striking that inhibition of Thr-55 phosphorylation restores p53 nuclear localization. Furthermore, Thr-55 phosphorylation induces the association of p53 with the nuclear exporter CRM1, thus providing new insight into the regulation of p53 nucleocytoplasmic shuttling.
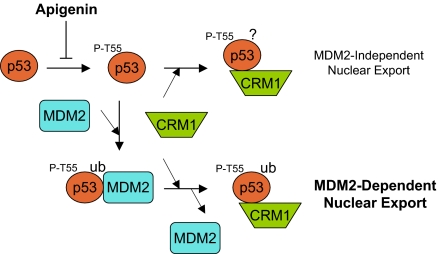
One of the most important regulators of p53 is MDM2, a ring domain E3 ligase that ubiquitinates p53, leading to p53 degradation and cytoplasmic localization. MDM2 has been shown to mediate monoubiquitination of p53 in the nucleus, which promotes p53 nuclear export by unmasking the NES of p53 (20, 23, 28, 29). Our studies establish that Thr-55 phosphorylation is required for MDM2-induced p53-CRM1 interaction and nuclear export. This is perhaps not surprising because Thr-55 phosphorylation has been shown to promote the p53-MDM2 interaction (8), which could lead to monoubiquitination of p53 and exposure of its NES. Interestingly, however, our data also suggest that Thr-55 phosphorylation enhances the p53-CRM1 interaction independent of MDM2. Thus, Thr-55 phosphorylation may play a dual role in access of p53 to the nuclear export machinery (Fig. 5). In the absence of MDM2, Thr-55 phosphorylation enhances the p53-CRM1 interaction, which leads to nuclear export of p53 in Mdm2-null cells. Because monoubiquitination of p53 has been shown to play a role in unmasking the p53 NES, other E3s may monoubiquitinate p53 in this process. In the presence of MDM2, however, Thr-55 phosphorylation probably largely affects the p53-MDM2 interaction, resulting in monoubiquitination of p53. In this setting, monoubiquitinated p53 unmasks its NES, binds to CRM1 and dissociates from MDM2 (29). Because MDM2 significantly enhances the p53-CRM1 interaction, this second mechanism probably represents a major pathway for nuclear export of p53 when MDM2 is present in the cell.
Fig. 5.
A proposed model for the regulation of p53 nuclear export by Thr-55 phosphorylation. In the absence of MDM2, Thr-55 phosphorylation enhances the p53-CRM1 interaction, which leads to nuclear export of p53 in MDM2-null cells. In the presence of MDM2, however, Thr-55 phosphorylation affects the p53-MDM2 interaction, resulting in monoubiquitination of p53, which unmasks p53 NES and promotes CRM1 binding.
Finally emerging evidence has begun to indicate that natural compounds play an important in cancer prevention and treatment. In the present study, we demonstrate that apigenin completely restores p53 nuclear localization in neuroblastoma cells through inhibition of Thr-55 phosphorylation. Apigenin is a natural flavonoid found in fruits and vegetables and has been shown to have antitumor effects in several human tumor cell lines (30). In fact, a recent study suggests that apigenin induces apoptosis of the human neuroblastoma cells and inhibits neuroblastoma cell xenograft tumor growth in a nonobese diabetic/severe combined immunodeficiency mouse model (31). Despite the progress that has been made in identifying the significance of apigenin in tumor suppression, little is known about its molecular targets. Our study establishes that apigenin specifically restores p53 nuclear localization and thus provides a molecular basis of using apigenin for targeting cancers caused by abnormal cytoplasm localization of wild-type p53. It is generally thought that the key to effective therapies is the identification of drugs that affect cancer cell but dispensable to normal cells. In this respect, apigenin has been shown to neither inhibit survival of primary sympathetic neurons (31) nor induce apoptosis of normal human cell line IMR90 (Fig. 4), suggesting that perhaps this dietary flavonoid is not toxic to nontransformed cells and thus could be used to specifically sensitize cancers caused by abnormal cytoplasm localization of wild-type p53.
Materials and Methods
Reagents and Transfection.
