Abstract
Understanding the causes and consequences of dispersal is a prerequisite for the effective management of natural populations. Rather than treating dispersal as a fixed trait, it should be considered a plastic process that responds to both genetic and environmental conditions. Here, we consider how the ambient temperature experienced by juvenile Erigone atra, a spider inhabiting crop habitat, influences adult dispersal. This species exhibits 2 distinct forms of dispersal, ballooning (long distance) and rappelling (short distance). Using a half-sib design we raised individuals under 4 different temperature regimes and quantified the spiders' propensity to balloon and to rappel. Additionally, as an indicator of investment in settlement, we determined the size of the webs build by the spiders following dispersal. The optimal temperature regimes for reproduction and overall dispersal investment were 20 °C and 25 °C. Propensity to perform short-distance movements was lowest at 15 °C, whereas for long-distance dispersal it was lowest at 30 °C. Plasticity in dispersal was in the direction predicted on the basis of the risks associated with seasonal changes in habitat availability; long-distance ballooning occurred more frequently under cooler, spring-like conditions and short-distance rappelling under warmer, summer-like conditions. Based on these findings, we conclude that thermal conditions during development provide juvenile spiders with information about the environmental conditions they are likely to encounter as adults and that this information influences the spider's dispersal strategy. Climate change may result in suboptimal adult dispersal behavior, with potentially deleterious population level consequences.
Keywords: behavior, body condition, plasticity, reaction norm, silk
The movement of dispersing individuals or propagules may have important consequences for gene flow, the genetic cohesions of species, the global persistence of species in the face of local extinction, speciation, inbreeding depression, the evolution of sociality, and the evolution of life history traits (1–7). The dispersal phenotype is predominantly treated as a fixed property in theoretical studies dealing with dispersal evolution and its consequences for population persistence in changing environments (1). However, empirical work has demonstrated high levels of dispersal plasticity (1). This is as expected according to the hypothesis that unless variation in habitat quality is highly unpredictable or information acquisition is costly (1, 3), the most successful strategy over evolutionary time has been for individuals to make dispersal decisions based on information (8) obtained during their lifetime (i.e., for individuals to adopt a conditional strategy). There is mounting evidence that strong selection pressures emerging from global change (i.e., land use changes, climate change, species invasions, pollution) are influencing the evolution of dispersal rate and dispersal distance (1). Correlative and experimental studies have demonstrated rapid evolution of morphological (e.g., wing and seed polymorphism; refs. 9–12), physiological (13), and behavioral (14) traits related to dispersal, but our understanding of the evolutionary ecology of plastic dispersal strategies under environmental change remains rudimentary. Empirical quantification of genotype × environment interactions by reaction norm analyses (15, 16) provides a highly promising approach for studying the adaptive potential of plastic dispersal strategies. These analyses present formidable challenges if dispersal is to be quantified under natural conditions or when dispersal morphology affects different types of movement (17). This underlines the importance of selecting model species carefully. The species we chose is a sheet-web spider from the family Linyphiidae, and because this species has a short generation time and distinctive dispersal behavior, it appears to be an especially appropriate subject for research on conditional dispersal strategies.
Like many other spiders, but also many mites and moth larvae (18), Linyphiidae disperse predominantly by using silk threads as either a sail (ballooning) for long-distance dispersal or as a bridging thread (rappelling) for short-distance dispersal. Both dispersal modes are preceded by tiptoe behavior (stretching out legs, raising the abdomen and making long silk threads; ref. 18). Ballooning spiders take off attached to the silk thread and can travel for distances of up to several hundred meters (19). When they rappel, spiders attach a thread to the substrate before takeoff, with the thread remaining attached while the spider bridges short distances. These behavioral components can be quantified under highly standardized laboratory conditions, making these spiders especially amenable to reaction–norm analysis (15, 16, 20–22).
The temperature experienced by juvenile ectotherms during development can influence their body condition and fitness (23–25) and may influence their adult life history in 2 distinct ways. First, under conditions that are suboptimal for development, inferior body condition may place a severe constraint on particular traits, including how much the animals invest in costly dispersal behaviors. Second, developing juveniles may use temperature to determine those life history characteristics that will be adopted by adults. Under conditions where there is seasonal variation in the life history characteristics that will be optimal, ambient temperature experienced during development might be an especially reliable indicator of the dispersal tactics that will be most advantageous for adults. In general, direct behavioral responses to thermal conditions experienced at an earlier life stage are poorly documented. However, in the Hymenoptera, adult oviposition and foraging behavior depend on juvenile thermal conditions (26, 27), but not on adult body condition.
