Abstract
Osteoblasts play an increasingly recognized role in supporting hematopoietic development and recently have been implicated in the regulation of B lymphopoiesis. Here we demonstrate that the heterotrimeric G protein α subunit Gsα is required in cells of the osteoblast lineage for normal postnatal B lymphocyte production. Deletion of Gsα early in the osteoblast lineage results in a 59% decrease in the percentage of B cell precursors in the bone marrow. Analysis of peripheral blood from mutant mice revealed a 67% decrease in the number of circulating B lymphocytes by 10 days of age. Strikingly, other mature hematopoietic lineages are not decreased significantly. Mice lacking Gsα in cells of the osteoblast lineage exhibit a reduction in pro-B and pre-B cells. Furthermore, interleukin (IL)-7 expression is attenuated in Gsα-deficient osteoblasts, and exogenous IL-7 is able to restore B cell precursor populations in the bone marrow of mutant mice. Finally, the defect in B lymphopoiesis can be rescued by transplantation into a WT microenvironment. These findings confirm that osteoblasts are an important component of the B lymphocyte niche and demonstrate in vivo that Gsα-dependent signaling pathways in cells of the osteoblast lineage extrinsically regulate bone marrow B lymphopoiesis, at least partially in an IL-7-dependent manner.
Keywords: B lymphocyte, G protein, osteoblast
Beginning in late embryogenesis, mammalian hematopoietic development occurs primarily in the bone marrow, where the presence of a supportive microenvironment, or niche, has long been postulated (1). The contribution of marrow stromal cells to this niche has been well documented and is supported by the identification of stromal cell lines that have the ability to support hematopoietic cell differentiation in vitro. The stroma of the bone marrow is composed of cells of various lineages, including osteoblasts, endothelial cells, fibroblasts, and adipocytes, but the relative contributions of each lineage have remained elusive. The importance of osteoblastic cells to the hematopoietic stem cell (HSC) niche has been demonstrated by several groups (2–5). In addition, a vascular component of the HSC niche has been proposed based on histological localization of stem cells (6). Whether or not osteoblastic and vascular cells represent distinct niches remains to be clarified.
Beyond its support for HSCs, the stromal microenvironment within the bone marrow appears to provide specific niches for more differentiated hematopoietic lineages, including B lymphocytes (7) and megakaryocytes (8). The relevance of extrinsic control of hematopoiesis to disease pathogenesis has been underscored by the finding that the microenvironment plays an integral role in the development of myeloproliferative syndromes (9, 10). Thus, the bone marrow may harbor distinct niches for differentiating cells of hematopoietic origin. The cellular constituents and relevant signaling molecules at each stage remain largely undefined, however.
A specific niche within the bone marrow for B lymphocyte differentiation was first proposed by Tokoyoda et al. (7), who demonstrated that within the bone marrow, B cell precursors are in direct contact with stromal cells that express CXCL12 or interleukin (IL)-7, two factors that play crucial roles in B lymphopoiesis. These stromal cells are located within the marrow space and do not co-localize with the osteoblasts lining the bone surface. Because the HSC niche has been proposed to lie along the endosteal surface (11, 12), these findings suggest that a B lymphocyte niche may reside in a distinct anatomic localization within the bone marrow. Moreover, Tokoyoda et al. (7) found that although prepro-B cells (the most immature identifiable population of B lymphocyte precursors) are in direct contact with cells expressing CXCL12, more differentiated pro-B cells instead contact stromal cells expressing IL-7. Thus, distinct subsets of stromal cells may be involved in regulating the transition of B lymphocytes from one stage of differentiation to the next.
Osteoblasts cultured from neonatal calvariae are capable of supporting all stages of B lymphocyte development from HSCs in vitro (13). Moreover, ablation of osteoblasts in vivo results in a rapid reduction in the number of B lymphocytes preceding the loss of HSCs (4, 13). These findings point to an integral role for osteoblastic cells in supporting B lymphopoiesis. Although mature osteoblasts line the bone surface, osteoblastic progenitors are present within the stromal cells of the bone marrow. In addition, cells of the osteoblast lineage can produce both CXCL12 and IL-7 (2, 13, 14). Therefore, immature osteoblast precursors within the marrow may play an important role in regulating B lymphocyte differentiation, perhaps in part through production of regulatory growth factors, such as CXCL12 and/or IL-7.
