Abstract
Lysophosphatidic acid (LPA) is a bioactive phospholipid and binds to its receptors, a family of G protein-coupled receptors (GPCR), which initiates multiple signaling cascades and leads to activation of several transcription factors, including NF-κB. Although LPA-induced signaling pathways have been intensively investigated, the molecular mechanism by which LPA activates NF-κB is not fully defined. In this work, we found that β-arrestin 2, but not β-arrestin 1, is required for LPA-induced NF-κB activation and interlukin-6 expression. Mechanistically, we found that β-arrestin 2 associated with CARMA3, a scaffold protein that plays an essential role in GPCR-induced NF-κB activation, suggesting that β-arrestin 2 may recruit CARMA3 to LPA receptors. Although β-arrestin 2 deficiency did not affect LPA-induced IKKα/β phosphorylation, it impaired LPA-induced IKK kinase activity, which is consistent with our previous findings that CARMA3 is required for IKKα/β activation but not for the inducible phosphorylation of IKKα/β. Together, our results provide the genetic evidence that β-arrestin 2 serves as a positive regulator in NF-κB signaling pathway by connecting CARMA3 to GPCRs.
Keywords: CARMA3, G protein-coupled receptor, IKK
Lysophosphatidic acid (LPA) is a major active constituent of serum and exerts hormone- and growth factor-like activities for proliferation, differentiation, survival, and chemotaxis on various types of cells (1). LPA induces its biological function by binding to its receptors on the cell surface. LPA receptors belong to the family of G protein-coupled receptors (GPCRs). GPCR is the largest family of cell surface receptors, and its members are expressed throughout the body and activated by a diverse array of ligands (2). Upon stimulation, GPCRs associate with the trimeric G protein complex, which consists of α, β, and γ subunits. The complexity of the signaling pathways initiated by various GPCR ligands results from the presence of numerous G proteins, including 18 α subunits that can be classified into four groups (Gs, Gi, Gq, G12/13), 12 β subunits, and 5 γ subunits (3). These G proteins independently or cooperatively activate their downstream signaling cascades. Stimulation of cells with LPA initiates a series of signaling cascades through these trimeric G proteins and leads to activation of several transcription factors, including nuclear factor κB (NF-κB) (2).
NF-κB is a family of transcription factors that exist as various homo- and heterodimers. Activation of NF-κB is through posttranslational mechanisms (4). In unstimulated cells, NF-κB is associated with a family of inhibitor protein, IκB, which masks the nuclear localization signal of NF-κB, thereby sequestering NF-κB in the cytoplasm. NF-κB can be activated by various receptors through the classical or the nonclassical pathway. Various stimulation signals activate the IKK complex, which phosphorylates IκBα and rapidly triggers a ubiquitination-mediated degradation of IκBα. The degradation of IκBα releases NF-κB, which translocates into the nucleus and binds to the cognate sequences in the promoter of its target genes (4), leading to the modulation of various biological responses, including cell survival and proliferation, immunity, and inflammation (5).
It has been shown that stimulation of cells with several GPCR ligands, such as LPA, can effectively induce the activation of NF-κB (2). However, the molecular mechanism of GPCR-induced NF-κB activation is not fully defined. Recently, we and others reported that GPCR-induced NF-κB is mediated through a CARMA3 [caspase recruit domain (CARD) and membrane-associated guanylate kinase homologs (MAGUK) domain-containing protein 3]-dependent signaling pathway (6), in which CARMA3 associates with its downstream components, Bcl10 and MALT1 (7–9), leading to activation of IKK. However, the signaling components/pathways, which link CARMA3 to GPCRs, remain to be identified. It has been shown that, in addition to the association with the trimeric G protein complex, GPCRs also associate with β-arrestins upon stimulation by their ligands such as LPA. β-Arrestins belong to a family of adaptor proteins that were initially considered as components to desensitize GPCR activation. However, more recent studies indicate that β-arrestins are also involved in mediating GPCR-induced signal transduction (10–17).
β-Arrestins are the members of the arrestin proteins. There are four isoforms of arrestin. Arrestin 1 and arrestin 4 regulate opsin, whereas arrestin 2 and 3, also named β-arrestin 1 and β-arrestin 2, respectively, regulate the function of most GPCRs and are expressed ubiquitously. Although β-arrestin 1 and 2 share ≈70% similar sequence and may be functionally redundant, it has been shown that these two isoforms may regulate different biological functions. It has been reported that β-arrestins are involved in various signaling pathways, leading to activation of ERK and JNK (17–19). Interestingly, several recent studies suggest that β-arrestin may also function as an inhibitory molecule to suppress NF-κB activation (20–23). However, the role of β-arrestin in GPCR-induced NF-κB has not been determined.
