Abstract
Extensive epidemiologic studies have suggested that adult disease risk is associated with adverse environmental conditions early in development. Although the mechanisms behind these relationships are unclear, an involvement of epigenetic dysregulation has been hypothesized. Here we show that individuals who were prenatally exposed to famine during the Dutch Hunger Winter in 1944–45 had, 6 decades later, less DNA methylation of the imprinted IGF2 gene compared with their unexposed, same-sex siblings. The association was specific for periconceptional exposure, reinforcing that very early mammalian development is a crucial period for establishing and maintaining epigenetic marks. These data are the first to contribute empirical support for the hypothesis that early-life environmental conditions can cause epigenetic changes in humans that persist throughout life.
Keywords: developmental origins, DNA methylation, insulin-like growth factor II, nutrition, periconception
Superimposed on the DNA sequence is a layer of epigenetic information that is heritable, particularly during mitosis, and controls the potential of a genomic region to be transcribed (1). Methyl groups coupled to cytosines in cytosine-guanine (CpG) dinucleotides and modifications of histones that package the DNA are the two main molecular marks that compose this information and regulate chromatin structure and DNA accessibility (2).
Animal studies have indicated that certain transient environmental influences can produce persistent changes in epigenetic marks that have life-long phenotypic consequences (3, 4). Early embryonic development is of special interest in this respect, because this is a crucial period for establishing and maintaining epigenetic marks (5). Indeed, culturing of preimplantation mice embryos found that epigenetic marks are susceptible to nutritional conditions in the very early stages of mammalian development (6, 7).
One of the rare opportunities for studying the relevance of such findings to humans is presented by individuals who were prenatally exposed to famine during the Dutch Hunger Winter (8). This period of famine was the consequence of a German-imposed food embargo in the western part of The Netherlands toward the end of World War II in the winter of 1944–45. During this period, registries and health care remained intact, so that individuals who were prenatally exposed to this famine can be traced. Moreover, the period of famine was clearly defined, and official food rations were documented. These unique features allow us to assess whether prenatal exposure to famine is associated with persistent epigenetic differences in humans.
One of the best-characterized epigenetically regulated loci is insulin-like growth factor II (IGF2). IGF2 is a key factor in human growth and development and is maternally imprinted (9). Imprinting is maintained through the IGF2 differentially methylated region (DMR), the hypomethylation of which leads to bi-allelic expression of IGF2 (10). We recently studied IGF2 DMR methylation in 372 twins (11). IGF2 DMR methylation is a normally distributed quantitative trait that is largely determined by genetic factors in both adolescence and middle age, indicating that the methylation mark is stable up to middle age. Thus, if affected by environmental conditions early in human development, altered IGF2 DMR methylation may be detected many years later.
Here we used our ongoing Hunger Winter Families Study (8) to investigate whether prenatal exposure to famine is associated with persistent differences in methylation of the IGF2 DMR. Our primary focus was exposure during periconception, thus ensuring that the exposure was present during the very early stages of development that are critical in epigenetic programming. To further investigate the role of timing, we also studied individuals who were exposed late in gestation.
Results
Periconceptional Exposure.
Our primary goal was to test whether periconceptional exposure to famine was associated with differences in IGF2 DMR methylation in adulthood. Toward this end, we selected the 60 individuals from the Hunger Winter Families Study who were conceived during the famine 6 decades ago. The exposure period thus included the very early stages of development. The exposed individuals were compared with their same-sex sibling to achieve partial genetic matching. Using a quantitative mass spectrometry–based method (12, 13), the methylation of five CpG dinucleotides within the IGF2 DMR was measured (11). Three CpG sites were measured individually, and two were measured simultaneously, because they could not be resolved due to their close proximity. All CpG sites but one were significantly less methylated among periconceptionally exposed individuals compared with their siblings (1.5 × 10−4 ≤ P ≤ 8.1 × 10−3; see Table 1). The average methylation fraction of the IGF2 DMR based on all five CpG sites was 0.488 among exposed siblings and 0.515 among unexposed siblings. Thus, periconceptional exposure was associated with a 5.2% lower methylation (P = 5.9 × 10−5), corresponding to 0.48 standard deviations (SDs) of the controls. The association was independent of sex (Pinteraction = 0.20).
Table 1.