In recent years, the contribution of signaling through the parathyroid hormone (PTH)/PTH-related peptide receptor (PPR) in osteoblastic cells to the regulation of HSC numbers was demonstrated in mice expressing a constitutively active form of PPR targeted to osteoblasts. These mice displayed a dramatic increase in trabecular bone, accompanied by an increase in HSC numbers (2). Intriguingly, the addition of PTH to calvarial osteoblast cultures was found to stimulate the support of B lymphopoiesis in vitro (13). Moreover, PTH is known to up-regulate production of both CXCL12 and IL-7 by osteoblastic cells in vitro (2, 13, 14), suggesting that signaling downstream of the PPR may be relevant to regulation of B lymphopoiesis in addition to HSCs.
The PPR is a G protein-coupled receptor (GPCR) that signals through multiple G proteins. One major downstream mediator of PPR signaling is Gsα, which activates adenylyl cyclase, increasing levels of adenosine 3′,5′-cyclic monophosphate (cAMP) (15). cAMP activates such effectors as protein kinase A (PKA), which in turn regulates gene transcription through phosphorylation of cAMP response element-binding proteins and other targets (16). Besides PPR, other GPCRs also activate Gsα in cells of the osteoblast lineage, including type 2 β adrenergic receptor (17), prostaglandin E2 receptors EP2R and EP4R (18), and thyroid-stimulating hormone receptor (19). Thus, the actions of Gsα on osteoblastic cells may reflect multiple inputs and may exceed the actions of any single ligand/receptor system. Indeed, deletion of Gsα in differentiated osteoblasts has demonstrated the importance of Gsα in trabecular bone (20). Because the aforementioned in vitro studies (13) suggest that signals downstream of the PPR might be able to regulate B lymphopoiesis, we hypothesized that Gsα may play an important role in regulating B lymphocyte development in vivo.
To test this hypothesis, we ablated Gsα in early osteoprogenitors using Cre recombinase driven by the promoter for osterix, a transcription factor expressed early in cells of the osteoblast lineage (21). The resulting mutant mice displayed a profound decrease in trabecular bone. Analysis of the bone marrow revealed a specific reduction in B cell precursors, with preservation of other hematopoietic lineages. The defect in B lymphopoiesis led to a lower number of precursors after the prepro-B cell stage. Our findings confirm that osteoblast lineage cells participate in a specific B lymphocyte niche within the bone marrow. Furthermore, we found that Gsα-dependent signaling pathways are crucial to the regulation of B lymphopoiesis by cells of the osteoblast lineage, and that IL-7 may be an important mediator of this process.
Results
Trabecular Bone Is Reduced in Mice with Conditional Knockout of Gsα in Osteoblast Precursors.
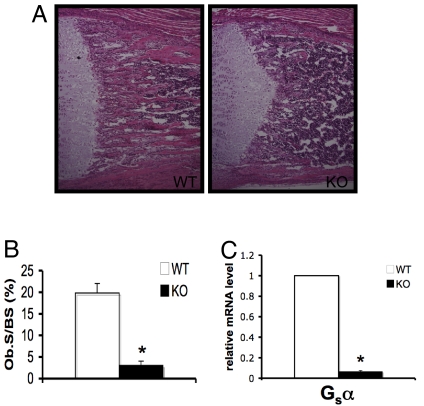
Gsα was ablated in osteoblast precursors using transgenic mice in which Cre recombinase is fused to green fluorescent protein (GFP), under the control of the promoter for osterix, a transcription factor expressed early in osteoblastogenesis (Osx1-GFP::Cre) (22). These mice were mated to mice carrying loxP sites flanking exon 1 of the gene encoding Gsα (23). The resulting mutant mice (Osx1-GFP::Cre+;Gsαfl/fl, designated GsαOsxKO mice) are born at the expected Mendelian ratio and are indistinguishable from control (Gsαfl/fl) littermates at birth; however, GsαOsxKO mice develop postnatal growth retardation [supporting information (SI) Fig. S1A and B] and early mortality, with most GsαOsxKO mice dying by postnatal day 14 and none surviving past the first month of life. GsαOsxKO mice display a dramatic reduction in the amount of trabecular bone (Fig. 1 A and B). GFP driven by osterix is expressed throughout the stages of osteoblast differentiation (22); thus, in Osx1-GFP::Cre+ mice, the GFP+ cell population will include most of the osteoblast lineage cells (Fig. S1C). To confirm efficient deletion of Gsα in the osteoblasts of GsαOsxKO mice, we isolated GFP+ osteoblastic cells by fluorescence-activated cell sorting (FACS) from mice carrying the Osx1-GFP::Cre+ transgene and their GsαOsxKO littermates. We found that Gsα mRNA levels were reduced by almost 90% in these cells in GsαOsxKO mice (Fig. 1C).
Fig. 1.
Trabecular bone is decreased in GsαOsxKO mice. (A) Hematoxylin and eosin–stained sections of proximal tibia at postnatal day 9 from WT and GsαOsxKO (KO) mice. (B) Osteoblast surface (ObS/BS) as a percentage of bone surface (n = 7 [WT] or 5 [KO]). (C) Quantitative real-time PCR for Gsα mRNA levels from GFP+ osteoblastic cells isolated by FACS. *P < .005.