Our objective was to determine how ambient temperature during ontogeny influences dispersal-related life history traits in Erigone atra, a Linyphiid spider of agricultural landscapes. In common with other agrobiont spiders, the species is adapted to life in a spatiotemporally dynamic landscape where productive crop habitat is only seasonally available. E. atra colonizes crop habitat when it becomes available (typically in the spring) and during breeding (often 2 generations), then abandoning the crop in late summer in favor of natural (noncrop) habitat for subsequent breeding and over-winter hibernation (28). This cycle of mass emigration from their hibernation sites in spring and subsequent immigration in autumn (29, 30) is most evident from the large amounts of gossamer (i.e., silk threads; ref. 31) present in late summer and autumn. In agricultural landscapes, natural (noncrop) habitat typically constitutes only a small proportion of an area. This may have important consequences for adaptive dispersal when spiders are moving to crop habitat compared with when they are moving to noncrop habitat. Spring dispersal from natural to crop habitat is less risky, as a large proportion of the landscape is suitable for the spider. Under these conditions, ballooning is an effective means of distributing individuals over a wide area of productive crop habitat. However, mortality risks are probably high for long-distance (uncontrolled) ballooning in landscapes when dispersal is toward less widespread noncrop hibernation sites (20, 22). High mortality costs associated with ballooning are therefore expected to select for the adoption of more controlled, lower-risk behavior, such as rappelling.
The hypothesis investigated was that E. atra uses the ambient temperature experienced during development for determining which dispersal tactics will be adopted after maturing (proximate mechanisms). The first prediction we considered is that maximal dispersal rates are obtained under juvenile developmental temperatures that result in optimal body condition. Second, we tested the prediction that a higher proportion of spiders balloon when they develop under cooler spring-like conditions when adult dispersal is toward crop habitat, whereas a higher proportion rappel when they develop under warmer summer-like conditions when adult dispersal will be toward the noncrop habitat. In following a half-sib breeding design, we were additionally able to test the hypothesis that any condition-dependent dispersal strategy has an innate basis (ultimate mechanisms).
Results
Fitness-Related Traits.
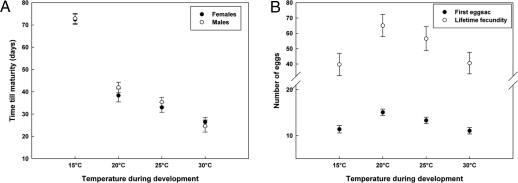
Juvenile development time was significantly influenced by temperature during development [F(3,21.1) = 184.27, P < 0.001]. Developmental time was shortest for individuals raised at 30 °C, was nearly twice as long at 20 °C, and was about 3 times as long at 15 °C (Fig. 1A). Effects of the spider's sex [F(1,352) = 0.67, P > 0.410] and the interaction of sex and temperature [F(3,351) = 0.68, P > 0.566] were not significant. Longevity after maturation differed between sexes [F(1,350) = 62.34, P < 0.001], but was not affected by temperature [F(3,47.5) = 2.49, P > 0.071] or by the interaction of temperature with sex [F(3,348) = 2.31, P > 0.075]. Females (57 ± 2 days) lived on average 18 days longer than males (39 ± 2 days).
Fig. 1.
Effect of temperature during development on fitness-related traits (mean values + SE) in E. atra. (A) Developmental time. (B) Egg production.
The total number of egg sacs, number of eggs within the first egg sac, and lifetime numbers of eggs were strongly correlated (rp ranged between 0.54 and 0.94; all df = 230, P < 0.001). Fecundity, in terms of the lifetime number of eggs, was highest for individuals reared at 20 °C and 25 °C [F(3,212) = 4.32, P < 0.006; Fig. 1B]. This is due both to the effect of temperature on the number of egg sacs [F(3,210) = 2.44, P < 0.065] and the influence of temperature on the number of eggs in the first egg sac [log-transformed data; F(3,212) = 3.90, P < 0.009].
Dispersal Motivation.