Wild-type p53, T55A, or T55D was amplified by PCR from pcDNA3.1-p53, pcDNA3-T55A, and pcDNA3-T55D (8, 32) and cloned into the pEGFP-C3 plasmid. To detect GFP signals, U2OS cells were transfected with 0.1 μg of GFP-p53, GFP-T55A, or GFP-T55D by using Lipofectamine (Invitrogen). For TAF1 knockdown, 2 21-nt siRNA duplexes (8) or a negative-control siRNA duplex (Qiagen) were introduced into cells by using Lipofectamine 2000 (Invitrogen). To test the p53-CRM1 association in U2OS cells, cells were pretreated with 20 μM MG132, then treated with either 40 μM apigenin (Sigma) for 3 h or 5 ng/ml LMB for 4 h. To test the p53-CRM1 association in H1299 cells, cells were transfected with 1 μg of various p53 expression vectors (p53, T55A, or T55D), treated with 40 μM apigenin for 3 h or 4 μM nutlin-3 (Cayman Chemical) for 24 h. To test the effect of MDM2 on the p53-CRM1 interaction, cells were transfected with 1 μg of various p53 expression vectors and 3 μg of wild-type MDM2 (pCHDM1B) or MDM2 RING-finger mutant expression vector (pCMV-C464A, Addgene).
Immunostaining, Immunoblotting, and Immunoprecipitation.
Immunostaining assays were performed with anti-p53 (FL393, Santa Cruz), and anti-actin (AC-15, Sigma) antibodies as described in ref. 25. Images were obtained with a Nikon E-800 fluorescence microscope. Antibodies used for immunoblotting were anti-p53 (DO-1, Santa Cruz), anti-TAF1 (6B3, Santa Cruz), anti-Bax (P-19, Santa Cruz), anti-CRM1 (H-300, Santa Cruz), anti-Parc (PRC069, Biolegend), anti-Vinculin (VIN-11–5, Sigma), anti-MDM2 (SMP-14, Santa Cruz), and anti-actin antibodies. To detect the p53-CRM1 association, 1.2 mL of cell lysate from 4 × 107 cells was incubated with 1 μg of anti-CRM1 antibody and the resulting immunocomplexes were analyzed by immunoblotting with anti-p53 antibody. To detect Thr-55 phosphorylation, the cell lysate was immunoprecipitated with phospho-specific antibody for Thr-55 (Ab202) (8) and immunoblotted with anti-p53 antibody.
Cellular Fractionation.
Approximately 2 × 107 cells were collected and resuspended in 400 μl of 1:5 diluted buffer A [50 mM Hepes (pH 7.4) 1 mM EDTA, 10 mM mannitol, 1 mM DTT, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 1 mM PMSF]. After incubation on ice for 10 min, cells were homogenized with 25 G needle for 10 strokes. After brief centrifugation at 6,000 × g at 4 °C, the supernatant (cytoplasmic fraction) was collected, and 100 μl of 5× buffer A was added. The pellet was washed with buffer A and then resuspended in 500 μL of l-buffer [50 mM Tris (pH 8.0), 120 mM NaCl, 0.5% Nonidet P-40, 1 mM DTT, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 1 mM PMSF]. After centrifugation, the supernatant (nuclear fraction) was collected.
Cell Apoptosis Analysis.
IMR32 or IMR90 cells were untreated or pretreated with either 40 μM apigenin or 5 ng/ml LMB for 3 h before treating cells with 10 μM etoposide for 14 h. 28 h after the etoposide treatment, cells were harvested, fixed, stained with propidium iodide, and analyzed by FACScan flow cytometry (Becton Dickinson) for apoptotic cells (subG1) according to DNA content.
Supplementary Material
Acknowledgments.
We thank W. Gu for providing IMR90 and IMR32 cell lines, D. Ethell for providing the SK-N-SH cell line, F.M. Sladek for providing pEGFP-C3 plasmid, J. Bachant and all members of our laboratory for helpful discussions, and an anonymous reviewer for an incisive suggestion. This work was supported by National Institutes of Health Grant CA075180 from the National Institute of Cancer.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804608105/DCSupplemental.