Ablation of Gsα in Osteoblast Precursors Leads to a Failure of B Lymphopoiesis.
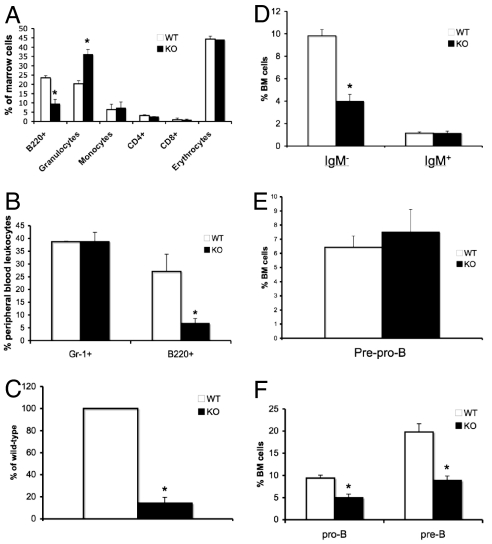
To determine whether the loss of Gsα alters the ability of osteoblast precursors to support hematopoietic development within the bone marrow, we analyzed mature hematopoietic lineages in the marrow using flow cytometry. Because the GsαOsxKO mice were significantly smaller than their control littermates, skeletal size and thus bone marrow cellularity also were decreased in GsαOsxKO mice (Fig. S1D); therefore, the distribution of hematopoietic lineages is reported as a percentage of bone marrow cells. In the bone marrow of the GsαOsxKO mice, we found a specific reduction in the B220+ population, which includes cells of the B lymphocyte lineage. A statistically significant increase in the percentage of CD11b+/GR-1+ granulocytes was found, whereas F480+/CD11b+ monocytes, Ter119+ erythrocytes, and CD4+ and CD8+ mature T lymphocytes were unaffected (Fig. 2A). This defect in bone marrow B lineage cell content was accompanied by a reduction of circulating B220+ cells. By postnatal day 10, there were 68% fewer B220+ cells in the peripheral blood of the GsαOsxKO mice; in contrast, the circulating Gr-1+ myeloid content remained unchanged (Fig. 2B). Furthermore, the spleens of GsαOsxKO mice were markedly smaller than those of their littermate controls. Even when corrected for body weight, a 50% reduction in the ratio of spleen to body weight was seen in the GsαOsxKO mice; other organs (e.g., kidney, heart, lung) remained unaffected (Fig. S2A and data not shown). Histological analysis demonstrated that white pulp, consisting of lymphocytes in a follicular arrangement, was dramatically reduced in the spleens of the GsαOsxKO mice (Fig. S2B). Consistent with this finding, the absolute number of B220+ cells within the spleen of GsαOsxKO mice was <20% of that in their WT littermates (Fig. 2C). This finding indicates that the ablation of osteoblast-specific Gsα led to a generalized decrease in B220+ cells, suggesting impaired bone marrow B lymphopoiesis.
Fig. 2.
B lymphopoiesis is impaired in GsαOsxKO mice. (A) Frequency of each lineage, shown as percentage of total marrow cells in postnatal day 10 bone marrow (n = 4). *, P < .005. (B) Gr-1+ myeloid cells and B220+ cells as a percentage of peripheral blood leukocytes at postnatal day 10 (n = 4). *, P < .005. (C) Number of splenic B220+ cells, as a percentage of the number of B220+ cells in WT littermates, is reduced in KO spleens at postnatal day 10 (n = 4). *, P < .001. (D) Percentage of bone marrow cells that are B220+/CD93+/IgM− (Left) or B220+/CD93+/IgM+ (Right) at postnatal day 8 (n = 10 WT or 7 KO). *, P < .05. (E) Percentage of B220+/CD19−/CD43+ prepro-B cells in bone marrow on postnatal day 8 (n = 6). (F) Percentages of pro-B (B220+/IgM−/CD43+) and pre-B (B220+/IgM−/CD43−) cells in bone marrow on postnatal day 8 (n = 3). *, P < .05.
B Lymphopoiesis Is Impaired Along the Pro-B to Pre-B Cell Transition.
