Abstract
Amphibians are a bellwether for environmental degradation, even in natural ecosystems such as Yellowstone National Park in the western United States, where species have been actively protected longer than anywhere else on Earth. We document that recent climatic warming and resultant wetland desiccation are causing severe declines in 4 once-common amphibian species native to Yellowstone. Climate monitoring over 6 decades, remote sensing, and repeated surveys of 49 ponds indicate that decreasing annual precipitation and increasing temperatures during the warmest months of the year have significantly altered the landscape and the local biological communities. Drought is now more common and more severe than at any time in the past century. Compared with 16 years ago, the number of permanently dry ponds in northern Yellowstone has increased 4-fold. Of the ponds that remain, the proportion supporting amphibians has declined significantly, as has the number of species found in each location. Our results indicate that climatic warming already has disrupted one of the best-protected ecosystems on our planet and that current assessments of species' vulnerability do not adequately consider such impacts.
Keywords: global warming, landscape change, remote sensing, amphibian community, drought
Amphibians are declining worldwide (1–5), and protected ecosystems figure prominently in their conservation. In this study, we document amphibian distress in remote areas of northern Yellowstone National Park in the western United States. This 8,893-km2 nature preserve in Wyoming, Montana, and Idaho has been protected by law longer than any other on Earth and is considered one of the world's most intact natural ecosystems (6). In this reserve, we have observed precipitous declines in populations of once-common amphibian species, and we link these declines to landscape and environmental changes resulting from global climatic change.
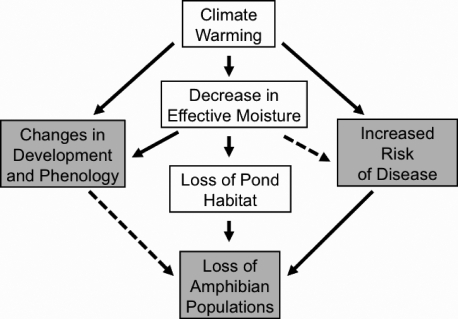
Pollution, pathogens, exotic species, UV irradiation, and habitat destruction all contribute to the current amphibian crisis (3, 7, 8), and climatic change poses an additional serious threat to populations (1, 5, 9–12). Changes in climate affect amphibian populations in many ways, three of which we detail here (Fig. 1). First, moderate increases in temperature may directly affect amphibian life history by altering behavior, phenology, reproduction, body condition, or survivorship (5, 9, 13, 14). Climate also changes the annual hydrological cycle and timing of events such as snow melt, which may lead to alterations in developmental timing of some species, causing stress and mortality (10, 14, 15). Increasingly rapid pond desiccation can further cause population die-offs. Second, climate may destroy the environments in which amphibians are found. Rising temperatures and decreasing precipitation can desiccate aquatic breeding habitats (16–19), preventing spawning. Warming and drying also impact terrestrial conditions. Decreased ambient moisture and leaf-litter availability can render terrestrial habitats more lethal to amphibians (1, 20), potentially restricting migration and connectivity. Third, rising temperatures can increase amphibian susceptibility to disease and infection (1, 21), a phenomenon currently decimating temperate and tropical populations (2, 21, 22).
Fig. 1.
Flow chart of global warming impacts on amphibian populations. These mechanisms are affecting amphibian populations worldwide and in particular within the Greater Yellowstone Ecosystem. Solid lines denote clearly established relationships; see introduction for details and citations.
Environmental impacts (including habitat degradation and disease) will continue to be enhanced by rising global temperatures, and amphibian species will continue to decline and disappear. Thus, it is crucial that we elucidate the ways in which climatic change and ensuing habitat change already have impacted amphibian species, particularly in locations such as Yellowstone National Park, where direct anthropogenic interference is not a primary conservation concern.
Our study focused on northern Yellowstone National Park, specifically the lower Lamar Valley, which houses dozens of small fishless ponds where amphibian habitat has been considered ideal for breeding and larval development (23). Lamar Valley is a glacially sculpted landscape, replete with moraines, kame terraces, and closed-basin kettle ponds. Many of these kettles are seasonal, recharged by groundwater and local surface runoff during the spring. The vulnerability of these ponds to climatic change depends on factors such as elevation, area, substrate, and proximity to large drainage catchments. Lamar Valley is free of any direct human impacts to the water table such as wells and other uses of these wetlands. The glacial substrate of Lamar Valley contains assorted-sized clasts, which contribute to an absorptive water table that is buffered against annual variation (17). However, in addition to widespread drought, decreasing snow pack and earlier runoff have likely induced large-scale changes to the hydrology of this region in recent decades (24).
