Abstract
Topical application of phorbol myristate acetate (PMA) elicits intense local inflammation that facilitates outgrowth of premalignant lesions in skin after carcinogen exposure. The inflammatory response to PMA treatment activates immune stimulatory mechanisms. However, we show here that PMA exposure also induces plasmacytoid dendritic cells (pDCs) in local draining lymph nodes (dLNs) to express indoleamine 2,3 dioxygenase (IDO), which confers T cell suppressor activity on pDCs. The induced IDO-mediated inhibitory activity in this subset of pDCs was potent, dominantly suppressing the T cell stimulatory activity of other DCs that comprise the major fraction of dLN DCs. IDO induction in pDCs depended on inflammatory signaling by means of IFN type I and II receptors, the TLR/IL-1 signaling adaptor MyD88, and on cellular stress responses to amino acid withdrawal by means of the integrated stress response kinase GCN2. Consistent with the hypothesis that T cell suppressive, IDO+ pDCs elicited by PMA exposure create local immune privilege that favors tumor development, IDO-deficient mice exhibited a robust tumor-resistant phenotype in the standard DMBA/PMA 2-stage carcinogenesis model of skin papilloma formation. Thus, IDO is a key immunosuppressive factor that facilitates tumor progression in this setting of chronic inflammation driven by repeated topical PMA exposure.
Keywords: dendritic cells, T cells, TPA, carcinogenesis, tryptophan
The underlying mechanisms that allow cells displaying neo-tumor or foreign pathogen antigens to persist in immunocompetent individuals are poorly understood. Local inflammation associated with chronic infections and tumors typically fails to stimulate T cell immunity and may even contribute to disease progression by actively suppressing adaptive immunity thus allowing potentially immunogenic infected and premalignant cells to persist (1, 2). Local suppression associated with chronic infections and developing tumors may also inhibit vaccine-induced immunity, in effect creating immune privileged sites (3).
In response to infections and other insults that provoke inflammation, cells of the innate immune system activate adaptive immunity by means of multiple immunostimulatory cytokines such as interferons (IFNs) and Toll-like receptor (TLR) ligands. However, in certain settings, IFNs and TLRs can also induce specialized pDCs, obtained from human peripheral blood mononuclear cell (PBMC) precursors (4, 5) and from mouse spleen (6–8) to respond to these inflammatory signals by expressing the tryptophan-catabolizing enzyme indoleamine IDO, which confers potent T cell suppressor activity on pDCs. IDO+ pDCs also promote the differentiation of naïve CD4 T cells into regulatory T cells (Tregs), and stimulate mature Tregs to acquire potent suppressor activity by means of a unique mechanism that depends on PD-1 signaling (9–11). Competence to express inducible IDO is restricted to a rare population of physiologic DCs in mouse spleen that coexpress the B cell marker CD19. Splenic CD19+ pDCs up-regulate IDO by means of a mechanism that depends on cell autonomous IFN type I signaling in response to CD80/86 (B7.1/2) or TLR9 ligation (12–14). CD19+ pDCs have also been found in inflamed dLNs associated with cutaneous growth of B16 melanomas (10). Unlike their counterparts in the spleen, CD19+ pDCs isolated from tumor dLNs constitutively express IDO and require no exogenous stimulation to mediate potent T cell suppression. The signaling mechanisms that stimulate pDCs to express IDO in this physiologic setting of chronic inflammation have yet to be defined.
PMA [12-O-tetradecanoylphorbol-13-acetate (TPA)] is a protein kinase C agonist commonly used as a nonantigen-dependent chemical stimulator of immune cells. Exposing skin to PMA promotes intense local inflammation that activates multiple immunostimulatory pathways. After a mutagenic insult, such as topical administration of the polycyclic hydrocarbon 7,12-dimethylbenz[a]anthracene (DMBA), sustained exposure to PMA drives papilloma development (15). In this report, we demonstrate that PMA induces T cell suppressive, IDO activity in pDCs from inflamed dLNs by activating “proinflammatory” pathways. In the context of PMA-induced inflammation on DMBA-initiated skin, we identified a strong requirement for IDO in supporting papilloma formation, thus establishing the physiological relevance of IDO induction in this setting. Thus, local IDO-mediated immune suppression is a key component of chronic cutaneous inflammation caused by repetitive topical exposure to PMA that supports the survival and outgrowth of mutagenized, premalignant cells.
Results
PMA-Induced Skin Inflammation Stimulates IDO Enzyme Activity in Local dLNs.