In this work, we have investigated the role of β-arrestins in NF-κB activation induced by a GPCR ligand, LPA. Surprisingly, we found that β-arrestin 2, but not β-arrestin 1, functions as a positive regulator for LPA-induced NF-κB activation, which is different from its negative role in NF-κB activation induced by other signaling pathways (20–23). Our results suggest that β-arrestin 2 mediates NF-κB activation through recruiting CARMA3 to LPA receptor. Together, our studies reveal a function of β-arrestin 2 in GPCR-induced signal transduction.
Results
Specific Requirement of β-Arrestin 2 for LPA-Induced NF-κB Activation.
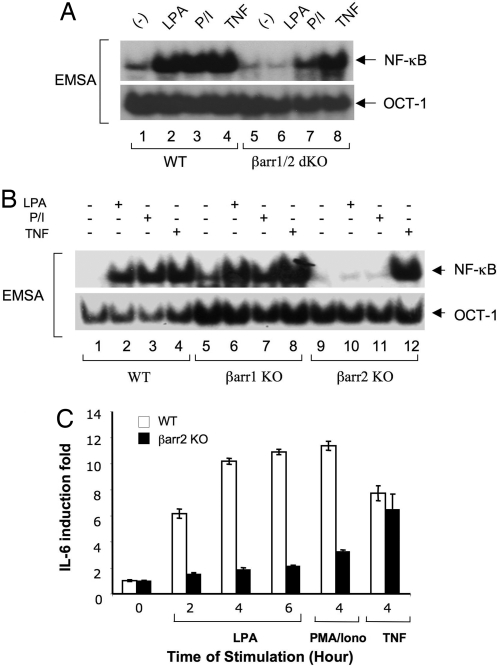
Earlier studies indicate that β-arrestins associate with GPCRs, which mediates receptor endocytosis, and also function as adaptors linking GPCRs to intracellular signaling pathways (10–16). More recently, it has been shown that β-arrestins can bind to IκBα, leading to suppressed NF-κB activation induced by TNFα, IL-1β, and UV light (20–23). Although various GPCR ligands such as LPA, angiotensin II (AngII), and endothelin-1, can recruit β-arrestins to their receptors and can also induce NF-κB activation, the role of β-arrestins in the NF-κB activation induced by these GPCRs has not been investigated. To address this question, we stimulated the mouse embryonic fibroblast (MEF) cells from β-arrestin 1/β-arrestin 2-double knockout (βarr1/2 dKO) mice with LPA and then examined NF-κB activation. Interestingly, we found that LPA stimulation failed to induce NF-κB activation in βarr1/2 dKO MEF cells (Fig. 1A), which contradicts the role in which β-arrestins functioned as a negative regulator of NF-κB.
Fig. 1.
β-Arrestin 2 is required in LPA- and PMA/Iono-induced NF-κB activation and in cytokine production. (A) Wild-type and βarr1/2 dKO MEF cells were stimulated with or without LPA (10 μM) or PMA (40 ng/ml) plus Ionomycin (100 ng/ml) for 60 min or TNFα (10 ng/ml) for 30 min. Nuclear extracts were prepared and subjected to EMSA by using 32P-labeled NF-κB or OCT-1 probes. (B) Wild-type, βarr1 KO, or βarr2 KO MEF cells were stimulated as in A. Nuclear extracts were prepared and subjected to EMSA by using 32P-labeled NF-κB or OCT-1 probes. (C) Wild-type or βarr2 KO MEF cells were serum-starved for 4 h and stimulated with or without LPA (10 μM), PMA (40 ng/ml) plus Iono (100 ng/ml) (P/I), or TNFα (10 ng/ml) for the indicated time points. Supernatants were collected, and IL-6 concentrations in the supernatants were measured by ELISA. All data were normalized to the value of unstimulated control in respective cells as the fold induction. Error bars indicated ±SD between triplicate experiments.
Because earlier studies indicated that PKC is involved in the NF-κB activation induced by LPA and other GPCR ligands (6), we also used the pharmacological PKC agonists, phorbol 12-myristate 13-acetate (PMA) plus Ionomycin (Iono), to stimulate these cells. Consistent with the role of β-arrestins in LPA-induced NF-κB activation, PMA/Iono-induced NF-κB activation was also significantly impaired in βarr1/2 dKO MEF cells (Fig. 1A). In contrast, TNFα effectively induced NF-κB activation in βarr1/2 dKO MEF cells (Fig. 1A), indicating that β-arrestins are selectively involved in GPCR-, but not TNFα-induced signal transduction.
