Abstract
One rabbit pathogenic Escherichia coli strain, belonging to serogroup O103, harbors a self-transferable 117-kb plasmid (pREC-1) encoding resistance to several antibiotics. The role of this R plasmid in the colonization of the digestive tract in specific-pathogen-free (E. coli O103-free) rabbits was studied. Five-week-old rabbits were inoculated with the wild-type strain, with its variant cured of the plasmid, with an E. coli K-12 strain, or with an untypeable E. coli strain from a healthy rabbit. No symptoms and no mortality were observed in animals inoculated with strains without the plasmid pREC-1, but 87.5% of the rabbits infected by the wild strain died, generally with bloody diarrhea, between days 5 and 15 postinfection. The weight gain of animals was strongly reduced. Transfer of the plasmid to the cured strain or to nonvirulent strains led these strains to induce the same pathology but with a lower mortality. Colonization of the gut by the O103 strain and symptoms of bloody diarrhea are thus related to the presence of the pREC-1 plasmid. The GV strain, which does not produce classical heat-labile enterotoxin or heat-stable enterotoxin and is not invasive, could be considered an enteropathogenic E. coli-like strain. The presence of a conjugative plasmid such as pREC-1 encoding both antibiotic resistance and virulence determinants in O103 E. coli from rabbits could represent a prominent epidemiological hazard under selective pressure by antibiotic therapy.
Full text
PDF
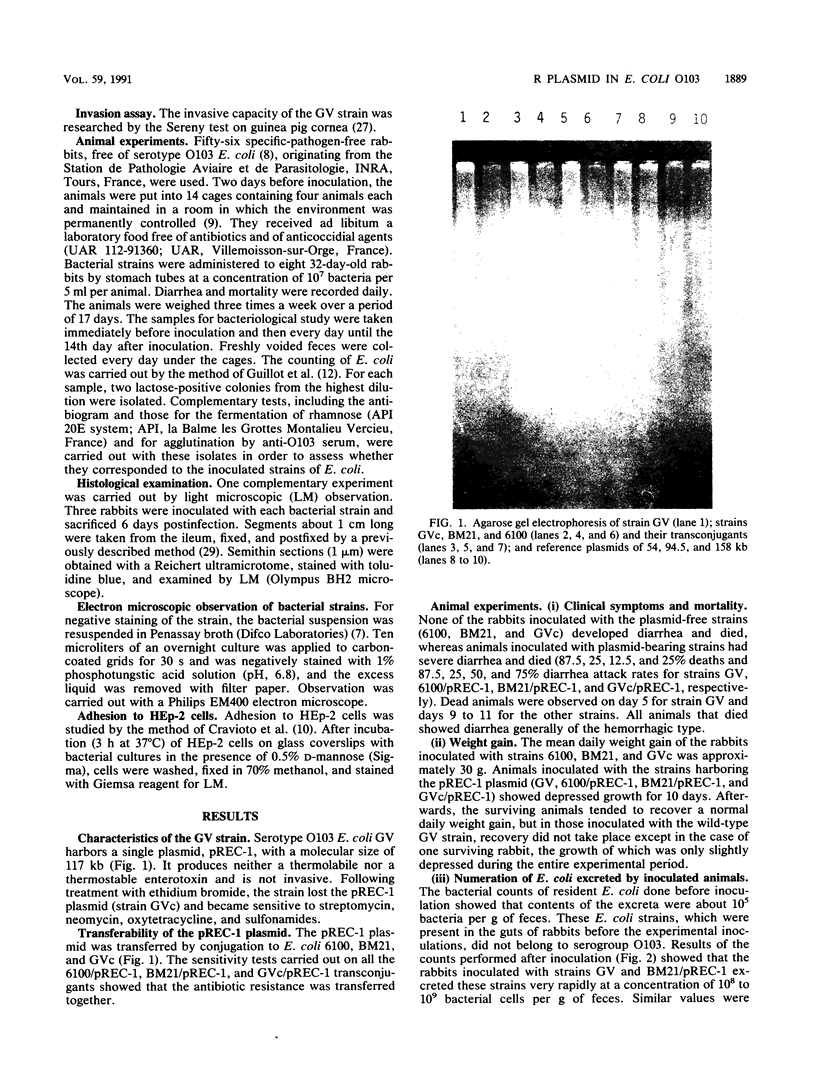
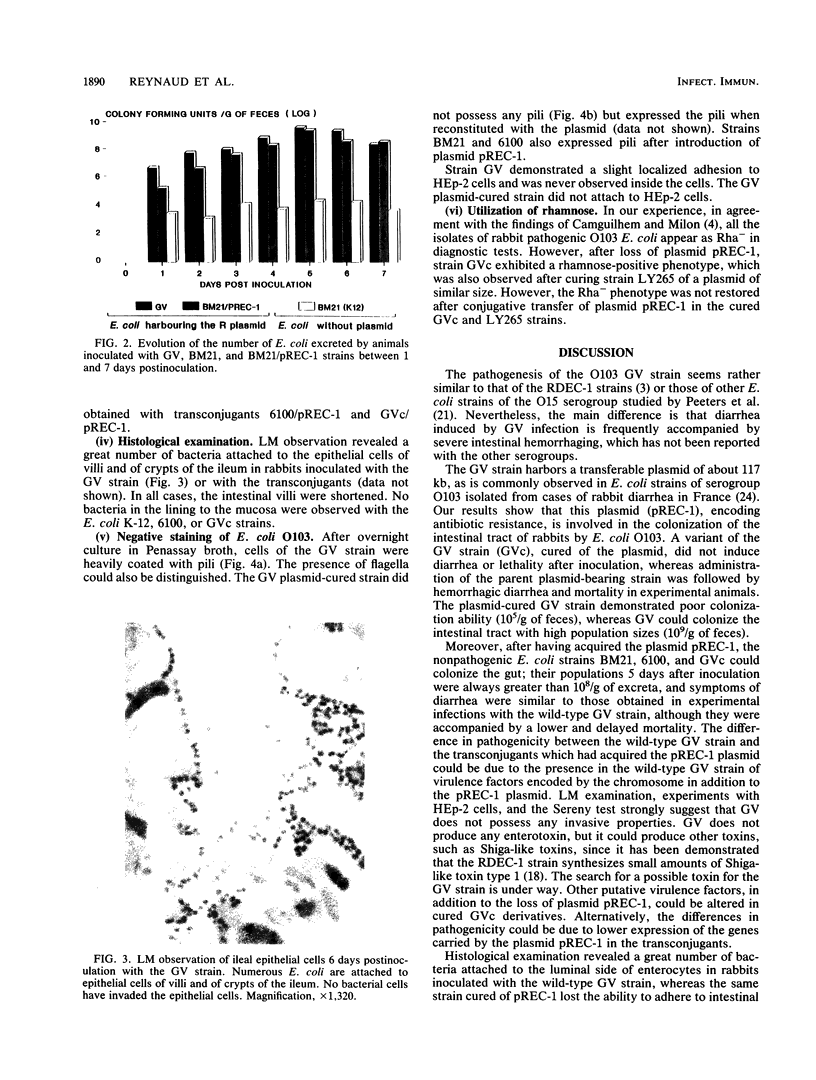


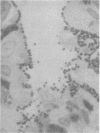
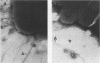
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Camguilhem R., Lebas F., Labie C. Reproduction expérimentale chez le lapin en engraissement d'une diarrhée provoquée par une souche de Escherichia coli de sérogroupe O-103. Ann Rech Vet. 1986;17(4):409–424. [PubMed] [Google Scholar]