References
- 1.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 2.Sharpless NE, DePinho RA. p53: Good cop/bad cop. Cell. 2002;110:9–12. doi: 10.1016/s0092-8674(02)00818-8. [DOI] [PubMed] [Google Scholar]
- 3.Moll UM, Riou G, Levine AJ. Two distinct mechanisms alter p53 in breast cancer: Mutation and nuclear exclusion. Proc Natl Acad Sci USA. 1992;89:7262–7266. doi: 10.1073/pnas.89.15.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moll UM, LaQuaglia M, Benard J, Riou G. Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc Natl Acad Sci USA. 1995;92:4407–4411. doi: 10.1073/pnas.92.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang SH, Clarke MF. Regulation of p53 localization. Eur J Biochem. 2001;268:2779–2783. doi: 10.1046/j.1432-1327.2001.02227.x. [DOI] [PubMed] [Google Scholar]
- 6.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 7.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 8.Li H-H, Li AG, Sheppard HM, Liu X. Phosphorylation on Thr55 by TAF1 mediates degradation of p53: A role for TAF1 in cell G1 progression. Mol Cell. 2004;13:867–878. doi: 10.1016/s1097-2765(04)00123-6. [DOI] [PubMed] [Google Scholar]
- 9.Sekiguchi T, Nohiro Y, Nakamura Y, Hisamoto N, Nishimoto T. The human CCG1 gene, essential for progression of the G1 phase, encodes a 210-kilodalton nuclear DNA-binding protein. Mol Cell Biol. 1991;11:3317–3325. doi: 10.1128/mcb.11.6.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekiguchi T, et al. Apoptosis is induced in BHK cells by the tsBN462/13 mutation in the CCG1/TAFII250 subunit of the TFIID basal transcription factor. Exp Cell Res. 1995;218:490–498. doi: 10.1006/excr.1995.1183. [DOI] [PubMed] [Google Scholar]
- 11.Wang EH, Tjian R. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science. 1994;263:811–814. doi: 10.1126/science.8303298. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien T, Tjian R. Functional analysis of the human TAFII250 N-terminal kinase domain. Mol Cell. 1998;1:905–911. doi: 10.1016/s1097-2765(00)80089-1. [DOI] [PubMed] [Google Scholar]
- 13.Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18:7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Keefe K, Li H, Zhang Y. Nucleocytoplasmic shuttling of p53 is essential for MDM2-mediated cytoplasmic degradation but not ubiquitination. Mol Cell Biol. 2003;23:6396–6405. doi: 10.1128/MCB.23.18.6396-6405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stommel JM, et al. A leucine-rich nuclear export signal in the p53 tetramerization domain: Regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999;18:1660–1672. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Xiong Y. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science. 2001;292:1910–1915. doi: 10.1126/science.1058637. [DOI] [PubMed] [Google Scholar]
- 17.Critchfield JW, Coligan JE, Folks TM, Butera ST. Casein kinase II is a selective target of HIV-1 transcriptional inhibitors. Proc Natl Acad Sci USA. 1997;94:6110–6115. doi: 10.1073/pnas.94.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai SC, Seto E. Regulation of histone deacetylase 2 by protein kinase CK2. J Biol Chem. 2002;277:31826–31833. doi: 10.1074/jbc.M204149200. [DOI] [PubMed] [Google Scholar]
- 19.Fiscella M, et al. The carboxy-terminal serine 392 phosphorylation site of human p53 is not required for wild-type activities. Oncogene. 1994;9:3249–3257. [PubMed] [Google Scholar]
- 20.Boyd D, Tsai KY, Jacks T. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat Cell Biol. 2000;9:563–568. doi: 10.1038/35023500. [DOI] [PubMed] [Google Scholar]
- 21.Kudo N, et al. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 22.Tao W, Levine AJ. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc Natl Acad Sci USA. 1999;96:3077–3080. doi: 10.1073/pnas.96.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geyer RK, Yu ZK, Maki CG. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat Cell Biol. 2000;9:569–573. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- 24.Vassilev LT, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;30:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 25.Li AG, et al. Mechanistic insights into maintenance of high p53 acetylation by PTEN. Mol Cell. 2006;23:575–587. doi: 10.1016/j.molcel.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Nikolaev A, Li M, Puskas N, Qin J, Gu W. Parc: A cytoplasmic anchor for p53. Cell. 2003;112:29–40. doi: 10.1016/s0092-8674(02)01255-2. [DOI] [PubMed] [Google Scholar]
- 27.Moll UM, et al. Cytoplasmic sequestration of wild-type p53 protein impairs the G1 checkpoint after DNA damage. Mol Cell Biol. 1996;16:1126–1137. doi: 10.1128/mcb.16.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, et al. Mono- versus polyubiquitination: Differential control of p53 fate by Mdm2. Science. 2003;302:1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- 29.Carter S, Bischof O, Dejean A, Vousden KH. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol. 2007;4:428–435. doi: 10.1038/ncb1562. [DOI] [PubMed] [Google Scholar]
- 30.Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Rad Biol Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 31.Torkin R, Lavoie J-F, Kaplan DR, Yeger H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol Cancer Ther. 2005;4:1–11. [PubMed] [Google Scholar]
- 32.Shouse GP, Cai X, Liu X. Serine-15 phosphorylation of p53 directs its interaction with B56γ and the tumor suppressor activity of B56γ-specific PP2A. Mol Cell Biol. 2008;28:448–456. doi: 10.1128/MCB.00983-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.