To determine further which β-arrestin isoform is involved in LPA-induced NF-κB activation, we examined NF-κB activation in β-arrestin 1-knockout (βarr1 KO) or β-arrestin 2-knockout (βarr2 KO) MEF cells. Interestingly, we found that LPA-induced NF-κB activation was selectively impaired in βarr2 KO, but not in βarr1 KO cells (Fig. 1B). Similarly, NF-κB activation induced by PMA/Iono was also defective in βarr2 KO but not in βarr1 KO cells (Fig. 1B), whereas TNFα-induced NF-κB activation in both βarr1 KO and βarr2 KO cells was comparable with that in wild-type MEF cells (Fig. 1B). Together, these data provide the genetic evidence that β-arrestin 2 is specifically involved in GPCR-induced NF-κB activation.
β-Arrestin 2 Is Required for LPA-Induced Cytokine Production.
It has been shown that LPA-induced cytokine expression depends on NF-κB activation (7, 8). With our findings that LPA-induced NF-κB activation depends on β-arrestin 2, we next examined whether β-arrestin 2-dependent NF-κB activation is required for LPA-induced cytokine expression. Because IL-6 is one of the major cytokines that are induced after LPA stimulation, we examined IL-6 production in wild-type and βarr2 KO MEF cells after the stimulation of LPA, PMA/Iono, and TNFα at different time points. Our results showed that IL-6 expression induced by LPA or PMA/Iono was significantly reduced in βarr2 KO cells (Fig. 1C), indicating that the β-arrestin 2-mediated NF-κB activation plays an essential role for LPA-induced cytokine production.
β-Arrestin 2 Functions Upstream of IKK.
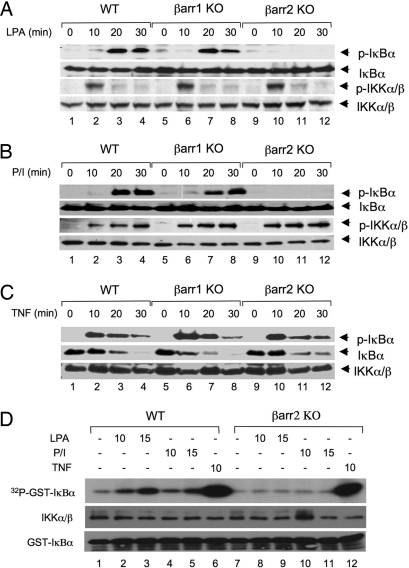
To determine whether β-arrestin 2 functions upstream of IKK, we examined LPA-induced IκBα phosphorylation. Consistent with the essential role of β-arrestin 2 in LPA-induced NF-κB activation, LPA-induced IκBα phosphorylation was defective in βarr2 KO, but not in βarr1 KO cells (Fig. 2A Top). Similarly, PMA/Iono-induced IκBα phosphorylation was defective in βarr2 KO, but not in βarr1 KO cells (Fig. 2B Top). In contrast to the role of β-arrestin 2 in the LPA-induced signaling pathway, TNFα-induced IκBα phosphorylation and degradation in βarr2 KO cells were comparable with those in wild-type and βarr1 KO cells (Fig. 2C Top). Interestingly, although LPA- and PMA/Iono-induced IκBα phosphorylation was defective in βarr2 KO cells, the signal-induced IKK phosphorylation was intact, indicating that the signal-induced IKK phosphorylation in βarr2 KO cells was not sufficient to activate IKK kinase activity (Fig. 2 A and B).
Fig. 2.
LPA- and PMA/Iono-induced IκBα phosphorylation and IKK kinase activity are dependent on β-arrestin 2. (A–C) Wild-type, βarr1 KO, and βarr2 KO MEF cells were stimulated with LPA (10 μM) (A), PMA (40 ng/ml) plus Ionomycin (100 ng/ml) (B), or TNFα (10 ng/ml) (C) for indicated time points. Phosphorylation of IκBα and/or IKK was examined by Western blotting with the indicated antibodies. (D) Wild-type or βarr2 KO MEF cells were stimulated with or without LPA (10 μM), PMA (40 ng/ml) plus Ionomycin (100 ng/ml) (P/I), or TNFα (10 ng/ml) for the indicated time points. The IKK complex was immunoprecipitated by using a mixture of IKKα and IKKγ antibodies and protein A–agarose. The immunoprecipitated complex was subjected to an in vitro kinase assay with GST-IκBα (1–62) as substrates. Parts of the lysates of the immunoprecipitated IKK complex and GST-IκBα (1–62) substrates were subjected to Western blotting as loading controls.