B lymphocyte development shifts to the bone marrow in late embryogenesis, with commitment of the common lymphoid progenitor to the B lymphocyte lineage marked by expression of B220, followed by sequential differentiation to prepro-B, pro-B, and pre-B cells (24). Immature, newly formed B lymphocytes, which express IgM, then migrate to the spleen, where further maturation occurs and expression of IgD is acquired. Therefore, a defect in the ability of the bone marrow to support B lymphopoiesis would be expected to affect B cell precursors. Because B220 expression is not exclusive to B lineage cells, we analyzed bone marrow cells for expression of the additional B lineage markers CD93 and IgM. Indeed, the reduction in B lineage cells in GsαOsxKO bone marrow was entirely due to loss of the IgM− precursor population (Fig. 2D). To confirm that osterix-driven Cre recombinase was not expressed in B lineage cells, we analyzed bone marrow cells for GFP expression by flow cytometry. We found that the GsαOsxKO bone marrow B220+ cells, as well as the other hematopoietic cell types, did not express high levels of GFP (Fig. S3A). We next isolated B220+/IgM− B cell precursors from bone marrow of WT and GsαOsxKO mice, and, using quantitative real-time PCR, demonstrated that Gsα mRNA levels remained unchanged in B lineage precursors in the GsαOsxKO mice (Fig. S3B). These results indicate that impaired B lymphopoiesis in the GsαOsxKO mice is an indirect effect of osteoblast-specific Gsα ablation, and not a consequence of ectopic Gsα ablation in the B lymphocyte lineage.
We next sought to determine at which stage B lymphocyte development is affected in the absence of osteoblast-specific Gsα. We found no decrease in B220+CD19−CD43+ prepro-B cells in the GsαOsxKO mice (Fig. 2E), suggesting that the blocking of B lymphopoiesis occurred at a later stage. Indeed, the percentages of both B220+IgM−CD43+ pro-B and B220+IgM−CD43− pre-B cells were significantly reduced in GsαOsxKO bone marrow (Fig. 2F). In contrast to the bone marrow, the fetal liver at e18.5 exhibited no difference in pro-B cells between the WT and GsαOsxKO mice (Fig. S3C). Thus, in the absence of Gsα in osteoblastic lineage cells, B lymphopoiesis was impaired along the pro-B cell to pre-B cell transition, specifically in the bone marrow.
Impaired B Lymphopoiesis Is Rescued by Transplantation into a WT Microenvironment.
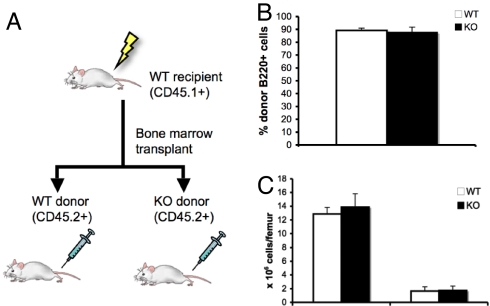
To confirm that the abnormal B lymphopoiesis in GsαOsxKO mice was due to a stromal defect resulting from Gsα deficiency in osteoblasts, we transplanted bone marrow from WT or GsαOsxKO mice into WT recipients. (Transplantation of WT or GsαOsxKO bone marrow into GsαOsxKO recipients is not feasible, because GsαOsxKO mice die before weaning.) WT CD45.1+ mice were irradiated and then underwent bone marrow transplantation, receiving either WT or GsαOsxKO bone marrow. Donor marrow from both WT and GsαOsxKO mice expressed CD45.2, allowing us to distinguish host-derived (CD45.1+) from donor-derived (CD45.2+) hematopoietic cells (Fig. 3A). We analyzed bone marrow 10 weeks posttransplantation and found successful engraftment; transplantation with either WT or GsαOsxKO bone marrow resulted in 90% leukocyte reconstitution by transplanted cells (Fig. 3B). Flow cytometric analysis of the bone marrow revealed that transplantation into a WT environment completely rescued bone marrow cellularity and B lymphopoiesis (Fig. 3C). Thus, the defective B lymphopoiesis in GsαOsxKO mice can be attributed to the loss of Gsα from the stromal microenvironment.
Fig. 3.
Transplantation into WT microenvironment rescues B lymphopoiesis of GsαOsxKO bone marrow. (A) Adult WT lethally irradiated CD45.1+ mice received bone marrow transplants from WT or KO CD45.2+ donors. (B) Percentage of donor (CD45.1+) B220+ cells in recipients of WT and KO bone marrow transplantation. (C) Number of total bone marrow mononuclear cells (Left) and B220+/IgM− immature B cell precursors (Right) in recipients of WT (n = 5) and KO (n = 4) bone marrow.
IL-7 Expression, but Not CXCL12 Expression, Is Decreased in Gsα-Deficient Osteoblasts.
