Abstract
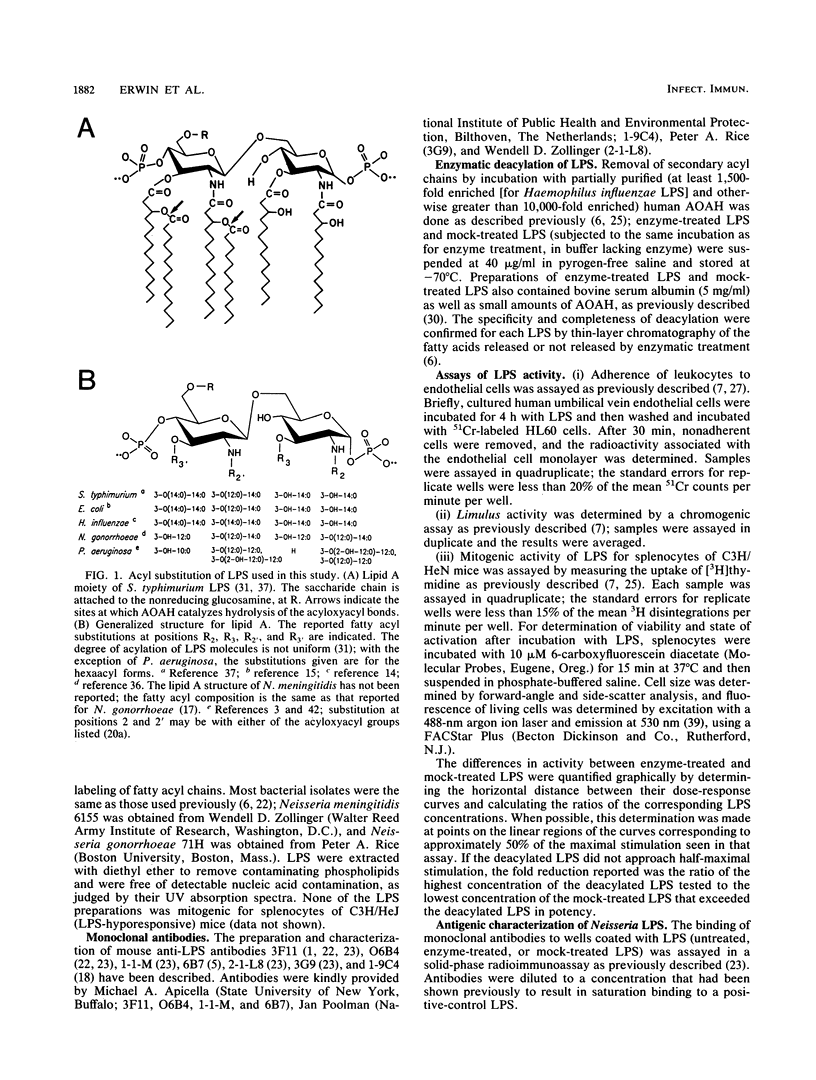
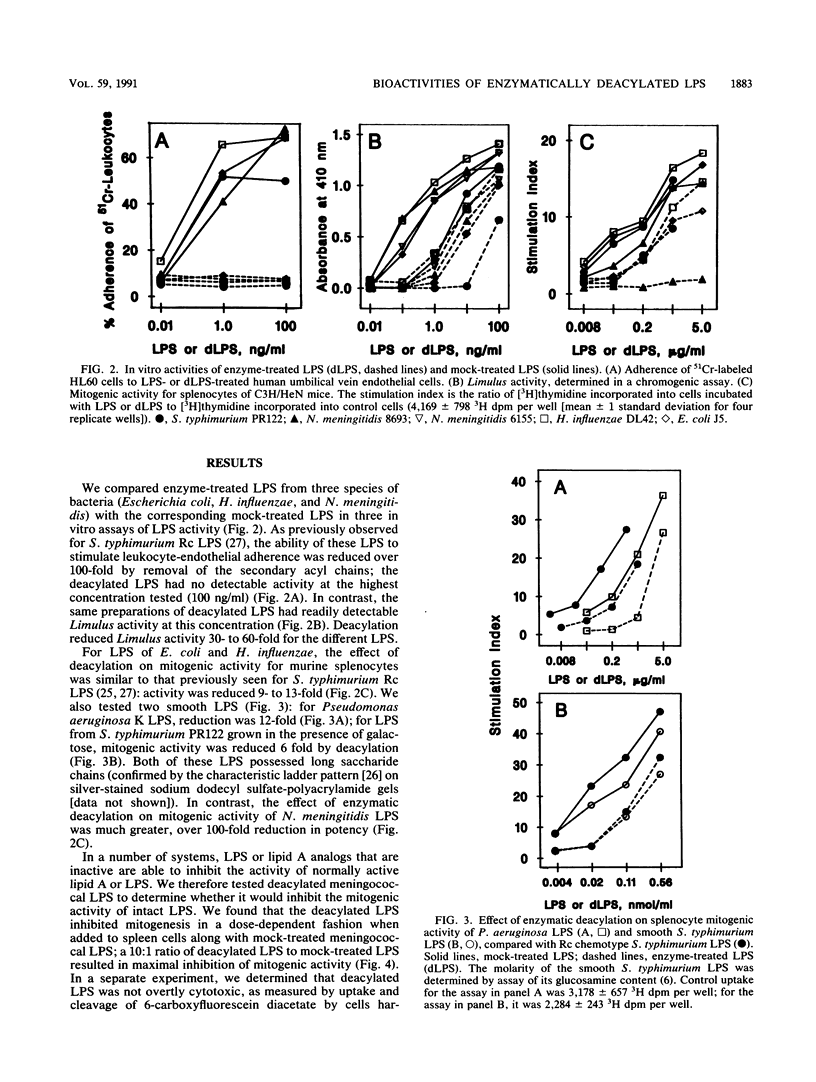
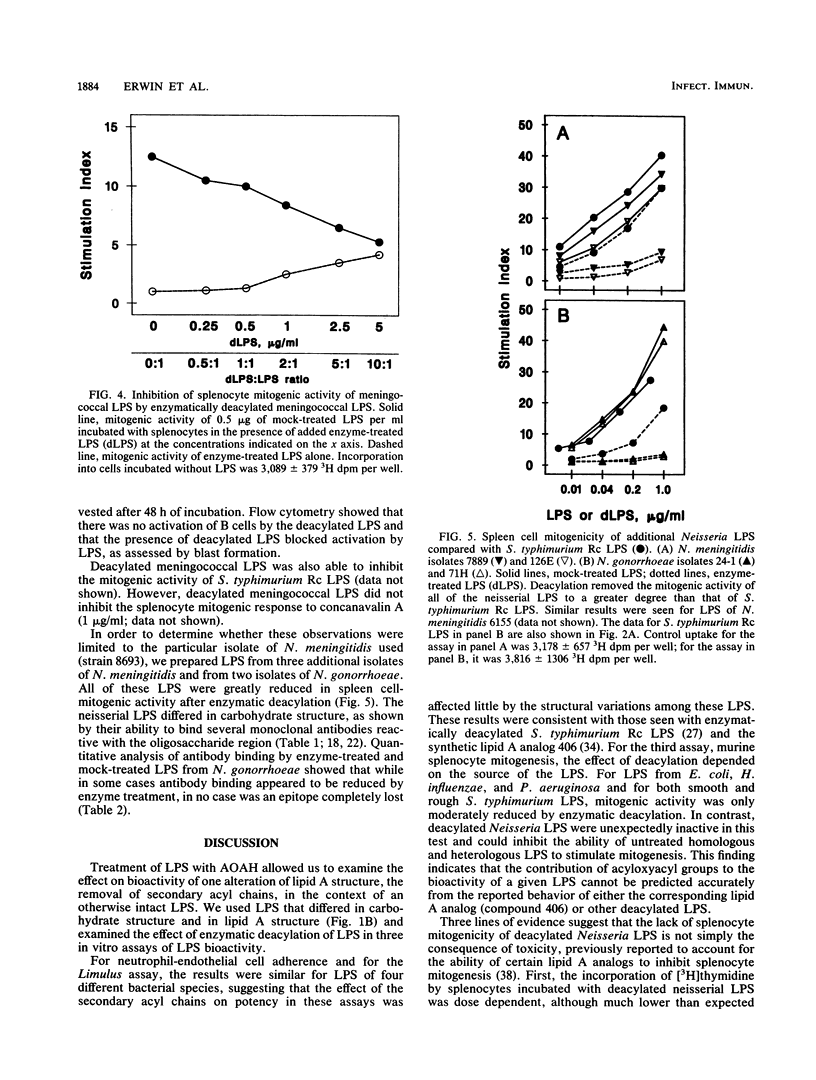
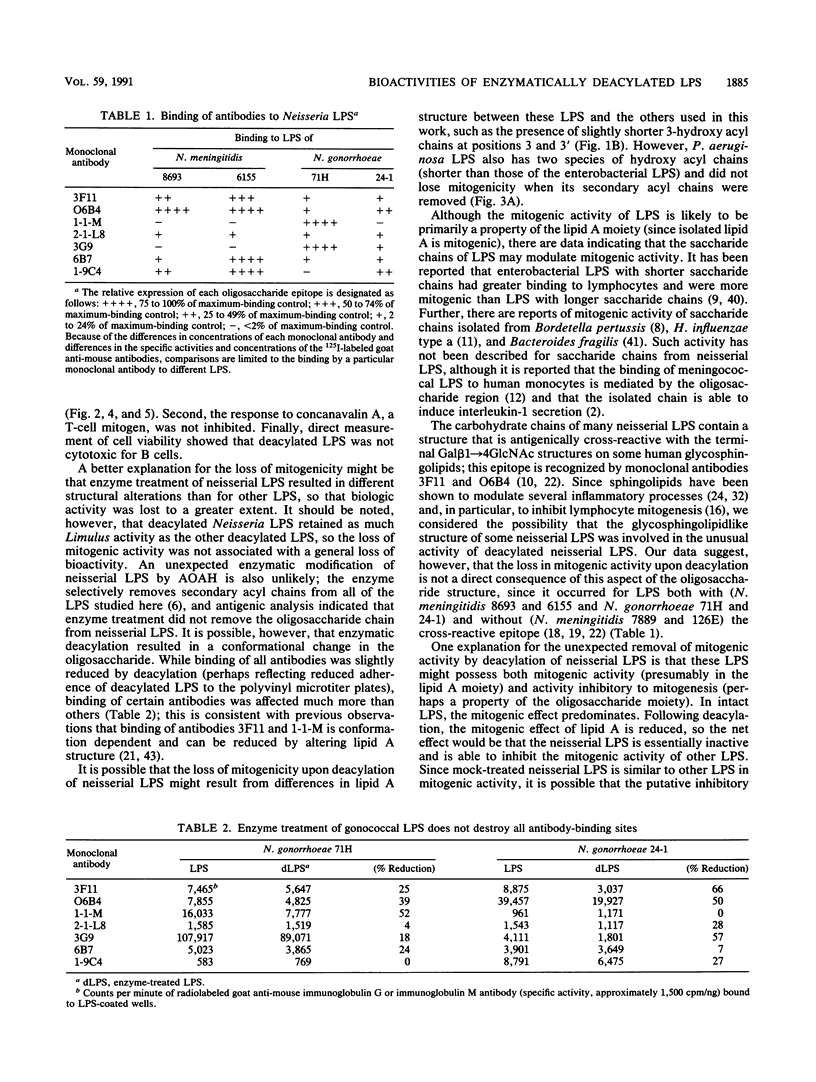
Acyloxyacyl hydrolase is a leukocyte enzyme that selectively removes the secondary acyl chains from the lipid A moiety of gram-negative bacterial lipopolysaccharides (LPS). As predicted by the reported contribution of secondary acyl chains to the bioactivities of lipid A analogs, enzymatic deacylation of Salmonella typhimurium Rc LPS substantially reduces its potency in the dermal Shwartzman reaction and in several in vitro assays that measure responses of human endothelial cells and neutrophils, whereas the potency of this LPS for inducing murine splenocyte mitogenesis is affected much less. In the experiments described here, we studied the impact of acyloxyacyl hydrolysis on the bioactivities of several LPS that differ from Salmonella LPS in carbohydrate and lipid A structures. Deacylated LPS from Escherichia coli, Haemophilus influenzae, Neisseria meningitidis, and S. typhimurium were similarly reduced in potency in the Limulus lysate test (30- to 60-fold reduction in potency relative to the corresponding mock-treated LPS), and the ability of all of these deacylated LPS to stimulate neutrophil adherence to human endothelial cells was reduced by a factor of 100 or more. For LPS from E. coli, H. influenzae, and Pseudomonas aeruginosa, the impact of deacylation on spleen cell mitogenesis was also similar to that observed for S. typhimurium LPS: deacylation reduced potency by less than 15-fold. Unexpectedly, the potency of Neisseria LPS in the murine splenocyte mitogenicity test was reduced over 100-fold by deacylation, and deacylated Neisseria LPS could block the mitogenic activity of Neisseria and Salmonella LPS. These studies indicate that the contribution of secondary acyl chains to the bioactivities of a given LPS cannot be predicted with confidence from the reported structure-activity relationships of lipid A or from the behavior of other deacylated LPS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Bennett K. M., Hermerath C. A., Roberts D. E. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1981 Dec;34(3):751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J. M., Haeffner-Cavaillon N. The role of serum in interleukin 1 production by human monocytes activated by endotoxins and their polysaccharide moieties. Immunol Lett. 1985;10(1):35–41. doi: 10.1016/0165-2478(85)90047-1. [DOI] [PubMed] [Google Scholar]
- Conrad R. S., Galanos C. Fatty acid alterations and polymyxin B binding by lipopolysaccharides from Pseudomonas aeruginosa adapted to polymyxin B resistance. Antimicrob Agents Chemother. 1989 Oct;33(10):1724–1728. doi: 10.1128/aac.33.10.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas K. C., Apicella M. A. Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect Immun. 1988 Feb;56(2):499–504. doi: 10.1128/iai.56.2.499-504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin A. L., Munford R. S. Comparison of lipopolysaccharides from Brazilian purpuric fever isolates and conjunctivitis isolates of Haemophilus influenzae biogroup aegyptius. Brazilian Purpuric Fever Study Group. J Clin Microbiol. 1989 Apr;27(4):762–767. doi: 10.1128/jcm.27.4.762-767.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin A. L., Munford R. S. Deacylation of structurally diverse lipopolysaccharides by human acyloxyacyl hydrolase. J Biol Chem. 1990 Sep 25;265(27):16444–16449. [PubMed] [Google Scholar]
- Girard R., Chaby R., Bordenave G. Mitogenic response of C3H/HeJ mouse lymphocytes to polyanionic polysaccharides obtained from Bordetella pertussis endotoxin and from other bacterial species. Infect Immun. 1981 Jan;31(1):122–128. doi: 10.1128/iai.31.1.122-128.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. A., Morrison D. C. Selective association of lipid-rich R-like lipopolysaccharide subunits with murine spleen cells. Mol Immunol. 