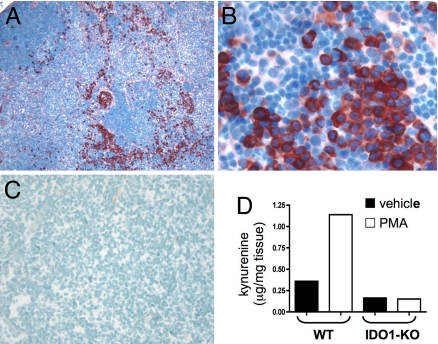
PMA was applied to dorsal skin of C57BL/6 (B6) strain mice to induce chronic inflammation. After 3 treatments over a period of 1 week, exposed skin was indurated, draining (inguinal) LNs were enlarged, and IDO-expressing cells with plasmacytoid morphology were present in dLNs (Fig. 1 A and B). IDO+ cells were dispersed throughout interfollicular (stromal) regions, but were largely absent from lymphoid follicles. As in previous analyses (8), few IDO+ cells were present in LNs from untreated mice (Fig. 1C). Elevated protein expression was accompanied by elevated enzyme activity, as demonstrated by a >3-fold increase in the level of the tryptophan catabolite kynurenine in dLNs from PMA-treated mice (Fig. 1D). IDO1, encoded by the IDO1 (Indo) gene, has a closely related family member encoded by the IDO2 (Indol1) gene (16, 17). No discernable increase in kynurenine was observed in IDO-deficient (IDO1-KO) mice exposed to PMA, indicating that loss of expression of the IDO1 gene product is sufficient to abrogate PMA-driven kynurenine production in dLNs, and that intact IDO2 alleles do not compensate for loss of IDO1.
Fig. 1.
Topical PMA treatment stimulates IDO1 expression in dLNs. (A–C) Inguinal dLN sections from PMA-treated (A and B) and untreated B6 (C) mice were stained with anti-IDO Ab (red chromogen; magnifications, 100x in A and C, 400x in B). (D) Kynurenine present in homogenized dLNs from PMA and acetone (vehicle) treated BALB/c (WT) and IDO1-KO mice was measured by LC/MS/MS as described in Methods.
The pDCs from dLNs of PMA-Treated Mice Possess Potent and Dominant T Cell Suppressor Activity.
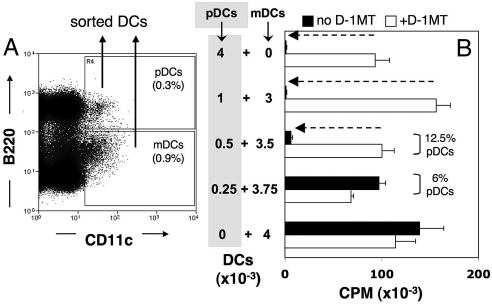
Next, we tested whether dLNs from PMA-treated mice contained pDCs with IDO-dependent suppressor activity. We used a flow cytometer to sort total pDCs (CD11c+B220+) and myeloid DCs (mDCs, CD11c+B220NEG) from inflamed inguinal LNs of PMA-treated mice (Fig. 2A). The functional dominance of IDO+ pDCs in culture (10, 13) obviated the need to purify rare CD19+ pDCs to detect IDO-mediated suppression ex vivo. In dLNs of PMA-treated B6 mice mDCs accounted for most (70–80%) of the DCs present in dLNs of PMA-treated mice (Fig. 2A). To evaluate whether dLN DCs possessed T cell suppressor activity by means of IDO, sorted DCs were cultured with sorted (CD8α+) OVA-specific (OT-1) T cells and OVA peptide in the presence or absence of 1-methyl-[d]-tryptophan (D-1MT), a biologically active inhibitor of IDO activity in DCs (18). Sorted dLN pDCs from PMA-treated mice did not stimulate OT-1 proliferation (Fig. 2B, Top bars); however, the same pDCs stimulated robust OT-1 proliferation in parallel wells containing D-1MT (filled and open bars, respectively). In striking contrast, sorted mDCs from the same dLNs stimulated robust OT-1 proliferation, which was not enhanced in the presence of D-1MT (Fig. 2B, Bottom bars). Also, the suppressor activity of pDCs was potent, completely obscuring the T cell stimulatory properties of mDCs in cultures containing as few as 500 pDCs mixed with 3,500 mDCs (Fig. 2B, Middle bars). As IDO-competent CD19+ pDCs comprise only a minor fraction of total pDCs in lymph nodes (<30%), these outcomes suggested that the suppressor activity of IDO+ pDCs predominated in mixtures containing a large excess of T cell stimulatory mDCs and other pDCs (≈95% of DCs). However, the T cell stimulatory properties of mDCs and other pDCs overcame the suppressor activity of IDO+ pDCs in mixtures containing only 250 pDCs mixed with 3750 mDCs (Fig. 2B). Thus, IDO-mediated T cell suppressor activity was an exclusive property of dLN pDCs from PMA-treated mice, and suppressor activity was potent and predominant over the T cell stimulatory properties of other dLN DCs. As expected, pDCs from inguinal LNs of untreated mice stimulated robust T cell proliferation in the absence of D-1MT (data not shown). These data are consistent with previous studies showing that IDO-competent DCs are a minor subset of pDCs in spleen and tumor dLNs (10, 13, 19), and reveal that potent IDO-mediated suppression is induced as a consequence of chemically induced inflammatory responses in skin.