E. atra raised at 15 °C on average attempted more climbing than those that developed at higher temperatures [F(3,34.28) = 25.21, P < 0.001]. Effects of sex [F(1,434.4) = 0.87, P > 0.350] and interactions with temperature [F(3,434.8) = 1.71, P > 0.162], fecundity [F(1,227.4) = 1.24, P > 0.260], or longevity [F(1,366.5) = 1.09, P > 0.191] were not significant. The tiptoe duration per trial was highest for females [F(1,363) = 15.28, P < 0.001], highest at developmental temperatures 20 °C and 25 °C [F(3,49) = 4.78, P < 0.006], and positively (r = 0.125 ± 0.060 SE) related to longevity [F(1,367) = 4.35, P < 0.037].
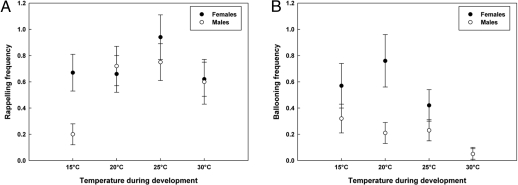
Females rappelled more often than males [F(1,437.7) = 4.46, P < 0.035]. Rappelling frequencies were lowest at 15 °C compared with other developmental temperatures [overall F(3,61.51) = 2.87, P < 0.038; Fig. 2A]. Females also ballooned more often than males [F(3,394.95) = 5.31, P < 0.004]. Ballooning occurred less frequently at 30 °C compared with other developmental temperatures [overall F(1,358.7) = 9.22, P < 0.002; Fig. 2B]. For ballooning, a positive relationship (r = 0.010 ± 0.004 SE) with longevity was found [F(1,367.7) = 4.32, P < 0.039]. Genotype*Environment [supporting information (SI) Table S1] interactions were large for climbing activity (σ = 0.116 ± 0.065 SE) and ballooning frequency (σ = 0.389 ± 0.187 SE).
Fig. 2.
Effect of temperature during development on dispersal motivation (mean values + SE) in E. atra. (A) Rappelling frequency. (B) Ballooning frequency.
Dispersal Distance.
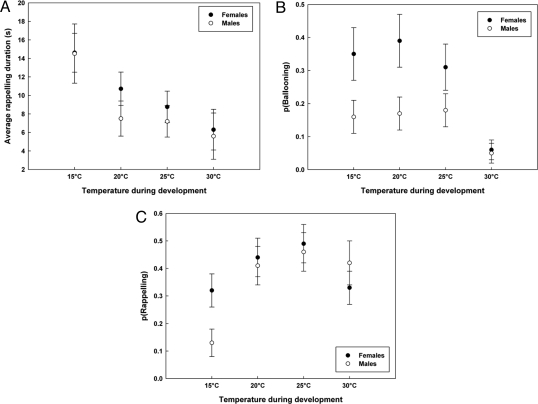
Duration of tiptoeing before ballooning was not significantly affected by any of the considered parameters (i.e., temperature, fecundity, longevity), whereas duration of the tiptoeing that preceded rappelling was significantly affected by temperature during development [F(3,143) = 4.30, P < 0.007; Fig. 3A), with the highest duration for individuals raised at 15 °C.
Fig. 3.
Effect of temperature during development on dispersal ability (mean values + SE) in E. atra. (A) Average rappelling tiptoe duration. (B) Probability of ballooning. (C) Probability of rappelling.
The probability of ballooning was highest for females [F(1,357.9) = 10.67, P < 0.002], lowest at developmental temperature of 30 °C [F(3,34.32) = 5.53, P < 0.004; Fig. 3B], and positively related to longevity [r = 0.013 ± 0.005 SE; F(1,352.7) = 5.14, P < 0.024]. Again, no interactions were significant, and no additive effects of fecundity on female behavior were found. In contrast, the probability of rappelling was not affected by sex [F(1,443.9) = 1.40, P > 0.237], but was significantly lower after development in 15 °C [F(3,50.37) = 5.65, P < 0.003; Fig. 3C]. In females, the effect of temperature disappeared [F(3,15.8) = 0.86, P > 0.481] when controlling for lifetime fecundity [r = 0.5341 ± 0.2563 SE; F(1,222.5) = 4.34, P < 0.045]. Similarity among kin due to paternal effects was restricted to the probability of ballooning (σ = 0.367 ± 0.225 SE; Table S1).
Settlement.