IGF2 DMR methylation among individuals periconceptionally exposed to famine and their unexposed, same-sex siblings
| IGF2 DMR methylation | Mean methylation fraction (SD) |
Relative change exposed | Difference in SDs | P | |||
|---|---|---|---|---|---|---|---|
| Exposed (n = 60) | Controls (n = 60) | ||||||
| Average | 0.488 | (0.047) | 0.515 | (0.055) | −5.2% | −0.48 | 5.9 × 10−5 |
| CpG 1 | 0.436 | (0.037) | 0.470 | (0.041) | −6.9% | −0.78 | 1.5 × 10−4 |
| CpG 2 and 3 | 0.451 | (0.033) | 0.473 | (0.055) | −4.7% | −0.41 | 8.1 × 10−3 |
| CpG 4 | 0.577 | (0.114) | 0.591 | (0.112) | −2.3% | −0.12 | .41 |
| CpG 5 | 0.491 | (0.061) | 0.529 | (0.068) | −7.2% | −0.56 | 1.4 × 10−3 |
P values were obtained using a linear mixed model and adjusted for age.
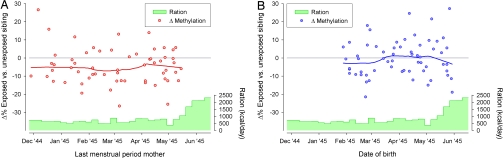
Fig. 1A displays the difference in IGF2 DMR methylation within sibships according to the estimated conception date of the famine-exposed individual. IGF2 DMR methylation was lowest in the famine-exposed individual among 72% (43/60) of sibships; this lower methylation was observed in conceptions across the famine period. Official daily rations were set weekly during the famine period and were the same for every individual. The average daily rations were 667 kcal (SD, 151) (Fig. 1A), and there was little variation in the percentage of calories from proteins (≈12%, of which 4% of animal origin), fat (19%), and carbohydrates (69%) (14).
Fig. 1.
Difference in IGF2 DMR methylation between individuals prenatally exposed to famine and their same-sex sibling. (A) Periconceptional exposure: Difference in methylation according to the mother's last menstrual period (a common estimate of conception) before conception of the famine-exposed individual. (B) Exposure late in gestation: Difference in methylation according to the date of birth of the famine-exposed individual. To describe the difference in methylation according to estimated conception and birth dates, a lowess curve (red or blue) is drawn. The average distributed rations (in kcal/day) between December 1944 and June 1945 are depicted in green.
As a technical validation, IGF2 DMR methylation was remeasured in 46 of 60 periconceptionally exposed individuals and their same-sex siblings, repeating the whole procedure from bisulfite treatment to quantification. A similarly lower 5.6% IGF2 DMR methylation was observed (P = 2.1 × 10−3), confirming our initial findings.
Late Gestational Exposure.
To further investigate the influence of timing, we selected the 62 individuals who were exposed to famine late in gestation for at least 10 weeks, so that they were born in or shortly after the famine. We found no difference in IGF2 DMR methylation between the exposed individuals and their unexposed siblings (Table 2; Fig. 1B).
Table 2.
IGF2 DMR methylation among individuals exposed to famine late in gestation and their unexposed, same-sex siblings
| IGF2 DMR methylation | Mean methylation fraction (SD) |
Relative change exposed | Difference in SDs | P | |||
|---|---|---|---|---|---|---|---|
| Exposed (n = 62) | Controls (n = 62) | ||||||
| Average | 0.514 | 0.045 | 0.519 | 0.036 | −0.9% | −0.12 | .64 |
| CpG 1 | 0.460 | 0.044 | 0.464 | 0.048 | −0.9% | −0.09 | .68 |
| CpG 2 and 3 | 0.462 | 0.039 | 0.471 | 0.039 | −1.7% | −0.21 | .46 |
| CpG 4 | 0.602 | 0.085 | 0.612 | 0.073 | −1.5% | −0.12 | .30 |
| CpG 5 | 0.529 | 0.060 | 0.531 | 0.060 | −0.3% | −0.02 | .77 |
P values were obtained using a linear mixed model and adjusted for age.
To formally test whether the association with lower IGF2 DMR methylation depended on the timing of exposure, we analyzed the periconceptional and late exposure groups together with all 122 controls in a single model (Table 3). Periconceptional exposure was associated with lower methylation (P = 1.5 × 10−5), whereas late exposure was not (P = 0.69). Furthermore, there was statistically significant evidence for an interaction between timing and exposure (Pinteraction = 4.7 × 10−3), indicating that the association was timing-specific.
Table 3.