Fig. 2.
PMA treatment stimulates dLN pDCs to acquire potent T cell suppressor activity by means of IDO. (A) An example of the sorting criteria used to select dLN pDCs (CD11c+B220+) and mDCs (CD11c+B220NEG) from PMA-treated mice. (B) Sorted dLN pDCs or mDCs were added separately (or as pDC:mDC mixtures in the proportions shown) to cultures containing OT-1 responder T cells and cognate OVA peptide with or without D-1MT. Thymidine incorporation was measured after 72 h. Dotted arrows indicate significant suppression (P < 0.05) due to IDO activity. Data shown is from quadruplicate cultures (error bars, SD = 1), and are representative of 5 (pDCs, mDCs) or 2 (pDC:mDC mixtures) separate experiments.
IDO Ablation Prevents pDCs from Acquiring Suppressor Activity After PMA Treatment.
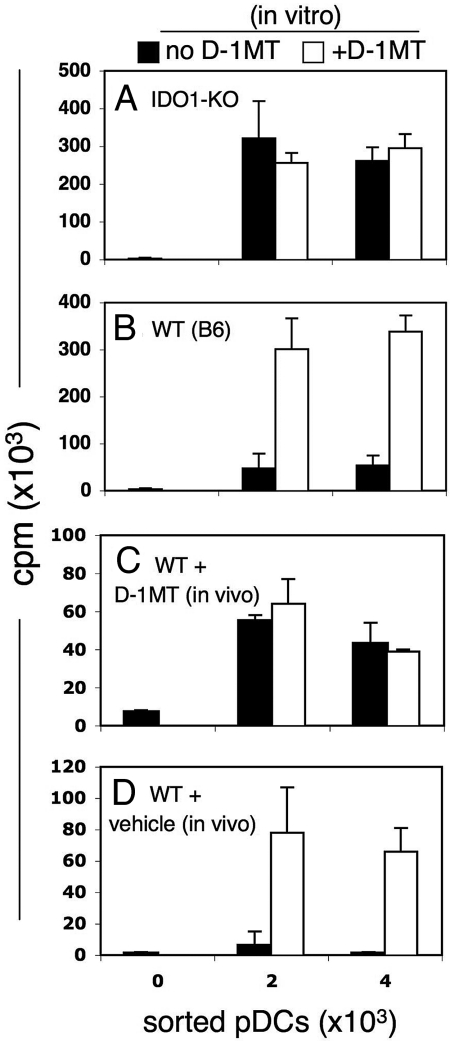
To test whether ablating IDO1, 1 of 2 genes encoding members of the IDO enzyme family (16, 17), affected PMA-induced, T cell suppressor activity of dLN pDCs, IDO1-KO mice were treated with PMA (20). Sorted pDCs from PMA-treated, IDO1-KO mice stimulated robust OT-1 responses that were not enhanced by adding D-1MT (Fig. 3A), whereas PMA-treated B6 (WT) mice treated in parallel possessed potent suppressor activity by means of IDO as before (Fig. 3B). Thus, the IDO1 gene product was necessary for pDCs to acquire suppressor activity after PMA treatment, consistent with its being required for increased kynurenine production in inflamed dLNs from PMA-treated mice (Fig. 1D). Also, intact IDO2 genes in these mice did not compensate for loss of IDO1-mediated suppressor activity in pDCs.
Fig. 3.
Genetic and pharmacologic ablation of IDO blocks induction of pDC suppressor activity. PMA was applied to skin of IDO1-KO (A); B6 (WT) mice (B–D). Some mice were also given (C) IDO inhibitor (D-1MT, 2 mg/mL) and sweetener or (D) sweetener only in drinking water starting 2 days before the first PMA treatment (day 0) until 2 days after the third PMA treatment (day 9). As before, sorted LN pDCs were incubated with OT-1 responders and OVA peptide with or without D-1MT. Data are representative of at least 2 experiments. Data shows significant suppression (B and D, P < 0.05) or no significant suppression (A and C, P > 0.05) and triplicate (or more) cultures were analyzed.
To complement this approach, we tested the hypothesis that IDO activity in dLN pDCs was induced during PMA treatment, and was not induced during culture ex vivo. B6 mice were provided with drinking water containing D-1MT (2 mg/mL, plus sweetener to enhance palatability) starting 3 days before the first PMA application (day 0), and continuing until mice were killed (day 9). Sorted dLN pDCs from mice treated with D-1MT (in vivo) and PMA stimulated robust OT-1 proliferation, which was not enhanced by adding IDO inhibitor ex vivo (Fig. 3C). As expected, PMA-treated mice provided with sweetened drinking water only (vehicle) yielded dLN pDCs with potent suppressor functions by means of IDO (Fig. 3D). These outcomes confirmed that IDO enzyme activity was essential during PMA treatment for pDCs in inflamed dLNs to mediate T cell suppression ex vivo. In summary, genetic and pharmacologic IDO ablation during PMA treatment prevented pDCs from acquiring suppressor activity.