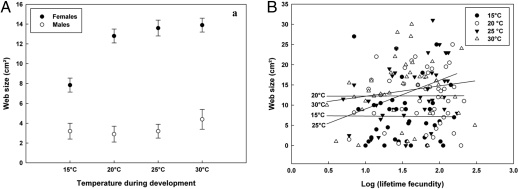
Females built larger webs than males [F(1,261) = 93.28, P < 0.001; Fig. 4A]. Effects of developmental temperature [F(3,261) = 3.51, P < 0.017] were only significant for females [interaction F(3,261) = 3.76, P < 0.012], with the smallest webs produced after development at 15 °C. Web size was positively related to longevity [r = 0.040 ± 0.017 SE; F(1,261) = 5.32, P < 0.022]. A significant temperature–fecundity interaction [F(3,217) = 3.27; P < 0.022] was found for females (Fig. 4B). Accordingly, only positive relationships between fecundity and web building were prominent at 25 °C (r = 2.34 ± 0.97 SE) and 30 °C (r = 2.56 ± 1.01 SE). At lower temperatures during development, no positive relationship between fecundity and web size was found. No parental effects were found (Table S1).
Fig. 4.
Effect of temperature during development on settlement (web size; mean values + SE) in E. atra. (A) Effect of temperature on both sexes. (B) Effect of temperature on female web size, controlled for lifetime fecundity.
Discussion
Our results demonstrate that thermal conditions during juvenile development can strongly influence adult dispersal behavior through effects on individual condition. Rappelling motivation and duration of the rappelling (short-distance movements) were lowest for individuals raised at 15 °C. In contrast, long-distance dispersal (ballooning) showed opposite patterns, with lowest motivation and ability for individuals raised at 30 °C. This suggests that the spider, when it matures, used the temperature it experienced as a juvenile as a basis for deciding which of the 2 dispersal tactics it adopts. Moreover, the chosen dispersal tactic is optimal according to the spatial patterns of habitat availability the spider experiences as an adult. Phenotypic plasticity was found to be responsible for most of the observed variation in dispersal strategy. However, for general predispersal activity and also for ballooning behavior, we found sire*temperature interactions, indicating genetic variation within the population for these characteristics. This suggests that natural selection may be able to act more readily on long-distance ballooning than on shorter-distance rappelling. The absence of correlations among the explored dispersal behaviors suggests that dispersal motivation, potential dispersal distance, and settlement are independently influenced by thermal conditions during juvenile development.
Our experiments revealed higher investments in rappelling when juveniles were exposed to summer temperatures. Higher investments in behaviors that are most likely to result in long-distance dispersal (i.e., longer rappelling threads and maximal ballooning propensity) were prevalent when individuals were raised at spring temperatures. Thermal conditions subsequently affect aeronautic dispersal both at the time of the dispersal event (higher ballooning initiations when ambient temperature is high; refs. 32, 33) and in life phases that precede the dispersal phase. Furthermore, plasticity in relation to thermal conditions during juvenile development appeared to be adaptive (i.e., beneficial) with respect to optimal seasonal dispersal movements. From earlier studies, we know that the costs of ballooning become higher when suitable habitat becomes scarcer, both in terms of area and isolation (15, 22). Our results add to this evidence because ballooning motivation declines when spiders are exposed to temperatures that represent the season with sparsely distributed winter habitat.
Our study highlights the importance of adaptive plasticity for species inhabiting a habitat with severe cycles of disturbance by using thermal conditions during development as a reliable (but probably not the only; ref. 34) source of information. Indeed, crop habitat shows a spatially homogeneous distribution in spring and early summer, rendering ballooning dispersal beneficial because unoccupied suitable habitat can be swiftly colonized without risking mortality due to landing in unsuitable habitat. This is clearly reflected by increased ballooning frequencies in individuals raised at temperatures of 15 °C to 20 °C, but also in the higher rappelling distances because longer threads are produced. In contrast, summer temperatures during juvenile development favor rappelling. This dispersal comprises local movements at low heights (above or even within the vegetation) and likely results in safer, short-distance dispersal when available habitat is rare (35, 36). However, short-distance displacements are documented to be less directional and more broadly distributed through variable changes in wind direction at short distances above the vegetation (37, 38), and therefore are less efficient for directional movements. It may consequently reflect higher tendencies to adopt a risk-spreading strategy at limited spatial scales—that is, as a response to avoid local disturbance (harvesting or ploughing in arable landscapes or flooding in natural habitats like regularly inundating wetlands) or overcrowding by moving more randomly over short distances until suitable habitat is reached. Moreover, our experiments indicate that high investments in gossamer production in autumn do not result from increased ballooning dispersal (31), but instead from increased investments in low-risk, short-distance mobility. These results also contradict the theory that aeronautic dispersal in agrobiont spiders is solely determined by the prevailing meteorological conditions during the life stage in which dispersal takes place (i.e., the dispersal window; ref. 39).