Northern Yellowstone supports 4 native amphibian species, and no exotic amphibians are present in the region. The most common amphibians are the blotched tiger salamander (Ambystoma tigrinum melanostictum), the boreal chorus frog (Pseudacris triseriata maculata), and the Colombia spotted frog (Rana luteiventris, formerly Rana pretiosa) (23). The boreal toad (Bufo boreas boreas) is also present but is less common (23).
Results
We observed severe reductions in the number and diversity of amphibian populations in northern Yellowstone National Park over the past 16 years. We document that amphibian decline is linked to regional changes in the hydrologic landscape and overall groundwater condition, which is driven by long-term, large-scale climatic trends.
Drought and Climatic Changes.
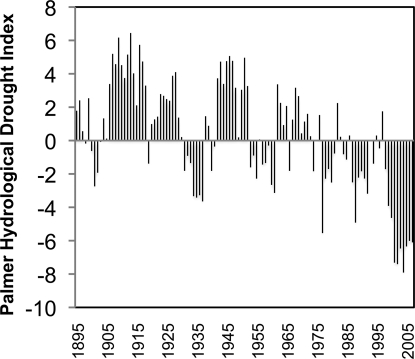
Over the last century, regional climatic changes synergistically have led to a significant decline in the Palmer Hydrological Drought Index (PHDI) (Fig. 2). The PHDI is a calculation of cumulative hydrological conditions that is based on effective moisture, with negative PHDI values indicating drought. The average yearly PHDI of the Yellowstone watershed indicates a century-long trend toward drought (Fig. 2, P ≪ 0.001). Parallel to the trends in Yellowstone, Wyoming PHDI has dropped significantly during the same period (R2 = 0.21, P ≪ 0.001, data not shown), as have the hydrological conditions of the entire western United States (24). Not only has the Yellowstone drainage experienced values from −6 to −8 over the past several years, the region as a whole has experienced drought for this same period. Average PHDI for Wyoming over the last 10 years was −2.4. In contrast, during the first half of the century, average PHDI in Wyoming was +2.1 (25). In Yellowstone, drought has become more frequent and more severe; indeed, the last 7 years experienced the most severe droughts on record (Fig. 2).
Fig. 2.
Annual PHDI of the Yellowstone Drainage region between 1895 and 2007 (25). Values above zero indicate a wet year, and values below zero indicate drought conditions. Best fit linear model of PHDI values (not shown) has slope = −0.057, adjusted R2 = 0.33, and P ≪ 0.001.
Analyses of data from 3 Yellowstone weather stations (26) since 1949 shows increases in average spring (March–May: P = 0.016) and summer (June–August: slope = 0.02, P = 0.035) temperatures (data not shown; see supporting information (SI) Figs. S1 and S2). Furthermore, over this time, we found an increase in maximum annual temperature (Fig. S1, P = 0.03) and a decrease in total annual precipitation (Fig. S2, P = 0.001). The decrease in precipitation was most severe during the winter months (December, January, and February; P ≪ 0.001) and has led to a reduction in snow pack. The decrease in annual precipitation is significant, even under a rigorous Bonferroni correction using an α-cutoff of 0.017 rather than 0.05.
Wetland Desiccation.
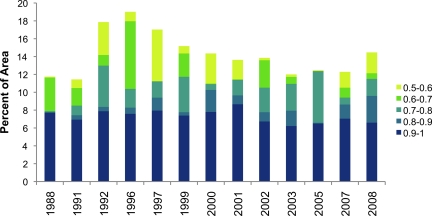
As a cumulative result of these changes in climate, the landscape across Lamar Valley has dried significantly (P < 0.001) from 1992 (27, 28) to 2008 (Figs. 3 and 4). The landscape proximate to and between the kettle ponds in Lamar Valley exhibited a substantial decrease in palustrine wetland (Fig. 4). In Lamar Valley, the wetlands are comprised largely of sedge (Carex spp.) marsh and wet meadows, both of which provide good amphibian habitat (23). Kettle pond permanence is determined by long-term hydrological conditions reflected in PHDI (17), and remote-sensing data were ground-truthed by field surveys of 49 ponds across northern Yellowstone. Direct field comparisons of pond hydration between 1992–1993 and 2006–2008 demonstrated a significant increase in the number of permanently dry ponds and a decrease in the number of hydrated ponds. Eight ponds that were either permanent or ephemeral in both 1992 and 1993 have been completely dry in 2006–2008 (locations in Table S1; observations in Table S2). Additionally, 11 more of the locations were dry in both 2006 and 2007, although they were rehydrated in 2008. Even though the spring of 2008 was the third wettest year on record, the number of hydrated ponds was still 17% less than the number hydrated in 1992–1993 (McNemar's x2 = 6.125, P = 0.013).