Timing of famine exposure during gestation, IGF2 DMR methylation, and birth weight
| Periconceptional exposure | Late gestational exposure | All controls | |
|---|---|---|---|
| n | 60 | 62 | 122 |
| Males, % | 46.7 | 45.2 | 45.9 |
| Mean age, years | 58.1 (SD, 0.35) | 58.8 (SD, 0.4) | 57.1 (SD, 5.5) |
| Birth weight, g | 3612 (SD, 648) | 3126 (SD, 408) | — |
| IGF2 DMR methylation | |||
| Average | 0.488 (SD, 0.047) | 0.514 (SD, 0.045) | 0.517 (SD, 0.047) |
| Pvs all controls | 1.5 × 10−5 | .69 | |
| Pinteraction | 4.7 × 10−3 |
P values were obtained using a linear mixed model and adjusted for age.
Birth Weight.
The mean birth weight of the 62 individuals exposed late in gestation was 3126 g (SD, 408), which is 296 g lower (95% confidence interval [CI], −420 to −170 g) than the mean (3422 g; SD, 464) of 324 reference births in 1943 at the same institutions (P = 4 × 10−6) (15). The lower birth weight underscores the impact of the famine during the Hunger Winter notwithstanding the absence of an association with IGF2 DMR methyl ation. The mean birth weight of the 60 individuals who were exposed periconceptionally was 3612 g (SD, 648), not lower that that of the reference births (95% CI, +15 to + 365 g; P = 0.03). IGF2 DMR methylation was not associated with birth weight (P = 0.39).
Age Association.
To put the association of periconceptional famine exposure with a 5.2% lower IGF2 DMR methylation into perspective, we assessed the relationship between age and IGF2 DMR methylation in the 122 control individuals. Within the age range studied (43–70 years), a 10-year-older age was associated with a 3.6% lower methylation (P = .015).
Discussion
Here we report that periconceptional exposure to famine during the Dutch Hunger Winter is associated with lower methylation of the IGF2 DMR 6 decades later. The hypomethylation that we observed is highly comparable to that found for the Nr3c1 and Ppara genes in offspring of female rats fed an isocaloric protein-deficient diet starting before pregnancy (−8.2% and −10.2% vs −5.2% in our human study) (16), although greater effects for the Agtr1b gene have been found in a similar rat model (17). These data from animal models are consistent with the interpretation that famine underlies the IGF2 hypomethylation that we observed and may be related to a deficiency in methyl donors, such as the amino acid methionine (3). An additional contribution of other stressors, such as cold and emotional stress (8), cannot be ruled out, however. Our study provides the first evidence that transient environmental conditions early in human gestation can be recorded as persistent changes in epigenetic information.
In contrast to periconceptional exposure to famine, exposure late in gestation was not associated with IGF2 DMR methylation. Epigenetic marks may be particularly vulnerable during the very early stage of mammalian development, which is a crucial period for establishing and maintaining epigenetic marks (5). Experiments in which mouse zygotes were cultured to blastocysts favor this hypothesis (6, 7). The timing dependence of the association that we observed also may relate to the timing of tissue development, however (18). We studied blood and adult blood cells stem from the hematopoietic system, which is established relatively early in mammalian development (eg, day 10.5 in the mouse embryo [cf. weeks 4–6 in human gestation] (19)). Detailed future studies are needed to establish whether the susceptibility of epigenetic marks is an intrinsic property of early mammalian development or a general feature of newly developing tissues throughout gestation. Our results do not exclude the occurrence of epigenetic changes later in development (20) or during aging (21).
The developmental origins hypothesis states that adverse conditions during development contribute to adult disease risk (22). Although the mechanisms behind these relationships are unclear, the involvement of epigenetic dysregulation has been proposed (22–24). Our findings are a key element in elaborating this hypothesis. Human studies on the developmental origins of health and disease often use low birth weight as a proxy for a compromised prenatal development (22). Our data indicate that such studies are not necessarily sufficient for testing the involvement of epigenetics and thus extend our previous finding that birth weight is a poor surrogate for nutritional status during gestation (15). Epigenetic differences were found among individuals who were exposed to famine early in gestation and had a normal birth weight. Exposure to famine late in gestation was associated with low birth weight, as expected, but not with epigenetic changes. To monitor the crucial stages of early development, assessing maternal lifestyle, especially regarding nutrition (25), and embryo growth using three-dimensional ultrasonography (26) may be more appropriate than assessing birth weight.
The current study presents a first example of an association between a periconceptional exposure and DNA methylation in humans. It will be of prime interest to investigate whether other exposures during early development that are more common in modern societies, including overnutrition (3) and assisted reproductive technologies (27), give rise to similar associations. In addition, the extent to which epigenetic marks at other genomic regions are vulnerable to such exposures remains to be established. A key area to explore in future studies will be to assess the phenotypic consequences of changes in epigenetic marks. Diseases that have been associated with early gestational exposure to famine, such as schizophrenia (28) and coronary heart disease (29), are of particular interest in this respect. Analogous to current studies in genetic epidemiology (30), such epigenetic epidemiologic studies may need to be large and to include replication. Understanding how epigenetic control depends on early exposure may shed light on the link between development and health over the lifespan and ultimately suggest new ways to prevent human disease.