The relationship between environmental thermal conditions and traits related to body condition (growth rates, body size, and fecundity) is generally parabolic (40), as observed for fecundity in thermophilic butterflies (24, 41) and spiders (23). Because silk production is an energy-demanding process (42), we expected a similar parabolic pattern for silk-related dispersal in relation to juvenile thermal conditions. Our experiments demonstrated the importance of body condition on the dispersal phenotype. In contrast to expectation, among-treatment variation was only pronounced for rappelling probability in females. Within-treatment variation explained more variation in the other behaviors. Dispersal in E. atra is subsequently dependent on body condition (43). In contrast with studies on insects with dimorphic wing development (10), we did not find a tradeoff between dispersal and fecundity, but instead a positive relationship between dispersal and fecundity under certain environmental conditions. This suggests that dispersers are nonrandom samples from a population, with the possibility that those individuals in the best condition are the best dispersers (11, 44). Apart from the probability of rappelling, effects of developmental temperature were additive. Thermal conditions consequently affected dispersal indirectly, through changes in individual body condition, and directly, probably through changes in neurological development (see, for example, ref. 26).
Female E. atra that experienced thermal spring conditions invested less in web building. Although this behavior covaries with body condition, as indirectly assessed by fitness-related parameters, it potentially reflects different “sampling” strategies of local prey availability. Because prey is on average less abundant in the beginning of the season, a more aggregated distribution can be expected (45). As agrobiont spiders select web locations according to prey availability, lower investments in web building (here shown to be only a partially condition-dependent strategy) could be regarded as a sampling strategy of microhabitat before foraging is optimized.
Our main result, that ambient temperature during juvenile development can influence the adult dispersal of organisms inhabiting spatiotemporally complex environments, has potentially important implications in the context of global warming. Any substantial increase in temperature may result in a mismatch between the dispersal strategy used by adults and the spatial patterns of habitat availability at different times of the year. For example, juveniles developing during the spring may become less likely to balloon as adults, and this could lead to damaging population-level consequences as less crop habitat is colonized. The genetic variation we observed for ballooning propensity suggests that adaptation to climate change may be possible, and an interesting avenue for future work would be to investigate the degree to which reaction norms are locally adapted to varying thermal conditions across the species' range. For species where dispersal is condition-dependent, and especially where temperature controls the propensity for long-distance dispersal, there may also be interesting considerations in terms of predicting range-shifting dynamics. For E. atra, not accounting for the temperature dependence in dispersal might result in an overestimation of the potential rate of range expansion, because the extent of long-distance ballooning seems likely to decline as temperatures increase. We argue that by using reaction norm analyses to gain an improved understanding of plastic life history characteristics, we will be better placed to develop robust ecological management strategies for a period of rapid environmental change.
Materials and Methods
Model Species and Aeronautic Dispersal.
E. atra (Blackwall, 1833) is one of the most common aeronautic spiders in Western Europe (18). It especially inhabits crops sown during autumn (46). The species adopts both ballooning and rappelling dispersal tactics after the initiation of tiptoe behavior. We deduced an individual's dispersal motivation from its propensity to exhibit this tiptoeing and its frequency of ballooning and rappelling during the trial. The potential covered dispersal distance is in the first instance determined by the performed dispersal mode (i.e., ballooning for long-distance dispersal and rappelling for short-distance dispersal; ref. 18). Second, the length of the thread is positively correlated with the duration of the tiptoeing (16). The length of the produced silk thread also correlates with the potential dispersal distance for rappelling, with longer rappelling threads resulting in larger covered distances. The thread length may additionally determine the length of the ballooning event because it is directly related to the drag velocity for individuals of similar weight (16). Finally, we determined the size of the produced sheet web after dispersal as an indicator for the energetic investment in settlement.
Experimental Design.