Fig. 3.
Time series of wetland probability surfaces of 20 of the 49 observed pond locations (red circles) in the Lamar Valley study area. Probabilities smoothed for display by cubic convolution given original pixel size of 30 m. Lakes are light blue; black polygons show the Lamar River.
Fig. 4.
Proportions of different categories of wetland probability in 500-m buffer zones around 49 current and former pond locations in northern Yellowstone National Park for 13 years between 1988–2008.
Flow records of the Lamar River at the Tower Ranger Station (29) further reveal the changing hydrodynamics of this landscape. Like many western United States drainages, peak flow of this river over the past 50 years has shifted earlier in the year because of earlier snowmelt (24, 30, 31). Furthermore, the amount of water flowing through the Lamar River during summer months (June–August) has declined significantly from the period 1924–1966 to the period 1989–2007 (Student's t test: t = 3.33, P < 0.001, data not shown), indicating that the hydrology of the region may be further compromised during the months when most wetland desiccation occurs and amphibians may be most susceptible.
Amphibian Declines.
Of the 46 hydrologically active ponds surveyed 1992–1993, 43 supported amphibian populations for at least one of the two years (27, 28). From 2006 to 2008, only 38 of these ponds contained water, even in a wet year (2008), as determined by spring precipitation, suggesting longer-term impacts to the hydrologic environment. After surveying 31 of the 38 active ponds to which we were permitted access, we found that only 21 supported amphibian populations for even one year during 2006–2008 (Table S2). Furthermore, of 11 ponds that were dry, 2006–2007, and rehydrated in 2008, only 6 were found to support any amphibians.
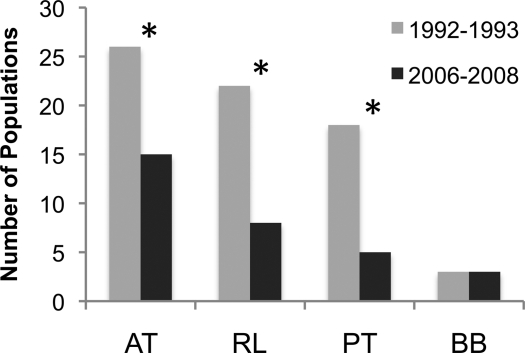
In this study, we found fewer amphibian species per hydrated pond than in 1992–1993, further suggesting that amphibians have been challenged by the environmental conditions of the past 16 years. The species richness of the surveyed communities has declined precipitously (Fig. 5; Paired t test: t = 5.49, P ≪ 0.001). All 3 of the most common amphibians suffered major declines in the number of populations (Fig. 6; McNemar's x2 test: A. tigrinum P = 0.005; P. triseriata P = 0.002; R. luteiventris P < 0.001). We did not observe a change in the number of B. boreas populations. However, B. boreas was extremely rare in all survey years, which limited our statistical power. Indeed, we observed only one adult of this species during 2006–2008.
Fig. 5.
Changes in species richness between 1992–1993 and 2006–2007. The graph shows number of locations containing 0, or at least 1, 2, 3, or 4 species of amphibians in 1992–1993 and 2006–2008. Of the 21 ponds lacking amphibians in 2006–2008, 11 were dry all 3 years. Changes in pond richness are highly significant (Paired t test: t = 5.6848, P = 1.214E-06).
Fig. 6.
Number of populations of each species present in 42 Yellowstone ponds during at least 1 year during 1992–1993 or 2006–2008. AT, Ambystoma tigrinum; BB, Bufo boreas; PT, Pseudacris triseriata; RL, Rana luteiventris. Asterisks denote statistical significance, determined by McNemar's Test (P <0.01).
Discussion
We have documented regional hydrologic changes in northern Yellowstone by climatic monitoring over a century, remote sensing over 20 years, and retrospective field surveys of ponds from 1992–1993 to 2006–2008. Climatic change has significantly altered this landscape and has resulted in a decline of wetlands and disappearances of more than half the amphibian populations present in northern Yellowstone just 15 years ago.
Over the past century, Yellowstone has experienced substantial changes in climate, precipitation, and hydrology. The long-term trend in this region is one of increasingly frequent and severe drought. We documented changes in regional climate over the past 6 decades, which included an increase in maximum annual temperature (Fig. S1), a startling decrease in total yearly precipitation (Fig. S2), and a robust trend toward drought (Fig. 2). The decrease in precipitation is driven largely by regional declines in winter precipitation and snow pack (30). The resulting decrease of local spring snowmelt reduces wetland presence and aquifer replenishment (17) that are crucial for hydrating the closed-basin kettles (19) characteristic of the Lamar Valley.