Multiple Inflammatory and Cell Stress Signals Induce dLN pDCs to Express IDO.
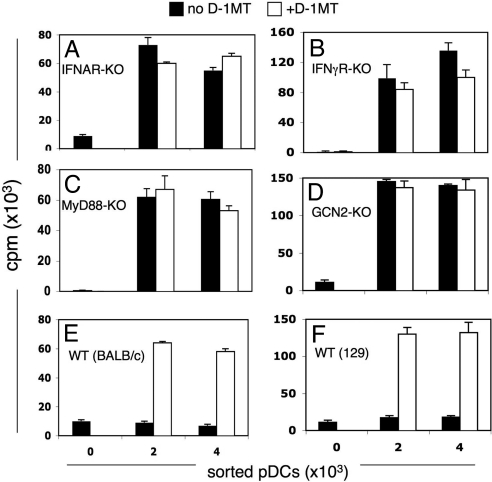
IFN type II (IFNγ) induces murine splenic pDCs to express IDO in a number of settings (7, 21), although IFN type I (IFNα) induces splenic pDCs to express IDO in some settings (19). In the case of IDO-competent splenic CD19+ pDCs, IFNα was the obligate upstream IDO inducer after B7 and TLR9 ligation, because IDO induction depended on signaling through IFN type I (IFNAR), but not IFN type II (IFNγR) receptors (12, 13). To evaluate whether IFN signals were required to induce pDCs to express IDO in dLNs of PMA-treated mice we treated mice with defective IFN type I (IFNAR-KO), type II (IFNγRα-KO) receptors with PMA and assessed the T cell stimulatory properties of dLN pDCs as before. Sorted dLN pDCs from PMA-treated IFNAR-KO mice (BALB/c strain background) stimulated robust DO11.10 T cell proliferation that was not enhanced by the presence of D-1MT in cultures (Fig. 4A). As with B6 (WT) mice, BALB/c mice treated with PMA yielded dLN pDCs with potent suppressor activity by means of IDO (Fig. 4E). Likewise dLN pDCs from PMA-treated IFNγRα-KO mice (129/Sv strain background) stimulated robust OT-1 proliferation with no evidence of IDO-mediated suppression (Fig. 4B), whereas pDCs from PMA-treated 129/Sv mice mediated potent suppression (Fig. 4F). These data revealed that inflammatory signals by means of IFN type I and II were required to induce pDCs to express functional IDO in inflamed dLNs of PMA-treated mice.
Fig. 4.
Inflammatory and cell stress signals induce pDCs to acquire suppressor activity by means of IDO. PMA was applied to the skin of (A) IFNAR-KO, (B) IFNRα-KO, (C) MyD88-KO (D), GCN2-KO mice, or WT (BALB/c, 129SvJ) mice. Sorted dLN pDCs from PMA-treated mice were cultured with responder DO11.10 (A and E) or OT-1 (B–D and F) T cells and cognate (OVA) peptide, and proliferation was assessed with or without D-1MT. No significant suppression by means of IDO was detected in KO mice (A–D, P > 0.05), whereas significant suppression was detected in WT mice (E and F, P < 0.05). Triplicate (or more) cultures were analyzed and experiments were repeated 2 or more times.
To further elucidate upstream inflammatory signaling requirements to induce pDCs to express IDO, MyD88-knockout (MyD88-KO) mice were treated with PMA. MyD88-KO mice are unable to mediate inflammatory signals from many TLR ligands or members of the IL1 cytokine family (22). Sorted dLN pDCs from PMA-treated MyD88-KO mice did not possess suppressor activity (Fig. 4C), showing that inflammatory signals from TLRs or IL1 were necessary to stimulate pDCs to express functional IDO in the setting of PMA-induced inflammation. Cell autonomous IFNα production by splenic pDCs after B7 ligation depended on cellular stress responses by means of the eIF2α kinase GCN2 (14), which is triggered when access to amino acids is limited (23). To test whether an intact GCN2 signaling pathway was essential for IDO induction in pDCs from dLNs, we exposed GCN2-KO mice to PMA. Sorted dLN pDCs from GCN2-KO mice (129/SvJ strain background) exhibited potent T cell stimulatory properties with no evidence of IDO-mediated suppression (Fig. 4D), indicating that GCN2 stress responses were essential to trigger IDO induction in pDCs. Collectively, data reported in Fig. 4 reveal that topical PMA treatment did not directly induce IDO in pDCs (i.e., by stimulating protein kinase C). Rather, PMA exposure activated inflammatory processes needed upstream of IDO, either cell autonomous signaling in IDO-competent pDCs themselves, or in other cells present in inflamed dLNs that provided critical signals to stimulate IDO-competent pDCs to express IDO.