A total of 520 individuals from 24 half-sib families were individually reared on moist plaster of Paris in Petri dishes with a diameter of 4 cm, which were randomly assigned to 4 thermal conditions (15 °C, 20 °C, 25 °C, and 30 °C) simulating climatological conditions during spring (15 °C), summer (20 °C–25 °C), and an extremely hot summer (30 °C). Until 1 week after maturation, spiders were fed ad libitum with Sinella curviseta (Collembola) and Drosophila melanogaster (Diptera). Spiders were checked every 2 days for molting (E. atra only shows full coloration the second day after molting).
Seven days after final molting, individuals were transferred to a wind tunnel with upward wind velocity of 1.2 ± 0.2 m/s and ambient temperature of 25 °C (16, 20, 21). They were placed on a platform with vertical wooden sticks (diameter 6 mm, height 20 cm) in a water bath. After acclimatizing for 1 hour, the general activity (number of climbings on the wooden sticks), duration of each tiptoe event, and frequency of ballooning or rappelling were scored during 15-min trials. During these trials, spiders were allowed to perform multiple tiptoeing. Therefore, individuals were gently put back on the platform (hence minimizing disturbance by the experimenter) after removal of the previously produced silk threads from the wooden sticks with a small brush. Individual variation in web-building investment was assessed by placing individuals in terrariums with a grid of vertical sticks of 0.2-mm diameter (1-cm2 grid size) to allow web attachment. Size of the web was assessed after standardized digitalization of the web surface (calculation of web surface after calibration of pixel numbers). Earlier work showed significant individual repeatability of the behaviors at longer time intervals, although aging and mating state have a strong influence (ref. 21; unpublished data). Web building is not constrained by prior production of silk for dispersal; lower investments are only recorded after sequential web destruction for >10 days in spiders under food deprivation (D.B., unpublished data). To maximize the number of independent trials, each individual was only tested once in unmated condition, 1 week after maturation. To test for condition-dependent dispersal, we recorded the following fitness-related traits of each experimental individual: developmental time until final molt and longevity after maturation (males and females), total lifetime numbers of egg sacs and eggs, and number of eggs in first egg sac.
Data Analysis.
Reaction norms for ballooning or rappelling incidence were modeled by multifactorial mixed models for binomially distributed data (logit-link function). Poisson models were applied to model frequencies of performed behaviors and numbers of egg sacs (log-link function). Models for normally distributed data were used for duration of tiptoe displays, age, and numbers of produced eggs (log-transformed). Because parameters showed different error distributions and were influenced by parental covariation, no multivariate techniques were applied. Instead, we first performed Spearman correlations to assess their correlated expression (SI Text). Because of strong correlations between the overall tiptoe frequency and the total duration of the tiptoeing within 1 trial and the frequencies of the 2 dispersal modes (SI Text), we omitted analyses for tiptoe frequency. Breeding temperature and sex were treated as categorical fixed factors. We allowed covariation with fitness-related traits to test for potential condition-dependent dispersal. Sire, dam(sire), and interactions with fixed factors were treated as random factors. Analyses of tiptoe duration for each dispersal event additionally took account of individual variation (repeated measure), nested within dam(sire) variation. We used the Satterthwaite procedure to approximate denominator degrees of freedom. Analyses were conducted with Proc Mixed (normal models) and Proc Glimmix (Poisson and Binomial models) (47).
Supplementary Material
Acknowledgments.
Gabriel Weyman provided valuable comments on an earlier draft of the manuscript. We are grateful to James Bell, Any Reynolds, and Pernille Thorbek for discussions on the relation between dispersal mode and experienced wind currents. Three anonymous reviewers provided extremely helpful comments to clarify the text. D.B. holds a postdoctorate fellowship at the Fund for Scientific Research–Flanders (FWO). This study was partially funded by FWO Grant G.0202.06.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806830105/DCSupplemental.