In northern Yellowstone, climatic and hydrological conditions have translated to a significant decrease in the annual amount of water flowing through the Lamar River, the number of wetlands (Figs. 3 and 4) and the number of active bodies of water (Table S2). Many locations that were once permanent or ephemeral bodies of water in 1992–1993 have not been rehydrated since 2006 and may have been dry even before 2002 (32). Although considerable decadal variability with respect to hydrological conditions has occurred (i.e., 1989 was the second driest year on record), such observations are likely a facet of a widespread and long-term trend toward desiccation (24) that is linked to global warming. The spring and summer of 2008 were particularly wet, as evidenced in the enhanced hydrological status of many of the ponds (Fig. 3 and Table S2), but even these notable levels of precipitation did not return all of the locations to their former hydrated status. This pattern suggests that occasional wet years are not sufficient to override the long-term drying trend.
The loss of pond habitat has been catastrophic to Yellowstone amphibian populations. All three of the most common native amphibians, A. tigrinum, P. triseriata, and R. luteiventris, have suffered declines since the early 1990s. The number of salamander populations has fallen by nearly half, the number of spotted frog populations has declined by 68%, and the number of chorus frog populations is down by 75%. Ironically, B. boreas, the only native amphibian highlighted by the ICUN as distressed (“near-threatened”, ref. 33), is the only species for which we did not detect a decline. Three populations of B. boreas were found in 1993, and three were found again in 2007–2008. All three of the 2007–2008 populations, however, were composed of only eggs or juveniles, and only 1 adult B. boreas was found during the entire 2006–2008 survey. This species is declining elsewhere in the region (22, 34), and low population presence may make declines difficult to measure. In addition to the decreases in number of amphibian populations, the diversity of amphibian communities has declined significantly. All but one of the ponds containing amphibians have suffered a reduction in species richness since 1992–1993 (Fig. 5), and no ponds now support all 4 species of amphibians (Table S2).
In the 1950s, studies from the Yellowstone region cited almost complete occupancy of wetlands by these common amphibians (35). In the early 1990s, 93% of ponds containing water for even part of the year supported amphibian populations at least one year 1992–1993. In stark contrast, over the three years of our retrospective study, we found not only that the number of hydrologically active ponds had declined by 20%, but that only 61% of remaining hydrologically active ponds contained amphibians for even one of the three years (Table S2). Particularly disturbing is the fact that, even though 2008 saw the refilling of 11 ponds that were dry the previous 2 years, only 6 of these were recolonized by amphibians.
Many habitats in this region now have very different characteristics than those observed 16 years ago. Six ponds that were permanent in 1992–1993 were ephemeral for 2 or more years in the 3-year period 2006–2008, a fact that may decrease the reproductive potential and fitness of local populations. Furthermore, remaining ponds now dry earlier and more rapidly, posing a grave threat to the amphibians living and breeding there. During the course of our study, we witnessed the loss of 4 amphibian communities because of drying (Pond 1 in 2006–2007, Pond 5 in 2007, and Pond 2 in 2008). Each event left hundreds of dried A. tigrinum corpses where the ponds were formerly located. The ponds dried over a few days, which was too fast for larvae to metamorphose and adults to migrate.
In addition to the deterioration and loss of pond habitats, changing hydroclimate within the intervening landscape (Fig. 3) may interfere with the terrestrial portions of amphibian life cycles. All 4 amphibians have generation times of several years and spend substantial portions of their lives on land. The conditions between breeding grounds determine the frequency of migration, and the genetic relatedness between A. tigrinum subpopulations across northern Yellowstone is correlated with the conditions of the intervening landscape (32). Current landscape trends may mediate increased terrestrial mortality and decreased migration and colonization.
Amphibians in Yellowstone are especially vulnerable to climatic changes in early spring (March–April) and late summer (July–August), when they are most active in terrestrial habitats (23). With the earlier arrival of spring and snowmelt in recent years (30), amphibians may be migrating and breeding earlier, which may further reduce survival (5, 9).