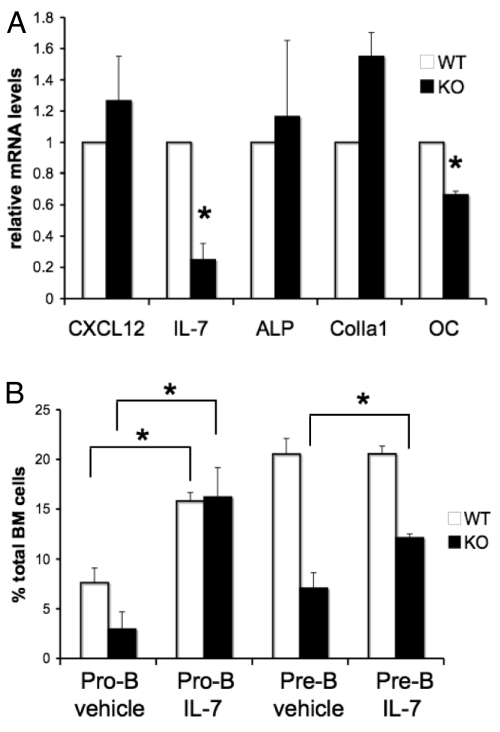
To identify potential mechanisms by which Gsα signaling in osteoblasts might regulate B lymphopoiesis, we investigated the expression of CXCL12 and IL-7 in WT and GsαOsxKO osteoblasts. Both CXCL12 and IL-7 production by osteoblastic cells can be stimulated by PTH (2, 13, 14); however, these cytokines regulate distinct stages of B cell development. CXCL12 is crucial for the production of prepro-B cells (25). In contrast, cells expressing IL-7 are in close contact with pro-B cells (7), and mice lacking IL-7 or the IL-7 receptor α subunit demonstrate impaired formation of pro-B and pre-B cells (26, 27). We isolated GFP+ osterix-expressing osteoblastic cells from WT and GsαOsxKO mice by FACS, purified total RNA, and performed quantitative real-time PCR for CXCL12 and IL-7 mRNA levels. Consistent with the finding that prepro-B cells were unaffected in the GsαOsxKO mice, no decrease in CXCL12 expression was seen in the GsαOsxKO osteoblasts. In contrast, IL-7 mRNA levels dropped to 19% of WT levels (Fig. 4A). This reduction is not simply a reflection of decreased osteoblast numbers in GFP+ cells from which RNA was isolated, because levels of alkaline phosphatase and type I collagen mRNA were not reduced in mutant mice. As expected, expression of osteocalcin, a known target of PKA-dependent signaling (28), also was specifically reduced in the GsαOsxKO mice (Fig. 4A).
Fig. 4.
IL-7 expression is reduced in GsαOsxKO osteoblasts. (A) CXCL12, IL-7, alkaline phosphatase (ALP), type I collagen (ColIa1), and osteocalcin (OC) mRNA levels in Osx-GFP:Cre+ osteoblasts from WT and KO littermates. *, P < .02. (B) Pro-B and pre-B cells as percentages of bone marrow cells in WT (n = 7) and KO (n = 3) mice injected with IL-7 or vehicle.
To determine whether IL-7 deficiency could explain the dramatic reduction in pro-B and pre-B cell content in GsαOsxKO mice, we administered recombinant murine IL-7 to WT and GsαOsxKO littermates on postnatal days 3–6. On postnatal day 7, a significant increase in pro-B cells in the WT mice receiving IL-7 was seen compared with the vehicle-injected mice (Fig. 4B). Importantly, in the GsαOsxKO mice, administration of IL-7 rescued pro-B cell production to WT levels. There was also a significant increase in pre-B cells in the GsαOsxKO mice given IL-7 compared with those given vehicle. The percentage of more differentiated pre-B cells in the IL-7-injected GsαOsxKO mice did not reach WT levels, likely due to the limited time course of treatment. These findings indicate that IL-7 can overcome, at least in part, the B cell precursor defect in GsαOsxKO mice, suggesting that IL-7 is an important mediator of Gsα-mediated regulation of B lymphopoiesis by osteoblasts.
Besides its critical role in supporting B lymphopoiesis, IL-7 also is necessary for thymic development of T lymphocytes (29). We assessed thymic and peripheral CD4 and CD8 T lymphocyte production and found no alterations in the GsαOsxKO mice (Fig. S4A and data not shown). Consistent with this finding, IL-7 levels were not decreased in thymus tissue from the GsαOsxKO mice (Fig. S4B). Thus, as a consequence of osteoblast-specific deletion of Gsα in the GsαOsxKO mice (which would be expected to affect the bone marrow microenvironment but not the thymic stroma), B lymphopoiesis was impaired, whereas T lymphopoiesis remained unaffected.
B Lymphopoiesis Is Attenuated in Mice with Constitutively Active PPR in Osteoblasts.