IDO1-Deficient Mice Are Resistant to Skin Tumor Formation After DMBA/PMA Treatment.
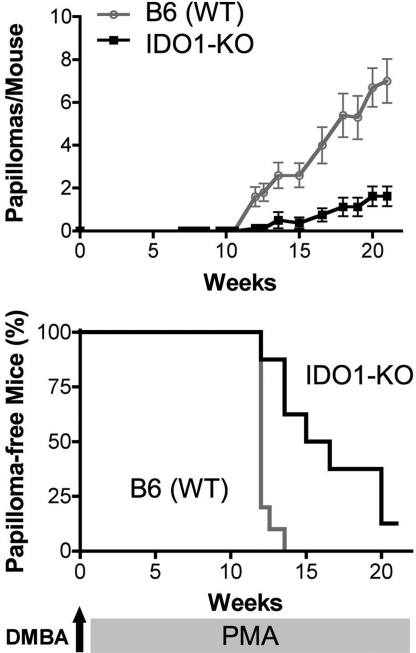
Based on our finding, in PMA-exposed skin that chronic inflammation elicits a potently T cell-suppressive class of pDCs through induction of IDO, we hypothesized that one pathophysiological consequence of IDO activity in this context might be to link chronic inflammation to tumor promotion, one pivotal aspect of which is to escape T cell-mediated antitumor immunity (24, 25). To investigate this issue, we evaluated the cancer susceptibility of IDO1-KO mice on a classical, DMBA/PMA (TPA) multistage skin carcinogenesis protocol. Papilloma incidence was dramatically reduced in B6-strain, IDO1-KO mice, with significantly fewer lesions arising by 10–20 weeks of PMA treatment when assessed either as the number of papillomas per mouse or the number of mice with papillomas (Fig. 5). A similar result was observed in a second strain background [BALB/c; supporting information (SI) Fig. S1], confirming the effect and arguing against a major role for strain-specific modifiers in determining the outcome. When applied as single agents, neither DMBA nor PMA, as administered under this protocol, produced any detectable papillomas, confirming the multistage nature of this model (data not shown).
Fig. 5.
IDO is crucial for PMA-driven skin carcinogenesis. B6 strain WT (n = 10) and IDO1-KO (n = 8) mice received a single topical application of 400 nM DMBA at week 0, followed by twice weekly applications of 10 μg of PMA from week 1 until week 20. Mice were evaluated for papillomas twice a week. (Upper) The numbers of papillomas per mouse over time plotted as mean values for each group ± SE. Significance (P < 0.0004) was determined a nonparametric 2-tailed Mann–Whitney test. (Lower) Number of mice remaining papilloma-free over time plotted as a survival curve. Significance (P < 0.0005) was determined by using a 2-group log-rank test (equivalent to the Mantel–Haenszel test).
After cessation of PMA promotion at 20 weeks, conversion of a subset of papillomas to squamous cell carcinomas was observed over an additional 10 week period in susceptible BALB/c but not in resistant B6 strain mice. Very few lesions developed in the BALB/c-strain, IDO1-KO mice relative to their wild type counterparts and the lesions that did develop were considerably smaller (Fig. S1). Pathologically, the lesions that developed in the IDO1-KO mice were identified as either early squamous cell carcinomas or squamous acanthomas: common reactive processes that arise in response to various insults that produce chronic irritation or inflammatory reactions, but which have the potential to develop into carcinomas with more aggressive growth potential. The lesions that formed in the wild-type mice were clearly more advanced and obviously transformed tumors than those in the IDO1-KO mice (data not shown). Most of the wild-type lesions were characterized as well differentiated carcinomas although one appeared to be a more malignant, poorly differentiated infiltrating squamous cell carcinoma. Thus, mice lacking IDO1 developed significantly fewer tumors and the few tumors that formed were at an earlier premalignant stage of development.
Discussion
IDO-mediated T cell suppression is essential for allogeneic pregnancy success in mice, and has been described in many clinically relevant settings including cancer, infectious, autoimmune, and allergic syndromes (26, 27). In this report, we demonstrate that intense local inflammation provoked by topical exposure to PMA stimulated a small subpopulation of pDCs to express IDO and elicit potent T cell suppression in skin dLNs. An intact IDO1 gene was essential for this response as was upstream signaling through IFN receptors, the TLR/IL-1 signaling adaptor MyD88, and signaling through the integrated stress response kinase GCN2. Physiologically, ablation of the IDO1 gene was associated with a significant reduction in chemical carcinogen-induced skin papilloma formation that develops in the context of chronic PMA-induced inflammation.