Methods
Study Population.
The design of and recruitment for the Hunger Winter Families Study were described previously (8). Individuals exposed to famine prenatally were recruited by identification and follow-up of live singleton births in 1945 and early 1946 at three institutions in famine-exposed cities (the midwifery training schools in Amsterdam and Rotterdam and the Leiden University hospital). As controls, same-sex siblings and unrelated individuals from the same institutions who were born before or conceived after the famine period were recruited. Clinical examination, including blood sampling, was completed for 311 exposed individuals, 311 same-sex siblings, and 349 unrelated controls. Birth weight was abstracted from birth records from the three institutions. No birth weight data are available for the same-sex siblings who were not born at these institutions.
For the current epigenetic study, we focused on exposed individuals and their siblings as controls to achieve partial genetic matching in view of the high heritability of IGF2 DMR methylation (11). From these, we selected the sibships with an individual exposed to famine periconceptionally and those with an individual exposed to famine late in gestation. Periconceptional exposure was defined as the mother's last menstrual period before conceiving the exposed individual between November 28, 1944 and May 15, 1945. This yielded 60 sibships. Exposure late in gestation was defined as a birth between January 28 and May 30, 1945, so that the duration of the famine exposure was at least 10 weeks. This yielded 62 sibships.
DNA Methylation.
Methylation of the IGF2 DMR was measured using genomic DNA from whole blood extracted using the salting-out method. One microgram of genomic DNA was bisulfite-treated using the EZ 96-DNA methylation kit (Zymo Research). Sibships were bisulfite-treated on the same plate. Three plates were used to process the 244 samples, each with an equal number of samples and a similar distribution in periconceptionally and late-exposed subjects. The region harboring the IGF2 DMR (chr11:2,126,035–2,126,372 in NCBI build 36.1) was amplified using primers described elsewhere (11). DNA methylation was measured using a mass spectrometry–based method (Epityper, Sequenom) (12), the quantitative accuracy (R2 duplicate measurements ≥ 0.98) and concordance with clonal polymerase chain reaction bisulfite sequencing of which has been reported previously (13, 31). All measurements were done in triplicate. CpG dinucleotides whose measurement was confounded by single nucleotide polymorphisms, as we discussed in a previous report (11), were discarded as part of quality control. The CpG dinucleotides reported in the current study were located at positions 41, 57 and 60, 202, and 251 bp in the amplicon targeting the IGF2 DMR. Methylation data were 93% complete. DNA methylation of five CpG dinucleotides could be measured, three individually and two as a pair because they were directly adjacent and could not be resolved individually.
Statistical Analysis.
The mean methylation fractions of individual CpGs and their SDs presented in the tables and figures are based on raw data. To obtain the average methylation of the whole IGF2 DMR presented in the tables and figures, missing methylation data were first imputed using estimates from linear mixed models, thereby exploiting the correlations among CpG sites (11). To test for differences between exposed individuals and their unexposed siblings, age-adjusted linear mixed models were applied to the raw data without imputation of missing values. These analyses accounted for age at examination, family relations, correlated methylation of CpG dinucleotides, and methylation data missing at random. Exposure status, CpG dinucleotide, and age were entered as fixed effects, and sibship was entered as a random effect. The model including both the periconceptional and the late-exposure groups was extended with a variable indicating timing of the exposure and an interaction term of exposure status times exposure time. To test for the association between IGF2 DMR methylation and birth weight, birth weight was added as a fixed effect. The linear mixed model may be viewed as an extension of the paired t-test; the model reduces to a paired t-test with identical outcomes if within-family methylation differences are assessed for a single CpG nucleotide and if data are complete and age adjustment is omitted. All P values are two-sided, and all statistical analyses were performed using SPSS 14.0.
Acknowledgments.