Because loss of Gsα signaling and reduced trabecular bone leads to a dramatic reduction in the numbers of bone marrow B lymphocytes, we investigated whether augmented Gsα signaling in relatively mature osteoblasts might result in increased B cell numbers. Transgenic mice expressing the constitutively active PTH/PTHrP receptor driven by a collagen I(α1) promoter fragment in differentiated osteoblasts are known to exhibit a significant increase in trabecular bone (30). The mutant receptor expressed in these mice leads to amplified cAMP accumulation downstream of Gsα activation. Surprisingly, 14-day-old constitutively active PPR transgenic mice also had a significantly reduced number of bone marrow B lymphocytes, due entirely to a decrease in the immature IgM− fraction (Fig. 5). These findings indicate that B cell numbers are not determined solely by bone mass or osteoblast number, and that tight regulation of signaling downstream of Gsα at different stages of osteoblastogenesis may be needed to allow optimal B lymphopoiesis.
Fig. 5.
Transgenic mice expressing the constitutively active PPR in osteoblasts have defective production of B220+IgM− lymphocytes. Shown are the frequencies of bone marrow leukocytes that are B220+, B220+/IgM−, or B220+/IgM+ in 2 week-old WT (n = 2) or transgenic (Tg, n = 4) mice. *, P < .005.
Discussion
We have found that ablation of Gsα early in the osteoblast lineage in vivo leads to a profound reduction in bone marrow B lineage cells. Other hematopoietic lineages are preserved, suggesting a specific impairment of B lymphopoiesis. Consistent with a defect stemming from an alteration in the bone marrow microenvironment, the production of B cell precursors is affected, resulting specifically in reduced numbers of pro-B and pre-B cells. We have further demonstrated that the abnormal B lymphopoiesis is due to the loss of Gsα from the stromal microenvironment. Osterix-Cre is not expressed in B lymphocytes, and Gsα mRNA levels are unchanged in B cell precursors isolated from GsαOsxKO mice. Moreover, transplantation of GsαOsxKO-derived bone marrow into a WT host completely restores B lymphopoiesis. Thus, the defect in B lymphopoiesis is extrinsic to the hematopoietic system.
These findings confirm that cells of the osteoblast lineage are indeed an important component of the B lymphocyte niche. Here we extend our current understanding of the interactions between cells of the osteoblast and hematopoietic lineages and present the first in vivo evidence for a relevant signaling pathway in osteoblastic cells that regulates B lymphopoiesis. The importance of Gsα-dependent signaling pathways to the HSC niche has been demonstrated by the finding that constitutively active PPR increases the numbers of HSCs in transgenic mice (2). In cultured cells, the constitutively active receptor mutation used in these transgenic mice signals predominantly through Gsα (31). Our findings demonstrate that signals downstream of Gsα in cells of the osteoblast lineage also are critical for the normal production of B lymphocytes by the bone marrow in early postnatal life.
Previous studies have found that a correlation between decreased osteoblasts and decreased B lymphocytes (4, 13). Because GsαOsxKO mice have fewer osteoblasts than their WT littermates, this may contribute to the loss of immature B cell precursors in mutant mice. B cell lymphopoiesis is not simply proportional to the number of osteoblasts, however. Mice lacking the proteoglycan biglycan have fewer osteoblasts than control mice, but no impairment of B lymphopoiesis (32). Conversely, the increased bone mass and numbers of osteoblasts found in constitutively active PPR transgenic mice do not lead to a greater number of B lymphocytes in bone marrow; rather, these mice also exhibit mildly impaired B lymphopoiesis. This somewhat surprising result may reflect changes in the subpopulations of cells of the osteoblast lineage needed to support B lymphopoiesis; alternatively, precise regulation of pathways downstream of Gsα-coupled receptors may be needed for adequate production of B lymphocytes in the bone marrow.
Of the downstream target(s) of Gsα-mediated signaling pathways critical for B lymphopoiesis, IL-7 appears to be a key mediator. IL-7 mRNA levels are decreased in cells of the osteoblast lineage in GsαOsxKO mice, and exogenous IL-7 completely restores pro-B cell production and significantly boosts pre-B cell numbers in these mice after only 3 days of treatment. Our results support the finding that PTH stimulates the production of IL-7 by stromal cells (13), demonstrating that PKA-dependent pathways play a significant role in regulating IL-7 expression. The upstream receptors coupling to Gsα to regulate the osteoblastic B lymphocyte niche remain undefined. Although PPR is an attractive candidate, other GPCRs that signal through Gsα have been identified in osteoblasts. In particular, the prostaglandin E2 receptors EP2R and EP4R are expressed in cells of the osteoblast lineage (18), and PGE2 has been reported to regulate the HSC niche (33).