Signaling mechanisms that induce IDO expression in CD19+ pDCs and engage their T cell suppressive capability have been elucidated by treating mice (or isolated splenic DCs) with reagents that induce IDO, and monitoring subsequent effects on T cell responses elicited ex vivo or in secondary recipients after DC adoptive transfer. However, the significance of these mechanisms with regard to the physiologic processes that induce IDO-competent pDCs to mediate suppression in tissues has not been investigated. In physiologic settings where IDO+ pDCs have been described, the mechanisms that create these nonhomeostatic situations have not been identified. Thus, IDO+ pDCs with constitutive suppressor activity were found in tumor-draining LNs of mice bearing s.c. melanomas, and pDC-associated IDO activity restrained type I diabetes progression in NOD female mice (28, 29). However, the mechanisms that stimulated IDO-competent pDCs to express IDO were not defined in either setting. Because IDO-competent pDCs can create local suppression only when induced to express IDO, defining physiologic conditions that stimulate this response is a key issue that must be addressed to understand how T cell responses are modulated by IDO. Identification of the well documented, proinflammatory agent PMA as a potent but indirect inducer of IDO in CD19+ pDCs, enabled us to investigate how IDO activity is integrated into the signaling milieu of chronic inflammation in conjunction with demonstrating biological relevance in this environment.
The finding that IFNAR and IFNγR signals were both necessary to up-regulate IDO in dLN pDCs after PMA treatment suggests that pDCs in this context may have more stringent requirements for upstream signaling than isolated splenic CD19+ pDCs, for which only IFNAR signaling was needed to induce IDO after B7 or TLR9 ligation (30). IFNγ signaling may be essential to induce upstream ligands expressed by other dLN cells that interact with CD19+ pDCs to induce IDO by means of cell autonomous IFNAR signaling. The requirement for intact MyD88 signaling suggests that TLR ligands (presumably endogenous) and/or IL1 cytokine family members are essential to induce pDCs to express IDO (22), although MyD88 may be required in other dLN cells to induce other upstream signals needed to stimulate pDCs. TLR ligands such as CpGs can induce splenic IDO-competent pDCs to express IDO (12, 31), although much lower CpG doses induce DC maturation associated with immunostimulatory effects (30). GCN2 kinase activity in effector and Tregs is a critical effector for IDO-mediated suppression (11, 32), but was also required for CD19+ pDCs to express IFNα and functional IDO after B7 ligation (14). Hence, GCN2 signaling to induce pDCs from PMA-treated mice to express IDO may be required in pDCs themselves, or in T cells acting as accessory cells. Thus, IDO may amplify its own expression by means of feed-forward signaling between GCN2 and IFNα signaling. Also, our finding that dLN pDCs from mice treated with D-1MT and PMA did not exhibit T cell suppressor activity when D-1MT was absent from ex vivo cultures suggests that the pDCs may have undergone irreversible differentiation to acquire T cell stimulatory functions in the absence of IDO activity in vivo, so that pDCs were unable to recover suppressor activity ex vivo even when IDO inhibitor was no longer present. Indeed, a role for IDO in regulating maturation of human DCs has recently been reported (33).
Topical PMA exposure activates a cascade of signaling pathways associated with immunostimulatory outcomes such as release of proinflammatory cytokines and growth factors by stromal and innate immune cells in inflamed skin lesions. DCs respond to these inflammatory cues by undergoing maturation and migrating to local dLNs where they present antigens acquired in inflammatory lesions in an immunostimulatory fashion. Hence, a common assumption is that intense inflammation stimulated by proinflammatory chemicals such as PMA has immune adjuvant effects. Indeed, proinflammatory immune cell signaling pathways involving MyD88 and the receptor for advanced glycation end-products (RAGE) are necessary for efficient tumor promotion by PMA (34, 35). The potent suppression created in inflamed dLNs by IDO+ pDCs runs counter to this perception. We hypothesize that chronic inflammation provoked by other insults will also activate local IDO-mediated suppression that attenuates effective T cell responses to antigens encountered in such settings. This hypothesis is consistent with the finding that IDO1 ablation significantly reduced the formation of cutaneous papillomas in DMBA/PMA treated mice. Although this experiment does not rule out the possibility of a cell autonomous role for IDO in neoplastic cells themselves, it is consistent with the finding that PMA drives expression of IDO in dLN pDCs that consequently acquire potent T cell suppressive activity. Therefore, this study provides a uniquely compelling demonstration of an immunosuppressive mechanism as an integral component of chronic inflammation that is directly linked to increased cancer susceptibility.