References
- 1.Ronce O. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol Syst. 2007;38:231–253. [Google Scholar]
- 2.Bowler DE, Benton TG. Causes and consequences of animal dispersal stragegies: Relating individual behaviour to spatial dynamics. Biol Rev. 2005;80:205–225. doi: 10.1017/s1464793104006645. [DOI] [PubMed] [Google Scholar]
- 3.Kokko H, López Sepulcre A. From individual dispersal to species ranges: Perspectives for a changing world. Science. 2006;313:789–790. doi: 10.1126/science.1128566. [DOI] [PubMed] [Google Scholar]
- 4.Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. Dispersal. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 5.Bullock JM, Kenward RE, Hails RS, editors. Dispersal Ecology. Oxford: Blackwell; 2002. [Google Scholar]
- 6.Hanski IA, Gaggiotti OE. Ecology, Genetics and Evolution of Metapopulations. San Diego: Academic; 2004. [Google Scholar]
- 7.Kremen C, et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for effects of land-use change. Ecol Lett. 2007;10:299–314. doi: 10.1111/j.1461-0248.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 8.Dall SR, Giraldeau LA, Olsson O, McNamara JM, Stephens DW. Information and its use by animals in evolutionary ecology. Trends Ecol Evol. 2005;20:187–193. doi: 10.1016/j.tree.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Imbert E. Capitulum characteristics in a seed heteromorphic plant, Crepis sancta (Asteraceae): Variance partitioning and inference for the evolution of dispersal rate. Heredity. 2001;86:78–86. doi: 10.1046/j.1365-2540.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- 10.Roff DA, Fairbarn DJ. The genetic basis of dispersal and migration and its consequences for the evolution of correlated traits. In: Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. Dispersal. Oxford: Oxford Univ Press; 2001. pp. 191–202. [Google Scholar]
- 11.Saastamoinen M. Mobility and lifetime fecundity in new versus old populations of the Glanville fritillary butterfly. Oecologia. 2007;153:569–578. doi: 10.1007/s00442-007-0772-5. [DOI] [PubMed] [Google Scholar]
- 12.Cheptou PO, Carrue O, Rouifed S, Cantarel A. Rapid evolution of seed dispersal in an urbanenvironment in the weed Crepis sancta. Proc Natl Acad Sci USA. 2008;105:3796–3799. doi: 10.1073/pnas.0708446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haag CR, Saastamoinen M, Marden JH, Hanski I. A candidate locus for variation in dispersal rate in a butterfly metapopulation. Proc R Soc London Ser B Biol Sci. 2005;272:2449–2456. doi: 10.1098/rspb.2005.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philips BL, Brown GP, Travis JM, Shine R. Reid's paradox revisited: The evolution of dispersal kernels during range expansion. Am Nat. 2008;172(Suppl 1):S34–S48. doi: 10.1086/588255. [DOI] [PubMed] [Google Scholar]
- 15.Bonte D, Bossuyt B, Lens L. Aerial dispersal plasticity under different wind velocities in a salt marsh wolf spider. Behav Ecol. 2007;18:438–443. [Google Scholar]
- 16.Bonte D, Lukac M, Lens L. Starvation affects two aerial dispersal decisions in Erigone-spiders in a different way. Basic Appl Ecol. 2008;9:308–315. [Google Scholar]
- 17.Van Dyck H, Baguette M. Dispersal behaviour in fragmented landscapes: Routine or special movements? Basic Appl Ecol. 2005;6:535–545. [Google Scholar]
- 18.Bell JR, Bohan DA, Shaw EM, Weyman GS. Ballooning dispersal using silk: World fauna, phylogenies, genetics and models. Bull Entomol Res. 2005;95:69–114. doi: 10.1079/ber2004350. [DOI] [PubMed] [Google Scholar]
- 19.Thomas CFG, Brain P, Jepson PC. Aerial activity of linyphiid spiders: Modelling dispersal distances from meteorology and behaviour. J Appl Ecol. 2003;40:912–927. [Google Scholar]
- 20.Bonte D, Vandenbroecke N, Lens L, Maelfait JP. Low propensity for aerial dispersal in specialist spiders from fragmented landscapes. Proc R Soc London Ser B Biol Sci. 2003;270:1601–1607. doi: 10.1098/rspb.2003.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonte D, De Blauwe I, Maelfait JP. Environmental and genetic background of tiptoe-initiating behaviour in the dwarfspider Erigone atra. Anim Behav. 2003;66:169–174. [Google Scholar]
- 22.Bonte D, Vanden Borre J, Lens L, Maelfait JP. Geographic variation in wolfspider dispersal behaviour is related to landscape structure. Anim Behav. 2006;72:655–662. [Google Scholar]