As a working model (Fig. 6), we hypothesize that during early postnatal B cell development, B lymphocyte precursors are in close proximity to osteoprogenitors in the marrow space. Stimulation of GPCRs leads to activation of the PKA-dependent pathway through Gsα, with up-regulation of target genes. One or more of these products, which likely include IL-7, may play important roles in B lymphopoiesis. Nagasawa (25) has proposed that whereas HSCs are initially in contact with terminally differentiated osteoblasts along the bone surface, as these cells mature, they migrate toward the central region of the marrow cavity, allowing more differentiated hematopoietic precursors to come in contact with immature stromal cells within the marrow. Whether the osteoblastic cells implicated in our findings are the same as the IL-7-expressing cells identified by Tokoyoda et al. (7) remains to be determined. Recently, Sapoznikov et al. (34) reported that perivascular clusters of dendritic cells within the bone marrow provide crucial survival signals to mature recirculating B lymphocytes. Thus, it appears increasingly plausible that each stage of B lymphocyte differentiation may occur in a specific niche, each with a potentially distinct anatomic localization.
Fig. 6.
Model of osteoblastic regulation of B lymphopoiesis. Within the bone marrow of early postnatal mice, B cell precursors are located in close proximity to cells of the osteoblast lineage. Stimulation of Gsα-coupled GPCRs on the osteoblast surface leads to up-regulation of PKA target genes. These may include genes encoding for factors that stimulate B lymphopoiesis, such as IL-7.
An increased mechanistic understanding of the interactions between the hematopoietic and skeletal systems in providing regulatory niches has potential therapeutic utility. The association of both osteoporosis and declining B cell numbers with age is well known (35, 36). The finding that bone mass may be related to B cell number, and that this may be regulated by signals downstream of Gsα, raises the possibility that treatments such as PTH, already approved for osteoporosis, may have beneficial effects for the immune system as well. In addition, it is now clear that the stromal microenvironment plays a key role in pathophysiologic processes. Given the propensity for malignant disorders of the B lymphocyte lineage (e.g., multiple myeloma) to involve the skeleton, clarifying the relevant signaling pathways may offer novel approaches with clinical benefits.
Methods
Experimental Animals.
Osx1-GFP::Cre (22) and Gsα(fl/fl) (23) mice have been described previously. Because these mice are of a mixed genetic background (C57BL/6 and CD1), WT littermates were used as controls for all experiments described. Genotyping was performed on genomic DNA isolated from tails, using previously published protocols. All animals were housed in the Center for Comparative Medicine at the Massachusetts General Hospital, and all experiments were approved by the hospital's Subcommittee on Research Animal Care.
Flow Cytometry Analysis.
Bone marrow, spleen, thymus, and hemolyzed peripheral blood cells were stained for antibodies to B lymphocytes (B220, IgM, and CD93), T lymphocytes (CD4 and CD8a), granulocytes (CD11b and Gr-1), and erythrocytes (Ter119), as described previously (9). B cell precursors were analyzed with antibodies to CD2, CD19, and CD43. Isotype-matched antibodies were used for controls. Antibodies were purchased from eBioscience. Flow cytometry was performed on a FACSCalibur cytometer (BD Biosciences).
Bone Marrow Transplantation.
The 8-week-old B6.SJL (CD45.1+) mice were irradiated, then transplanted with 3 × 106 BM mononuclear cells from WT or GsαOsxKO mice (CD45.2+) (n = 5 for each group). At 10 weeks posttransplantation, peripheral blood, bone marrow, and spleen cells were obtained for analysis of CD45.2+ cells within the B lymphocyte lineage.
Isolation of Osteoblastic Cells by FACS.
Osteoblastic cells were harvested from neonatal calvariae of GsαOsxKO and control (Osx1-GFP::Cre+; Gsα+/+) mice by serial collagenase digestion (37). Fractions 3–6 were pooled and resuspended in phosphate-buffered saline (PBS) with 2% fetal bovine serum. Then ≈30,000 Osx1-GFP+CD45− cells were isolated per genotype by FACS using a FACS Aria sorter (BD Biosciences).
Quantitative Real-Time PCR.
Total RNA was isolated from cells using the RNeasy kit (Qiagen) and cDNA was synthesized with the SuperScript III First Strand synthesis system for real-time PCR (Invitrogen). Quantitative real-time PCR was performed using primers for Gsα (38), CXCL12 (39), IL-7 (40), ALP (41), ColIa1 (42), and OSC (39) according to previously published protocols, with mRNA levels normalized relative to β-actin expression. Total RNA samples subjected to cDNA synthesis reactions in the absence of reverse transcriptase were included as negative controls.
IL-7 Administration.