There is growing recognition that inflammatory processes nurture developing malignancies in tissues before overt tumors are established (36). Local inflammation triggers the release of factors that can support the outgrowth of premalignant cells (37), whereas inflammation-associated immune activation may eliminate tumors or promote malignant progression by positively selecting for immune escape variants, a process collectively referred to as “immunoediting” (38). However, the assumption that inflammation exclusively drives anti-tumor immunity is clearly an oversimplification, because the immune environment associated with chronic inflammation likely contains multiple counterbalancing signals including some that are inherently immunosuppressive. Therefore, inflammation may also facilitate tumor progression by creating local immune tolerance (24, 39), which may also impede effective immunotherapy. In an inflammatory environment, both tumor-derived factors as well as stromal cell components may contribute to the establishment of tumoral immune tolerance, providing several potential targets for therapeutic intervention (40). Indeed, IDO has previously been identified as a tractable target for the development of effective preclinical cancer treatment strategies (41). Its identification here as an integral component of chronic inflammation elicited by PMA treatment further enhances its attractiveness as a drug target by suggesting that IDO has a critical role in fostering the outgrowth of cancers from the earliest neoplastic lesions.
Methods
Mice.
Mice were bred at the Medical College of Georgia or the Lankenau Institute for Medical Research in barrier facilities, and were free of common mouse pathogen infections. All experimental procedures performed on mice were approved by the local Institutional Animal Care and Use Committee.
Antibodies.
Polyclonal rabbit anti-mouse IDO Ab was generated by using a synthetic peptide (CLRSVKDTTEKALLSWP). Other antibodies were obtained from Becton Dickinson: PerCp-CD4 (no. 553052), PE-CD4 (no. 553653), PerCp-CD8α (no. 553036), APC-CD11c (no. 550261), FITC-CD45R/B220 (no. 553088), and PE-CD25 (no. 553866).
PMA Treatment.
Shaved mice (3 per group) were treated 3 times with PMA (10 μg; days 0, 3, and 7) and killed on day 9 to harvest inguinal LNs (6 per group). Some mice were provided with sweetened drinking water containing IDO inhibitor (2 mg/mL) during PMA exposure.
Immunohistochemistry (IDO).
Formalin-fixed paraffin tissue sections (4 μm) were treated with citrate buffer to retrieve antigen, preblocked with goat serum and incubated with anti-IDO Ab (1:4,000). Biotinylated goat anti-rabbit, IgG streptavidin-HRP (Biogenex), and AEC chromagen (Dakocytomation) were used to detect primary antibody. Hematoxylin was used as a counterstain.
Kynurenine Assay.
Lymph nodes from PMA-treated animals were harvested 6 h after the last of 3 topical PMA treatments (10 μg; days 0, 4, and 7). Pooled inguinal dLNs from 3 mice were homogenized in water (1:4 wt/vol) and subjected to 3 rounds of freeze thaw lysis. Deproteinated lysates were analyzed by HPLC coupled to electrospray ionization tandem mass spectroscopy (LC/MS/MS) analysis as described (42). Quantitation of kynurenine was based on analysis of 2 daughter ions.
FACS.
LN cells were stained with mAbs and sorted by using a Mo-Flo (Cytomation) flow cytometer to isolate pDCs (CD11c+B220+), mDCs (CD11c+B220NEG) as described (8, 10, 11, 13). Responder CD8α+ T cells were sorted from spleen of OT-1 (or CD4+ T cells from DO11.10) TCR transgenic mice. Cell purities after sorting were typically 95–99%. Sorted DCs were collected in complete medium on ice, and added promptly to cultures to preserve cell viability and suppressor activity.
DC Suppression Assays.
Sorted DCs from pooled LNs were cultured (4,000, 2,000 per well) with sorted OT-1 T cells (105 per well) and OVA peptide (SIINFEKL, 100 nM) as described (11). Parallel cultures contained D-1MT (200 μM). Proliferation was assessed by thymidine incorporation (72 h) in cultures replicated 3–6 times contingent on pDC yields. Data shown are means of replicate cultures (1 sd), and all experiments were performed on 2 or more occasions. To assess suppressor activity of pDCs from IFNAR-KO mice (BALB/c backgrounds), we modified this procedure to use OVA-specific DO11.10 T cells.
DMBA/PMA Carcinogenesis Model.
Mice were shaved 2 days before applying 400 nmol of DMBA (in 200 μL of acetone) to shaved dorsal skin under yellow lighting. Mice were kept in the dark for 24 h and transferred to clean cages. One week after DMBA application, the same dorsal area was painted with 10 μg of PMA in 200 μL of acetone twice weekly for 20 weeks. Mice were evaluated for papillomas twice a week.
Statistical Analysis.
Data were subjected to 2-tailed paired t tests (T cell response assays) or as described in Fig. 5.
Supplementary Material
Acknowledgments.
We thank Doris McCool (MCG) for providing mice used (at Medical College of Georgia) in this study and Anita Wylds MCG and Erika Sutanto-Ward LIMR for excellent technical support. This work was supported by National Institutes of Health (NIH) Grants AI63402 (to A.L.M.) and CA112431 and CA096651 (to D.H.M.) and by a Concern Foundation Grant (A.J.M.). A.J.M. is also supported by grants from the DoD Breast Cancer Research Program (BC044350), and the Lance Armstrong Foundation. G.C.P. is supported by NIH Grants CA109542, CA82222, and CA100123, with additional support provided by the Lankenau Hospital Foundation.