1984 Aug;21(8):689–697. doi: 10.1016/0161-5890(84)90021-x. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Schneider H., Mandrell R. E., Yamasaki R., Jarvis G. A., Kim J. J., Gibson B. W., Hamadeh R., Apicella M. A. Lipooligosaccharides: the principal glycolipids of the neisserial outer membrane. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S287–S295. doi: 10.1093/cid/10.supplement_2.s287. [DOI] [PubMed] [Google Scholar]
- Guenounou M., Raichvarg D., Hatat D., Brossard C., Agneray J. In vitro immunological activities of the polysaccharide fraction from Haemophilus influenzae type a endotoxin. Infect Immun. 1982 May;36(2):603–608. doi: 10.1128/iai.36.2.603-608.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Etievant M., Lebbar S., Szabo L. Specific binding of endotoxin to human monocytes and mouse macrophages: serum requirement. Cell Immunol. 1985 Mar;91(1):119–131. doi: 10.1016/0008-8749(85)90037-1. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Munford R. S. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6671–6675. doi: 10.1073/pnas.80.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander I. M., Lindner B., Brade H., Altmann K., Lindberg A. A., Rietschel E. T., Zähringer U. Chemical structure of the lipopolysaccharide of Haemophilus influenzae strain I-69 Rd-/b+. Description of a novel deep-rough chemotype. Eur J Biochem. 1988 Nov 15;177(3):483–492. doi: 10.1111/j.1432-1033.1988.tb14398.x. [DOI] [PubMed] [Google Scholar]
- Jeng K. C., Chen T. L., Lan J. L. Gangliosides suppression of murine lymphoproliferation and interleukin 1 production. Immunol Lett. 1988 Dec;19(4):335–340. doi: 10.1016/0165-2478(88)90164-2. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Hawes G. B., Adams G. A., Kenny C. P. The chemical composition and serological reactions of lipopolysaccharides from serogroups A,B,X, and Y Neisseria meningitidis. Can J Biochem. 