- 23.Li D, Jackson RR. How temperature affects development and reproduction in spiders: A review. J Therm Biol. 1996;21:245–274. [Google Scholar]
- 24.Karlsson B, Van Dyck H. Does habitat fragmentation affect temperature-related life-history traits? A laboratory test with a woodland butterfly. Proc R Soc Ser B Biol Sci. 2005;272:1257–1263. doi: 10.1098/rspb.2005.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamaiilé-Jammes S, Massot M, Aragon P, Clobert J. Global warming and positive fitness response in mountain populations of common lizards Lacerta vivipara. Global Change Biol. 2006;12:392–402. [Google Scholar]
- 26.Tautz J, Maier S, Groh S, Rossler W, Brockmann A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc Natl Acad Sci USA. 2003;100:7343–7347. doi: 10.1073/pnas.1232346100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Baaren J, Outreman Y, Boivin G. Effect of low temperature exposure on oviposition behaviour and patch exploitation strategy in parasitic wasps. Anim Behav. 2005;70:153–163. [Google Scholar]
- 28.Sunderland K. Mechanisms underlying the effects of spiders on pest populations. J Arachnol. 1999;27:308–316. [Google Scholar]
- 29.Schmidt MH, Tscharntke T. Landscape context of sheetweb spider (Araneae: Linyphiidae) abundance in cereal fields. J Biogeogr. 2005;32:467–473. [Google Scholar]
- 30.Schmidt M, Thies C, Nentwig W, Tscharntke T. Contrasting responses of arable spiders to the landscape matrix at different spatial scales. J Biogeogr. 2008;35:157–166. [Google Scholar]
- 31.Toft S. Two functions of gossamer dispersal in spiders? Acta Jutlandica. 1995;70:257–268. [Google Scholar]
- 32.Richter CJ. Aerial dispersal in relation to habitat in eight wolf spider species (Pardosa, Araneae, Lycosidae) Oecologia. 1970;5:200–214. doi: 10.1007/BF00344884. [DOI] [PubMed] [Google Scholar]
- 33.Thorbek P, Sunderland KD, Topping CJ. Eggsac development rates and phenology of agrobiont linyphiid spiders in relation to temperature. Entomol Exp Appl. 2003;109:89–101. [Google Scholar]
- 34.Bradshaw WE, Holzaphel CM. Evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. [DOI] [PubMed] [Google Scholar]
- 35.Murrell DJ, Travis JMJ, Dytham C. The evolution of dispersal distance in spatially-structured populations. Oikos. 2002;97:229–236. [Google Scholar]
- 36.Rousset F, Gandon S. Evolution of the distribution of dispersal distance under distance-dependent cost of dispersal. J Evol Biol. 2002;15:515–523. [Google Scholar]
- 37.Suter RB. An aerial lottery: The physics of ballooning in a chaotic atmosphere. J Arachnol. 1999;27:281–293. [Google Scholar]
- 38.Finnigan J. Turbulence in plant canopies. Annu Rev Fluid Mech. 2000;32:519–571. [Google Scholar]
- 39.Weyman GS, Jepson PC, Sunderland KD. Do seasonal changes in numbers of aerially dispersing spiders reflect population density on the ground or variation in ballooning motivation? Oecologia. 1995;101:487–493. doi: 10.1007/BF00329428. [DOI] [PubMed] [Google Scholar]
- 40.Roff DA. The Evolution of Life Histories, Theory and Analysis. New York: Chapman & Hall; 1992. [Google Scholar]
- 41.Karlson B, Wiklund C. Butterfly life-history and temperature adaptations: Dry, open habitats select for increased fecundity and longevity. J Anim Ecol. 2005;74:99–104. [Google Scholar]
- 42.Sherman PM. The orb-web: an energetic and behavioural estimator of a spider's dynamic foraging and reproductive strategies. Anim Behav. 1994;48:19–34. [Google Scholar]
- 43.Ims RA, Hjermann DO. Condition-dependent dispersal. In: Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. Dispersal. Oxford: Oxford Univ Press; 2001. pp. 203–216. [Google Scholar]
- 44.Le Galliard JF, Ferrière R, Clobert J. Effect of patch occupancy on immigration in the Common Lizard. J Anim Ecol. 2005;74:241–249. [Google Scholar]
- 45.Harwood JD, Sunderland KD, Symondson WOC. Living where the food is: Web-location by linyphiid spiders in relation to prey availability in winter wheat. J Appl Ecol. 2001;38:88–99. [Google Scholar]
- 46.Downie I, et al. Modelling populations of Erigone atra and E. dentipalpis (Araneae: Linyphiidae) across an agricultural gradient in Scotland. Agr Ecosys Environm. 2000;80:15–28. [Google Scholar]
- 47.SAS 9.1. NC: Cary; 2003. SAS Institute Inc. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.