We thank the participants of the Hunger Winter Families Study, TNO Quality of Life for contact tracing, the staff of the Gerontology and Geriatrics Study Center at the Leiden University Medical Center for performing the clinical examinations, Marja Kersbergen and Margot van Schie for extracting genomic DNA, and Dennis Kremer for technical assistance. This work was supported by grants from the Netherlands Heart Foundation (2006B083 to B.T.H.), the U.S. National Institutes of Health (RO1-HL067914 to L.H.L.), the Netherlands Organization for Scientific Research NWO (911–03-016 to P.E.S.), and the European Union–funded Network of Excellence LifeSpan (FP6 036894).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Waterland RA, Jirtle RL. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinclair KD, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A. 2007;104:19351–19356. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 6.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 7.Morgan HD, Jin XL, Li A, Whitelaw E, O'Neill C. The culture of zygotes to the blastocyst stage changes the postnatal expression of an epigentically labile allele, agouti viable yellow, in mice. Biol Reprod. 2008;79:618–623. doi: 10.1095/biolreprod.108.068213. [DOI] [PubMed] [Google Scholar]
- 8.Lumey LH, et al. Cohort profile: The Dutch Hunger Winter Families Study. Int J Epidemiol. 2007;36:1196–1204. doi: 10.1093/ije/dym126. [DOI] [PubMed] [Google Scholar]
- 9.Smith FM, Garfield AS, Ward A. Regulation of growth and metabolism by imprinted genes. Cytogenet Genome Res. 2006;113:279–291. doi: 10.1159/000090843. [DOI] [PubMed] [Google Scholar]
- 10.Cui H, et al. Loss of IGF2 imprinting: A potential marker of colorectal cancer risk. Science. 2003;299:1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 11.Heijmans BT, Kremer D, Tobi EW, Boomsma DI, Slagboom PE. Heritable rather than age-related environmental and stochastic factors dominate variation in DNA methylation of the human IGF2/H19 locus. Hum Mol Genet. 2007;16:547–554. doi: 10.1093/hmg/ddm010. [DOI] [PubMed] [Google Scholar]
- 12.Ehrich M, et al. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci USA. 2005;102:15785–15790. doi: 10.1073/pnas.0507816102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coolen MW, Statham AL, Gardiner-Garden M, Clark SJ. Genomic profiling of CpG methylation and allelic specificity using quantitative high-throughput mass spectrometry: Critical evaluation and improvements. Nucleic Acids Res. 2007;35:e119. doi: 10.1093/nar/gkm662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger GCE, Drummond JC, Sandstead HR. Malnutrition and Starvation in Western Netherlands, September 1944–July 1945, Part 1. The Hague: General State Printing Office; 1948. [Google Scholar]
- 15.Stein AD, Zybert PA, van de Bor M, Lumey LH. Intrauterine famine exposure and body proportions at birth: The Dutch Hunger Winter. Int J Epidemiol. 2004;33:831–836. doi: 10.1093/ije/dyh083. [DOI] [PubMed] [Google Scholar]
- 16.Burdge GC, et al. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr. 2007;97:435–439. doi: 10.1017/S0007114507352392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet. 2006;15:705–716. doi: 10.1093/hmg/ddi484. [DOI] [PubMed] [Google Scholar]
- 19.Dzierzak E, Speck NA. Of lineage and legacy: The development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 21.Bjornsson HT, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299:2877–2883. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
- 23.Cutfield WS, Hofman PL, Mitchell M, Morison IM. Could epigenetics play a role in the developmental origins of health and disease? Pediatr Res. 2007;61:68R–75R. doi: 10.1203/pdr.0b013e318045764c. [DOI] [PubMed] [Google Scholar]
- 24.McClellan JM, Susser E, King MC. Maternal famine, de novo mutations, and schizophrenia. JAMA. 2006;296:582–584. doi: 10.1001/jama.296.5.582. [DOI] [PubMed] [Google Scholar]
- 25.Verkleij-Hagoort AC, et al. Validation of the assessment of folate and vitamin B12 intake in women of reproductive age: The method of triads. Eur J Clin Nutr. 2007;61:610–615. doi: 10.1038/sj.ejcn.1602581. [DOI] [PubMed] [Google Scholar]
- 26.Verwoerd-Dikkeboom CM, Koning AH, van der Spek PJ, Exalto N, Steegers EA. Embryonic staging using a 3D virtual reality system. Hum Reprod. 2008;23:1479–1484. doi: 10.1093/humrep/den023. [DOI] [PubMed] [Google Scholar]
- 27.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susser E, et al. Schizophrenia after prenatal famine: Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 29.Painter RC, et al. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2006;84:322–327. doi: 10.1093/ajcn/84.1.322. [DOI] [PubMed] [Google Scholar]
- 30.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrich M, et al. Cytosine methylation profiling of cancer cell lines. Proc Natl Acad Sci USA. 2008;105:4844–4849. doi: 10.1073/pnas.0712251105. [DOI] [PMC free article] [PubMed] [Google Scholar]