Recombinant murine IL-7 (R&D Systems) or vehicle (PBS + 0.1% bovine serum albumin) was injected at a dose of 100 ng twice daily on postnatal days 3–6. The mice were euthanized on postnatal day 7, and their bone marrow was analyzed for B cell precursors.
Statistics.
Statistical analyses were performed using a two-tailed Student's t test. All values are expressed as mean ± standard error of the mean.
Supplementary Material
Acknowledgments.
We thank Drs. Sean Wu, Carl Walkley, and Heather Fleming for discussion and helpful suggestions; Dr. Natalie Sims for quantitation of osteoblast surfaces; David Dombkowski and Laura Prickett for assistance with FACS sorting; James Oh for technical assistance; and the MGH Center for Comparative Medicine staff for caring for the mice in this study. This work was supported in part by National Institutes of Health Grants AR053781 (to J.Y.W.), DK071773 (to L.E.P.), HL081030 (to H.M.K. and D.T.S.), DK117940 (to H.M.K.), and DK056246 (to A.P.M.), and by the National Institute of Diabetes and Digestive and Kidney Diseases' Intramural Research Program (M.C. and L.S.W.). S.R. was supported by postdoctoral fellowships from the National Health and Medical Research Council of Australia (301299) and the Arthritis Foundation (401683).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802898105/DCSupplemental.
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, et al. Identification of the hematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 4.Visnjic D, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 5.Arai F, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Tokoyoda K, et al. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Avecilla ST, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 9.Walkley CR, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walkley CR, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46:65–72. [PubMed] [Google Scholar]
- 12.Gong JK. Endosteal marrow: a rich source of hematopoietic stem cells. Science. 1978;199:1443–1445. doi: 10.1126/science.75570. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 14.Jung Y, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts: a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Juppner H, et al. A G protein–linked receptor for parathyroid hormone and parathyroid hormone–related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 16.Pearman AT, et al. Parathyroid hormone induces c-fos promoter activity in osteoblastic cells through phosphorylated cAMP response element (CRE)-binding protein binding to the major CRE. J Biol Chem. 1996;271:25715–25721. doi: 10.1074/jbc.271.41.25715. [DOI] [PubMed] [Google Scholar]
- 17.Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 18.Suda M, et al. Prostaglandin E receptor subtypes in mouse osteoblastic cell line. Endocrinology. 1996;137:1698–1705. doi: 10.1210/endo.137.5.8612504. [DOI] [PubMed] [Google Scholar]
- 19.Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto A, et al. Deficiency of the G-protein alpha-subunit G(s)alpha in osteoblasts leads to differential effects on trabecular and cortical bone. J Biol Chem. 2005;280:21369–21375. doi: 10.1074/jbc.M500346200. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima K, et al. The novel zinc finger–containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 22.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, et al. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. J Clin Invest. 2005;115:3217–3227. doi: 10.1172/JCI24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy RR, et al. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6:107–116. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 26.Peschon JJ, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Freeden-Jeffry U, et al. Lymphopenia in interleukin (IL)-7 gene–deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boguslawski G, et al. Activation of osteocalcin transcription involves interaction of protein kinase A– and protein kinase C–dependent pathways. J Biol Chem. 2000;275:999–1006. doi: 10.1074/jbc.275.2.999. [DOI] [PubMed] [Google Scholar]
- 29.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 30.Calvi LM, et al. Activated parathyroid hormone/parathyroid hormone–related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107:277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schipani E, Kruse K, Juppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- 32.Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin–mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 33.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sapoznikov A., et al. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat Immunol. 2008;9:388–395. doi: 10.1038/ni1571. [DOI] [PubMed] [Google Scholar]
- 35.Hamrick MW, et al. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006;39:845–853. doi: 10.1016/j.bone.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Miller JP, Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Semin Immunol. 2005;17:321–329. doi: 10.1016/j.smim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Yang D, et al. CBP/p300-interacting protein CITED1 modulates parathyroid hormone regulation of osteoblastic differentiation. Endocrinology. 2008;149:1728–1735. doi: 10.1210/en.2007-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastepe M, et al. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc Natl Acad Sci U S A. 2004;101:14794–14799. doi: 10.1073/pnas.0405091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semerad CL, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blin-Wakkach C, et al. Hematological defects in the oc/oc mouse, a model of infantile malignant osteopetrosis. Leukemia. 2004;18:1505–1511. doi: 10.1038/sj.leu.2403449. [DOI] [PubMed] [Google Scholar]
- 41.Kenner L, et al. Mice lacking JunB are osteopenic due to cell-autonomous osteoblast and osteoclast defects. J Cell Biol. 2004;164:613–623. doi: 10.1083/jcb.200308155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobreva G, et al. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125:971–986. doi: 10.1016/j.cell.2006.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.