1973 Oct;51(10):1347–1354. doi: 10.1139/o73-178. [DOI] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Griffiss J. M. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect Immun. 1989 Feb;57(2):602–608. doi: 10.1128/iai.57.2.602-608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Hu Z., Westerink M. A., Poolman J. T., Griffiss J. M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988 Oct;56(10):2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach N. L., Yee E., Munford R. S., Raetz C. R., Harlan J. M. Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J Exp Med. 1990 Jul 1;172(1):77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Griffiss J. M., Macher B. A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988 Jul 1;168(1):107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus D. M. A review of the immunogenic and immuno-modulatory properties of glycosphingolipids. Mol Immunol. 1984 Nov;21(11):1083–1091. doi: 10.1016/0161-5890(84)90118-4. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986 Oct 10;234(4773):203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogare A. R., Yarbrough W. C., Jr A comparison of the effects of intact and deacylated lipopolysaccharide on human polymorphonuclear leukocytes. J Immunol. 1990 Feb 15;144(4):1404–1410. [PubMed] [Google Scholar]
- Pohlman T. H., Munford R. S., Harlan J. M. Deacylated lipopolysaccharide inhibits neutrophil adherence to endothelium induced by lipopolysaccharide in vitro. J Exp Med. 1987 May 1;165(5):1393–1402. doi: 10.1084/jem.165.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Textor J. A. Activation and inhibition of Limulus amebocyte lysate coagulation by chemically defined substructures of lipid A. Infect Immun. 1985 Aug;49(2):286–290. doi: 10.1128/iai.49.2.286-290.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackow E. C., Astiz M. E., Kim Y. B., Weil M. H. Monophosphoryl lipid A blocks the hemodynamic effects of lethal endotoxemia. J Lab Clin Med. 1989 Jan;113(1):112–117. [PubMed] [Google Scholar]