Although climate and hydrological changes are likely the major drivers of the observed declines, they are not the only factors at work. Atmospheric contamination may have contributed to the declines (36), and disease has likely played a substantial role. Indeed, pathogen outbreaks of chytridiomycosis (caused by the fungus Batrachochytrium dendrobatidis) and ranavirus have been documented in all 4 of these species (37–39). During the course of our study, we observed mass mortalities of A. tigrinum populations at 3 different ponds (Ponds 33, 34, and 47). Two of these ponds (33 and 34) experienced such catastrophic eradications in both 2006 and 2007. These may have been caused by a pathogen outbreak (39, 40), by lethal water temperatures, or by the concentration of minerals during pond drying. Disease-related eradications in this area were first observed in this area in 2000 (41), and no such eradications were observed in the 1990s. Given the role that climate may play in amphibian epidemics (1, 21), it is likely that these mortalities and the ones that we observed were facilitated, if not directly caused, by the stress of environmental changes.
Although some studies demonstrate that climatic conditions may contribute to amphibian decline, the relationship is still controversial (4, 42). It has been suggested that B. dendrobatidis causes amphibian declines independent of environmental change (4, 43), but evidence suggests that climate and environmental change enhance declines in manifold ways (1, 11, 42, 44, 45). Global amphibian decline is due to a synergy of factors, and we have shown that environmental change has caused the decline of Yellowstone amphibians.
Although populations and conditions fluctuate annually, both the baseline and follow-up surveys for this study were conducted over multiple years. The noise of annual hydrological fluctuations is dampened by our use of multiple years of survey data and by multiple lines of evidence showing substantial landscape change over this time period, which was particularly conservative, because we conducted a 3-year follow up to a 2-year baseline (46). Further, we considered an amphibian species present if we found it during even 1 of 3 years, and amphibian occupancy was noted even if only 1 individual was found in a pond. Although the baseline studies were performed during years wetter than the follow-up surveys, 1992 and 1993 were not exceptionally wet on a decadal scale (Fig. 2 and Fig. S2), and indeed followed several years of drought from 1989–1991 (see Figs. 2–4), which may have damaged amphibian populations even before the baseline measurement. Additionally, multiple studies have documented amphibian decline in this area (37–39, 47), corroborating our results.
Amphibians in northern Yellowstone have experienced substantial climatic fluctuations in the past. The last several thousand years have seen considerable preanthropogenic climatic fluctuations, and A. tigrinum was able to persist in large numbers through both warm and dry periods (48). However, recent climatic changes have been more severe than those of the Medieval Warm Period (49). During the last century, the region has witnessed multiple periods of drought, including the 1989–1991 drought and the notorious Dust Bowl of the 1930s (evident in Fig. 2), but amphibians have endured. With ongoing anthropogenic climatic change and hydrological desiccation, amphibians in Yellowstone now face conditions far more threatening than any seen during the last century, or indeed throughout late Holocene.
The extinction vulnerability of species is usually assessed by breadth of habitat occupancy, extent of geographic range, population number, and population sizes. Our results emphasize that global warming and its associated impacts challenge even common and widespread species. The IUCN has listed A. tigrinum, R. luteiventris, and P. triseriata as species of least concern, stating that they are unlikely to be declining fast enough to qualify as threatened (50). Over the course of less than 2 decades, we found that all 3 of these native amphibians have experienced severe declines, which are unlikely to have been caused purely by annual fluctuation or exclusively by disease. Our data suggest that climatic change is causing substantial changes to the hydrologic landscape and that amphibian populations are being damaged by both habitat loss and other climate-associated mechanisms.
In Yellowstone, the local hydrological changes contributing to amphibian declines are not isolated or local events and are attributable to regional and global changes in climate (24), which are likely to continue, if not accelerate, in coming decades (51). We have shown that the kettle pond amphibians have been impacted by climate in Yellowstone, which cascades to other species in these communities. Amphibian predators, such as herons, coyotes, and birds of prey, will be directly influenced by amphibian decline, and other species will be negatively impacted by wetland loss. Climate-linked impacts to other species in the Yellowstone ecosystem are also becoming prevalent (30, 52, 53). Precipitous declines of purportedly unthreatened amphibians in the world's oldest nature reserve indicate that the ecological effects of global warming are even more profound and are happening more rapidly than previously anticipated.
Materials and Methods
Climate Analysis.
To document climatic change over the last century in this region, we used publicly available climate and hydrological record databases (25, 26, 29). To characterize recent changes specifically in the study area, we used monthly temperature and precipitation data recorded between 1948–2008 (n = 60 years) from 3 weather stations in Yellowstone National Park (26) (see Figs. S1 and S2 for analysis details). PHDI values of the Yellowstone Drainage Basin from 1895–2007 were obtained from the National Climatic Data Center (25). Trends in conditions were characterized by linear models; one-tailed P values are given, where appropriate. Lamar River stream flow data were obtained through the National Water Information System (29).
Remote Sensing Analysis.