To confirm further the requirement of β-arrestin 2 for IKK activity, we examined the kinase activity of the IKK complex in wild-type or βarr2 KO cells after the stimulation with LPA, PMA/Iono, or TNFα. Although LPA or PMA/Iono could induce IKK activity in wild-type cells, they failed to activate IKK in βarr2 KO cells (Fig. 2D). In contrast, TNFα could effectively activate IKK activity in both wild-type and βarr2 KO cells (Fig. 2D). In contrast to IKK activation, we found that ERK and JNK MAP kinases were effectively induced with a similar kinetics after the treatment of LPA [supporting information (SI) Fig. S1A] or PMA/Iono (Fig. S1B) in all of these cells. Together, these results indicate that β-arrestin 2 is specifically involved in regulation of IKK but not MAP kinase activation in LPA-induced signaling pathway.
β-Arrestin 2, but Not β-Arrestin 1, Can Rescue the Defect of LPA-Induced NF-κB Activation in Cells Deficient in Both β-Arrestin 1 and 2.
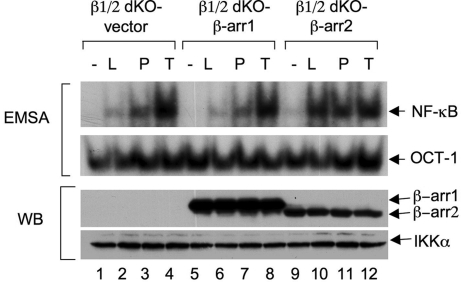
The specific defect of β-arrestin 2, but not β-arrestin 1, in LPA-induced NF-κB activation surprised us because β-arrestin 1 and β-arrestin 2 are structurally very similar. Therefore, we decided to verify further the functional requirement of β-arrestin 2 for LPA-induced NF-κB activation. βarr1/2 dKO cells were reconstituted with either β-arrestin 1 (Fig. 3, lanes 5–8) or β-arrestin 2 (Fig. 3, lanes 9–12). Consistent with above results, only β-arrestin 2, but not β-arrestin 1, could rescue the defect for LPA- and PMA/Iono-induced NF-κB activation (Fig. 3, lanes 10 and 11), whereas this reconstitution did not affect TNFα-induced NF-κB activation (Fig. 3, lanes 4, 8, and 12). Together, these results further confirm that β-arrestin 2 is selectively involved in LPA-induced NF-κB activation.
Fig. 3.
β-Arrestin 2, but not β-arrestin 1, can rescue the defect of LPA-induced NF-κB activation in βarr1/2 dKO cells. βArr1/2 dKO MEF cells were reconstituted with a retroviral vector encoding FLAG-tagged β-arrestin 1, β-arrestin 2, or empty vector, respectively. The resulting cells were stimulated with or without LPA, PMA/Iono, or TNFα. The nuclear extracts from these cells were subjected to EMSA with 32P-labeled NF-κB and OCT-1 probes. Cytosolic extracts were subjected to SDS/PAGE and Western blotting with the indicated antibodies.
Association of β-Arrestins with CARMA3.
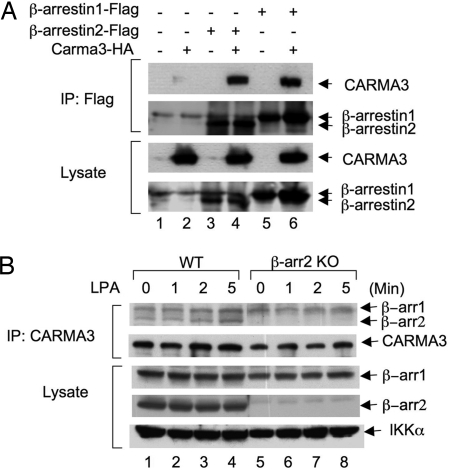
Our recent studies show that CARMA3, a scaffold protein, is required for GPCR-induced NF-κB activation (6). However, how CARMA3 is linked to GPCR complexes remains to be determined. Because previous data indicate that β-arrestin 2 is associated with GPCR, we hypothesize that β-arrestin 2 may link CARMA3 to GPCR complexes. Consistent with our hypothesis, we found that CARMA3 associated with β-arrestin 2 when they were coexpressed in human embryonic kidney 293T (HEK293T) cells (Fig. 4A and Fig. S2). However, although β-arrestin 1 was functionally not required for LPA-induced NF-κB activation (Fig. 1B), it also associated with CARMA3 under this condition (Fig. 4A and Fig. S2).
Fig. 4.