- Riedo F. X., Munford R. S., Campbell W. B., Reisch J. S., Chien K. R., Gerard R. D. Deacylated lipopolysaccharide inhibits plasminogen activator inhibitor-1, prostacyclin, and prostaglandin E2 induction by lipopolysaccharide but not by tumor necrosis factor-alpha. J Immunol. 1990 May 1;144(9):3506–3512. [PubMed] [Google Scholar]
- Rietschel E. T., Wollenweber H. W., Russa R., Brade H., Zähringer U. Concepts of the chemical structure of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):432–438. doi: 10.1093/clinids/6.4.432. [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Gobran L., Morrison D. C. Modulation of murine macrophage metabolism by glycolipids: inhibition of LPS-induced metabolism by specific gangliosides. J Leukoc Biol. 1986 Oct;40(4):367–379. doi: 10.1002/jlb.40.4.367. [DOI] [PubMed] [Google Scholar]
- Strain S. M., Armitage I. M., Anderson L., Takayama K., Qureshi N., Raetz C. R. Location of polar substituents and fatty acyl chains on lipid A precursors from a 3-deoxy-D-manno-octulosonic acid-deficient mutant of Salmonella typhimurium. Studies by 1H, 13C, and 31P nuclear magnetic resonance. J Biol Chem. 1985 Dec 25;260(30):16089–16098. [PubMed] [Google Scholar]
- Takada H., Kotani S. Structural requirements of lipid A for endotoxicity and other biological activities. Crit Rev Microbiol. 1989;16(6):477–523. doi: 10.3109/10408418909104475. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Beutler B., Kirkland T. N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989 Apr;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Hyver K., Honovich J., Cotter R. J., Mascagni P., Schneider H. Characterization of a structural series of lipid A obtained from the lipopolysaccharides of Neisseria gonorrhoeae. Combined laser desorption and fast atom bombardment mass spectral analysis of high performance liquid