We obtained cloudless Landsat Thematic Mapper images of Yellowstone National Park (Landsat path 38/row 29) between 1988 and 2008 to analyze the landscape hydrologic trends in this region. Probabilities of palustrine wetland presence in 30m-resolution pixels were estimated following previously described methods (54, 55). These techniques reliably predict wetland presence regardless of whether images are from early or late summer (54, 55), and because all images were taken during this seasonal period, it was not necessary to further correct for time of year. To assess changes in wetland status in the vicinity of the 49 focal ponds, we drew a 500-m buffer zone around each pond and analyzed the proportion of wetland probabilities greater than 0.5 within these zones for each year (see Fig. 4 for locations of certain ponds and Table S1 for coordinates of all focal ponds).
Population and Habitat Surveys.
We collected amphibian habitat and population data from northern Yellowstone National Park during 3 summers, from mid-June through July 2006, from June through August 2007, and from June through July 2008. Our study area included many of the fishless bodies of water in northern Yellowstone National Park, particularly in lower Lamar Valley, where we targeted 42 sites that had been surveyed in 1992–1993 (27, 28), which included Ice Lake near Gardiner, MT, and 2 ponds on Mount Everts (see Table S1 for coordinates and details of all locations). As in the initial studies, we systematically assessed the conditions of all ponds and wetlands in June and monitored them for amphibians over the remaining weeks (Table S2). After a perimeter search, which was performed during each visit to every site, we used one or more of several common methods to capture amphibians. We generally performed active dip-netting of the pond area in the mid-afternoon using hip waders and a large net for 1 h or more—a procedure used frequently in 1992 and 1993 (28). Other methods included the use of unbaited funnel minnow traps, which we set overnight for at least 10 h, and pitfall traps with drift fences, spaced at regular intervals around the ponds. Used at Ponds 36 and 38 in 2007, the pitfall traps were effective at trapping R. luteiventris and A. tigrinum during the late summer. Seven of the forty-nine ponds surveyed in the initial surveys were not surveyed for amphibians during our field studies, because of seasonal closures surrounding trumpeter swan nesting sites and other limitations (Ponds 11–14, 23, and 46; see Table S2). Although we were able to observe the hydration status of these locations, these particular ponds are excluded from species analyses. Until pond drying prevented aquatic sampling, each of the 49 ponds was surveyed no less than 4 times (mean = 8 times) each summer 2006–2008, using at least one of the first two described methods. We scored a species as present at a locality if we observed egg masses, larvae, or adults of that species (living or dead). To test for changes in proportion of hydrated and amphibian-supporting sites, we used McNemar's x2 test on contingency tables. To test for changes in the number of species present in each site (i.e., species richness), we used a paired t test (n = 42). Tests were performed in the program R v.2.4.1.
Supplementary Material
Acknowledgments.
We thank National Park Service personnel C. Hendrix, T. Oliff, and J. Varley; A.T. Eamsherangkoon, L.C.M. Ha and family, Y.J. Lee, W. Love-Anderegg and family, L.M. Palumbi and family, S. Winfree, and S.G. Hayes-Williams for assistance with fieldwork; A.P. Wagner, D. Ragosa, and J. Boik for statistical advice; A. Barnosky, J. Blois, P. Marquet, S. Palumbi, A. Rominger, T. Root, S. Schneider, J. Varley, and an anonymous reviewer for comments on the manuscript; J.A. Pounds, whose insightful comments as a reviewer substantially improved this work and who suggested inclusion of both the flow-through data and the drought analysis; and S.R. Hill and R.E. Moore, without whom our retrospective study would not have been possible. This research was funded by the Sigma Xi, The Scientific Research Society, and the Stanford Center for Evolutionary Biology.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809090105/DCSupplemental.