Footnotes
Conflict of interest statement: A.J.M., D.H.M., G.C.P., and A.L.M. are members of the Scientific Advisory Board for NewLink Genetics Inc. and receive consulting income from this source.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806173105/DCSupplemental.
References
- 1.Peters N, Sacks D. Immune privilege in sites of chronic infection: Leishmania and regulatory T cells. Immunological Reviews. 2006;213:159–179. doi: 10.1111/j.1600-065X.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 2.Gajewski TF, et al. Immune resistance orchestrated by the tumor microenvironment. Immunological Reviews. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 3.Mellor AL, Munn DH. Creating immune privilege: Active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 4.Munn DH, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 5.Munn DH, Sharma MD, Mellor AL. Ligation of B7–1/B7–2 by Human CD4(+) T Cells Triggers Indoleamine 2,3-Dioxygenase Activity in Dendritic Cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 6.Grohmann U, et al. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J Immunol. 2001;166:277–283. doi: 10.4049/jimmunol.166.1.277. [DOI] [PubMed] [Google Scholar]
- 7.Grohmann U, et al. IL-6 inhibits the tolerogenic function of CD8alpha(+) dendritic cells expressing indoleamine 2,3-dioxygenase. J Immunol. 2001;167:708–714. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 8.Mellor AL, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 9.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 10.Munn DH, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma MD, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:1–13. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellor AL, et al. Cutting Edge: CpG Oligonucleotides Induce Splenic CD19+ Dendritic Cells to Acquire Potent Indoleamine 2,3-Dioxygenase-Dependent T Cell Regulatory Functions via IFN Type 1 Signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 13.Baban B, et al. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 14.Manlapat AM, Kahler DJ, Chandler PR, Munn DH, Mellor AL. Cell autonomous control of interferon type I expression by indoleamine 2,3-dioxygenase in regulatory CD19+ dendritic cells. Eur J Immunol. 2007;37:1064–1071. doi: 10.1002/eji.200636690. [DOI] [PubMed] [Google Scholar]
- 15.DiGiovanni J, et al. Further genetic analyses of skin tumor promoter susceptibility using inbred and recombinant inbred mice. Carcinogenesis. 1992;13:525–531. doi: 10.1093/carcin/13.4.525. [DOI] [PubMed] [Google Scholar]
- 16.Ball HJ, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Metz R, et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 18.Hou D, et al. Stereoisomers of 1-methyl-tryptophan as pharmacologic inhibitors of indoleamine 2,3-dioxygenase. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 19.Fallarino F, et al. Ligand and cytokine dependence of the immunosuppressive pathway of tryptophan catabolism in plasmacytoid dendritic cells. Int Immunol. 2005;17:1429–1438. doi: 10.1093/intimm/dxh321. [DOI] [PubMed] [Google Scholar]
- 20.Baban B, et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Grohmann, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi O, Akira S. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Top Microbiol Immunol. 2002;270:155–167. doi: 10.1007/978-3-642-59430-4_10. [DOI] [PubMed] [Google Scholar]
- 23.Harding HP, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 24.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:803–804. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 25.Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 26.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: Much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 27.Mellor AL, Munn DH. IDO expression in dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 28.Munn DH. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18:221–225. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 30.Asselin-Paturel C, et al. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wingender G, et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur J Immunol. 2006;36:12–20. doi: 10.1002/eji.200535602. [DOI] [PubMed] [Google Scholar]
- 32.Munn DH, et al. GCN2 Kinase in T Cells Mediates Proliferative Arrest and Anergy Induction in Response to Indoleamine 2,3-Dioxygenase. Immunity. 2005;22:1–10. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Hill M, et al. IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells. Eur J Immunol. 2007;37:3054–3062. doi: 10.1002/eji.200636704. [DOI] [PubMed] [Google Scholar]
- 34.Gebhardt C, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swann JB, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peek RM, Jr, Mohla S, DuBois RN. Inflammation in the genesis and perpetuation of cancer: Summary and recommendations from a national cancer institute-sponsored meeting. Cancer Res. 2005;65:8583–8586. doi: 10.1158/0008-5472.CAN-05-1777. [DOI] [PubMed] [Google Scholar]
- 37.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Munn DH, Mellor AL. Tumor Draining Lymph Nodes as a Site of Tolerance Induction. Immunological Reviews. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 40.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer. 2006;6:613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 41.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 42.Amirkhani A, Heldin E, Markides KE, Bergquist J. Quantitation of tryptophan, kynurenine and kynurenic acid in human plasma by capillary liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780:381–387. doi: 10.1016/s1570-0232(02)00572-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.