chromatography-purified dimethyl derivatives. J Biol Chem. 1986 Aug 15;261(23):10624–10631. [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P. Complete structure of lipid A obtained from the lipopolysaccharides of the heptoseless mutant of Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12801–12803. [PubMed] [Google Scholar]
- Tanamoto K., Galanos C., Lüderitz O., Kusumoto S., Shiba T. Mitogenic activities of synthetic lipid A analogs and suppression of mitogenicity of lipid A. Infect Immun. 1984 May;44(2):427–433. doi: 10.1128/iai.44.2.427-433.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A. Intracellularly trapped pH indicators. Soc Gen Physiol Ser. 1986;40:311–325. [PubMed] [Google Scholar]
- Vukajlovich S. W., Morrison D. C. Activation of murine spleen cells by lipid A: negative modulation of lipid A mitogenic activity by O-antigen polysaccharide. J Immunol. 1985 Oct;135(4):2546–2550. [PubMed] [Google Scholar]
- Williamson S. I., Wannemuehler M. J., Jirillo E., Pritchard D. G., Michalek S. M., McGhee J. R. LPS regulation of the immune response: separate mechanisms for murine B cell activation by lipid A (direct) and polysaccharide (macrophage-dependent) derived from Bacteroides LPS. J Immunol. 1984 Nov;133(5):2294–2300. [PubMed] [Google Scholar]
- Wollenweber H. W., Seydel U., Lindner B., Lüderitz O., Rietschel E. T. Nature and location of amide-bound (R)-3-acyloxyacyl groups in lipid A of lipopolysaccharides from various gram-negative bacteria. Eur J Biochem. 1984 Dec 3;145(2):265–272. doi: 10.1111/j.1432-1033.1984.tb08547.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki R., Schneider H., Griffiss J. M., Mandrell R. Epitope expression of gonococcal lipooligosaccharide (LOS). Importance of the lipoidal moiety for expression of an epitope that exists in the oligosaccharide moiety of LOS. Mol Immunol. 1988 Aug;25(8):799–809. doi: 10.1016/0161-5890(88)90116-2. [DOI] [PubMed] [Google Scholar]