References
- 1.Pounds JA, et al. Widespread amphibian extinctions from endemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 2.Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;3:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 3.Collins JP, Storfer A. Global amphibian declines: Sorting the hypotheses. Divers Distrib. 2003;9:89–98. [Google Scholar]
- 4.Skerratt LF, et al. Spread of Chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- 5.Reading CJ. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia. 2007;151:125–131. doi: 10.1007/s00442-006-0558-1. [DOI] [PubMed] [Google Scholar]
- 6.Noss RF, Carroll C, Vance-Borland K, Wuerthner G. A multicriteria assessment of the irreplaceability and vulnerability of sites in the Greater Yellowstone Ecosystem. Conserv Biol. 2002;16:895. [Google Scholar]
- 7.Alford RA, Richards SJ. Global amphibian declines: A problem in applied ecology. Annu Rev Ecol Syst. 1999;30:133–165. [Google Scholar]
- 8.Blaustein AR, Romansic JM, Kiesecker JM, Hatch AC. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers Distrib. 2003;9:123–140. [Google Scholar]
- 9.Beebee TJC. Amphibian breeding and climate. Nature. 1995;374:219–220. [Google Scholar]
- 10.Wake DB. Climate change implicated in amphibian and lizard declines. Proc Natl Acad Sci USA. 2007;104:8201–8202. doi: 10.1073/pnas.0702506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alford RA, Bradfield KS, Richards SJ. Global warming and amphibian losses. Nature. 2007;447:E3–E4. doi: 10.1038/nature05940. [DOI] [PubMed] [Google Scholar]
- 12.Carey C, Alexander MA. Climate change and amphibian declines: Is there a link? Divers Distrib. 2003;9:111–121. [Google Scholar]
- 13.Blaustein AR, et al. Amphibian breeding and climate change. Conservation Biology. 2001;15:1804–1809. [Google Scholar]
- 14.Semlitsch RD, Wilbur HM. Effects of pond drying time on metamorphosis and survival in the salamander Ambystoma talpoideum. Copeia. 1988;1988:978–983. [Google Scholar]
- 15.McCoy KA, Harris RN. Integrating developmental stability analysis and current amphibian monitoring techniques: An experimental evaluation with the salamander Ambystoma maculatum. Herpetologica. 2003;59:22–36. [Google Scholar]
- 16.Pounds JA, Fogden MPL, Campbell JH. Biological response to climate change on a tropical mountain. Nature. 1999;398:611–616. [Google Scholar]
- 17.Winter TC. The vulnerability of wetlands to climate change: A hydrologic landscape perspective. J Am Water Resour As. 2000;36:305–310. [Google Scholar]
- 18.Daszak P, et al. Amphibian population declines at Savannah River site are linked to climate, not Chytridiomycosis. Ecology. 2005;86:3232–3237. [Google Scholar]
- 19.Price JS, Branfireun BA, Waddington JM, Devito KJ. Advances in Canadian wetland hydrology, 1999–2003. Hydrol Process. 2005;19:201–214. [Google Scholar]
- 20.Whitfield SM, et al. Amphibian and reptile declines over 35 years at La Selva, Costa Rica. Proc Natl Acad Sci USA. 2007;104:8352–8356. doi: 10.1073/pnas.0611256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muths E, Pilliod DS, Livo LJ. Distribution and environmental limitations of an amphibian pathogen in the Rocky Mountains. Biol Cons. 2008;141:1484–1492. [Google Scholar]
- 22.Muths E, Corn PS, Pessier AP, Green DE. Evidence for disease-related amphibian decline in Colorado. Biol Conserv. 2003;110:357–365. [Google Scholar]
- 23.Koch ED, Peterson CR. Amphibians & Reptiles of Yellowstone and Grand Teton National Parks. Salt Lake City: Univ of Utah Press; 1995. [Google Scholar]
- 24.Barnett TP, et al. Human-induced changes in the hydrology of the western United States. Science. 2008;319:1080. doi: 10.1126/science.1152538. [DOI] [PubMed] [Google Scholar]
- 25.National Climatic Data Center. State Wide Palmer Drought Indices 1895–2008. Asheville, NC: National Oceanic and Atmospheric Administration; 2008. [Google Scholar]
- 26.Western Regional Climate Center. Monthly Station Averages for Temperature and Precipitation 1948–2007 for Lake Yellowstone WY, Tower Falls WY and Yellowstone Park WY. Reno, NV: Western Regional Climate Center; 2008. [Google Scholar]
- 27.Hill SR. Bozeman: Montana State University; 1995. Migratory chronology of adult tiger salamanders (Ambystoma tigrinum) and survey of larvae of the tiger salamander in the northern range of Yellowstone National Park. MS thesis. [Google Scholar]
- 28.Hill SR, Moore RE. Herpetological Survey in the Northern Range of Yellowstone National Park. Yellowstone National Park: Investigator's Annual Reports; 1994. [Google Scholar]