- Camguilhem R., Milon A. Biotypes and O serogroups of Escherichia coli involved in intestinal infections of weaned rabbits: clues to diagnosis of pathogenic strains. J Clin Microbiol. 1989 Apr;27(4):743–747. doi: 10.1128/jcm.27.4.743-747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Formal S. B., Schad P. A., Boedeker E. C. Genetic transfer of a mucosal adherence factor (R1) from an enteropathogenic Escherichia coli strain into a Shigella flexneri strain and the phenotypic suppression of this adherence factor. J Infect Dis. 1983 Apr;147(4):711–723. doi: 10.1093/infdis/147.4.711. [DOI] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot J. F., Chaslus-Dancla E., Lafont J. P. Spontaneous implantation of antibiotic-resistant Enterobacteriaceae in the digestive tract of chickens in the absence of selective pressure. Antimicrob Agents Chemother. 1977 Dec;12(6):697–702. doi: 10.1128/aac.12.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C. L., Palchaudhuri S., Maas W. K. Naturally occurring plasmid carrying genes for enterotoxin production and drug resistance. Science. 1977 Oct 14;198(4313):198–199. doi: 10.1126/science.333581. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milon A., Esslinger J., Camguilhem R. Adhesion of Escherichia coli strains isolated from diarrheic weaned rabbits to intestinal villi and HeLa cells. Infect Immun. 1990 Aug;58(8):2690–2695. doi: 10.1128/iai.58.8.2690-2695.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., LaVeck G. D., Thompson M. R., Formal S. B. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J Infect Dis. 1982 Dec;146(6):763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- Okerman L. Enteric infections caused by non-enterotoxigenic Escherichia coli in animals: occurrence and pathogenicity mechanisms. A review. Vet Microbiol. 1987 May;14(1):33–46. doi: 10.1016/0378-1135(87)90050-2. [DOI] [PubMed] [Google Scholar]
- Peeters J. E., Charlier G. J., Raeymaekers R. Scanning and transmission electron microscopy of attaching effacing Escherichia coli in weanling rabbits. Vet Pathol. 1985 Jan;22(1):54–59. doi: 10.1177/030098588502200109. [DOI] [PubMed] [Google Scholar]
- Peeters J. E., Geeroms R., Glorieux B. Experimental Escherichia coli enteropathy in weanling rabbits: clinical manifestations and pathological findings. J Comp Pathol. 1984 Oct;94(4):521–528. doi: 10.1016/0021-9975(84)90056-2. [DOI] [PubMed] [Google Scholar]
- Peeters J. E., Pohl P., Okerman L., Devriese L. A. Pathogenic properties of Escherichia coli strains isolated from diarrheic commercial rabbits. J Clin Microbiol. 1984 Jul;20(1):34–39. doi: 10.1128/jcm.20.1.34-39.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas S. B., Calzolari A., La Torre J. L., Ghittoni N. E., Vásquez C. Involvement of a plasmid in Escherichia coli envelope alterations. J Bacteriol. 1983 Jul;155(1):402–406. doi: 10.1128/jb.155.1.402-406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- SERENY B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4(4):367–376. [PubMed] [Google Scholar]
- TAYLOR J., WILKINS M. P., PAYNE J. M. Relation of rabbit gut reaction to enteropathogenic Escherichia coli. Br J Exp Pathol. 1961 Feb;42:43–52. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Inman L. R., O'Hanley P. D., Cantey J. R., Lushbaugh W. B. Scanning and transmission electron microscopic study of Escherichia coli O15 (RDEC-1) enteric infection in rabbits. Infect Immun. 1978 Feb;19(2):686–694. doi: 10.1128/iai.19.2.686-694.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. J., Hart A., Batt R. M., McDougall C., McLean L. Ultrastructural and biochemical changes in human jejunal mucosa associated with enteropathogenic Escherichia coli (0111) infection. J Pediatr Gastroenterol Nutr. 1986 Jan;5(1):70–73. doi: 10.1097/00005176-198601000-00013. [DOI] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. I. Transfer of resistance factors by conjugation. J Bacteriol. 1961 May;81:669–678. doi: 10.1128/jb.81.5.669-678.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Andrews G. P., Fritz D. L., Sjogren R. W., Jr, Boedeker E. C. Characterization of the plasmid from Escherichia coli RDEC-1 that mediates expression of adhesin AF/R1 and evidence that AF/R1 pili promote but are not essential for enteropathogenic disease. Infect Immun. 1988 Aug;56(8):1846–1857. doi: 10.1128/iai.56.8.1846-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Boedeker E. C. Cloning of the genes for AF/R1 pili from rabbit enteroadherent Escherichia coli RDEC-1 and DNA sequence of the major structural subunit. Infect Immun. 1990 Apr;58(4):1124–1128. doi: 10.1128/iai.58.4.1124-1128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]