- 29.National Water Information System. Real-Time Water Data for Montana. Reston, VA: United States Geological Survey; 2008. [Google Scholar]
- 30.Wilmers CC, Getz WM. Gray wolves as climate change buffers in Yellowstone. PLoS Biol. 2005;3:e92. doi: 10.1371/journal.pbio.0030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart I, Cayan DR, Dettinger M. Changes toward earlier streamflow timing across western North America. J Climate. 2005;18:1136–1155. [Google Scholar]
- 32.Spear SF, Peterson CR, Matocq MD, Storfer A. Landscape genetics of the blotched tiger salamander (Ambystoma tigrinum melanostictum) Mol Ecol. 2005;14:2553–2564. doi: 10.1111/j.1365-294X.2005.02573.x. [DOI] [PubMed] [Google Scholar]
- 33.International Union for the Conservation of Nature and Natural Resources. Amphibian Reports. Gland, Switzerland: World Conservation Union; 2007. [Google Scholar]
- 34.Kiesecker JM, Blaustein AR, Belden LK. Complex causes of amphibian population declines. Nature. 2001;401:681–684. doi: 10.1038/35070552. [DOI] [PubMed] [Google Scholar]
- 35.Turner FB. Reptiles and Amphibians of Yellowstone National Park. Yellowstone Park: Yellowstone National Park; 1955. [Google Scholar]
- 36.Service NP. Western Airborne Contaminants Assessment Project. Washington, DC: U.S. Department of the Interior; 2008. [Google Scholar]
- 37.Patla DA. Pocatello, ID: Idaho State University; 1997. Changes in a population of spotted frogs in Yellowstone National Park between 1953 and 1995: The effects of habitat modification. MS thesis. [Google Scholar]
- 38.Patla DA, Peterson CR. Amphibian Diversity, Distribution, and Habitat Use in the Yellowstone Lake Basin. Yellowstone National Park, WY: Yellowstone Lake Conference Program Committee; 2002. [Google Scholar]
- 39.Corn PS. Amphibians and disease: Implications for conservation in the Greater Yellowstone Ecosystem. Yellowstone Sci. 2007;15:11–16. [Google Scholar]
- 40.Worthylake KM, Hovingh P. Mass mortality of salamanders (Ambystoma tigrinum) by bacteria (Acinetobacter) in an oligotrophic seepage mountain lake. Great Basin Nat. 1989;49:364–372. [Google Scholar]
- 41.Patla DA, Peterson CR. Amphibian and Reptile Inventory and Monitoring: Grand Teton and Yellowstone National Parks. Mammoth, WY: 2000–2003 Final Report; 2004. [Google Scholar]
- 42.Pounds JA, Coloma LA. Beware the lone killer. Nature Rep Clim Change. 2008;2:57–59. [Google Scholar]
- 43.Lips KR, Diffendorfer J, Mendelson JRI, Sears MW. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008;6:e72. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pounds JA, et al. Global warming and amphibian losses: The proximate cause of frog declines? (Reply) Nature. 2007;447:5–6. [Google Scholar]
- 45.Di Rosa I, Simoncelli F, Fagotti A, Pascolini R. Ecology: The proximate cause of frog declines? Nature. 2007;447:E4–5. doi: 10.1038/nature05941. [DOI] [PubMed] [Google Scholar]
- 46.Skelly DK, Yurewicz KL, Werner EE, Relyea RA. Estimating decline and distributional change in amphibians. Conserv Biol. 2003;17:744–751. [Google Scholar]
- 47.Spear SF, Peterson CR, Matocq MD, Storfer A. Molecular evidence for historical and recent population size reductions of tiger salamanders (Ambystoma tigrinum) in Yellowstone National Park. Conserv Genet. 2006;7:605–611. [Google Scholar]
- 48.Bruzgul JE, Long W, Hadly EA. Temporal response of the tiger salamander (Ambystoma tigrinum) to 3,000 years of climatic variation. BMC Ecol. 2005;5:7. doi: 10.1186/1472-6785-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters RL, Lovejoy TE. Global Warming and Biological Diversity. New Haven, CT: Yale Univ Press; 1994. [Google Scholar]
- 50.Hammerson G. Red List of Threatened Species. International Union for the Conservation of Nature and Natural Resources; 2007. [Google Scholar]
- 51.Working Group I. IPCC Fourth Assessment Report. Geneva, Switzerland: United Nations Intergovernmental Panel on Climate Change; 2007. [Google Scholar]
- 52.Schneider SH, Root TL. Wildlife Responses to Climate Change: North American Case Studies. Washington, DC: Island Press; 2001. [Google Scholar]
- 53.Romme WH, Turner MG. Implications of global climate change for biogeographic patterns in the greater Yellowstone ecosystem. Conservation Biol. 1991;5:373–386. [Google Scholar]
- 54.Wright CK. Bozeman: Montana State University; 2004. Remote sensing of wetlands in Yellowstone National Park. PhD thesis. [Google Scholar]
- 55.Wright C, Gallant A. Improved wetland remote sensing in Yellowstone National Park using classification trees to combine TM imagery and ancillary environmental data. Remote Sens Environ. 2006;107:582–605. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.