Interaction of CARMA3 with β-arrestins. (A) HEK293T cells were transfected with expression vectors encoding HA-tagged CARMA3, FLAG-tagged β-arrestin 1, or FLAG-tagged β-arrestin 2 at different combinations. Twenty hours after transfection, cell lysates were subjected to immunoprecipitation (IP) with anti-FLAG antibody-conjugated beads, and immunoprecipitated proteins and cell lysates were subjected to SDS/PAGE and analyzed by immunoblotting with the indicated antibodies. (B) Wild-type or β-arrestin 2 KO MEF cells (15-cm plate, 80% confluent) were stimulated with or without LPA for different periods of time and then scraped off and lysed. The resulting lysates were subjected to immunoprecipitation with CARMA3 antibody-conjugated beads. The obtained immunocomplexes were subjected to SDS/PAGE and Western blotting with β-arrestin 1 and β-arrestin 2 antibodies or CARMA3 antibodies as indicated. For detection of coimmunoprecipitated β-arrestins, rabbit IgG TrueBlot was used as secondary antibody.
To confirm these associations further, we examined the subcellular localization of β-arrestin 2 and CARMA3 by fusing CARMA3 to green fluorescent protein (GFP) while fusing β-arrestin 2 to red fluorescent protein (RFP) and then expressed these proteins in different combinations in HeLa cells. Consistent with their physical association, we found that GFP-CARMA3 and RFP-β-arrestin 2 were colocalized in the cytoplasm (Fig. S3). In contrast, GFP-CARMA3 did not colocalize with RFP alone, whereas RFP-β-arrestin 2 did not colocalize with GFP alone (Fig. S3).
To determine whether endogenous CARMA3 and β-arrestins associate in a signal-dependent manner, we immunoprecipitated endogenous CARMA3 from wild-type or β-arrestin 2 KO MEF cells. We found that CARMA3 coprecipitated with both β-arrestin 2 and β-arrestin 1 (Fig. 4B). Although this interaction was weak, it is specific because IgG control bead did not pull down β-arrestin 2 (Fig. S4). However, LPA stimulation slightly enhanced the interaction between CARMA3 and β-arrestin 2 (Fig. 4B and Fig. S4). Together, these data suggest that CARMA3 forms a complex with β-arrestins.
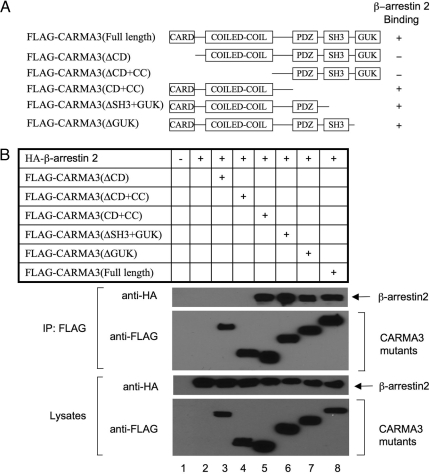
To identify the interacting domains of CARMA3 and β-arrestin 2, we constructed a series of CARMA3 deletion mutants (Fig. 5A). They were expressed in HEK293T cells together with β-arrestin 2. We found that deletion of the CARD domain resulted in complete loss of its binding to β-arrestin 2 (Fig. 5B), suggesting that the CARD domain is involved in the interaction. Reciprocally, we also constructed a set of β-arrestin 2 deletion mutants (Fig. S5A) and expressed these mutants with CARMA3 in HEK293T cells. We found that β-arrestin 2 deletion mutants containing amino acids 1–320 and 60–410 associate with CARMA3 with a high affinity (Fig. S5B). However, β-arrestin 2 mutants 1–240 and 240–410 interacted with CARMA3 with a low affinity, suggesting that residues 60–320 of β-arrestin 2 contribute to CARMA3 binding.
Fig. 5.
The domain of CARMA3 interacted with β-arrestin 2. (A) Schematic diagram of full-length and deletion mutants of CARMA3. CC, coil-coiled domain; SH3, Src homology 3 domain; PDZ, PDZ domain; GUK, guanylate kinase domain. (B) FLAG-tagged full-length or deletion mutants of CARMA3 together with HA-tagged β-arrestin 2 was transfected into HEK293T cells. Twenty hours after transfection, cell lysates were subjected to immunoprecipitation (IP) with anti-FLAG antibody-conjugated beads, and immunoprecipitated proteins and cell lysates were subjected to SDS/PAGE and analyzed by immunoblotting with the indicated antibodies.
β-Arrestin 2 Enhances the Interaction between CARMA3 and LPA Receptor.
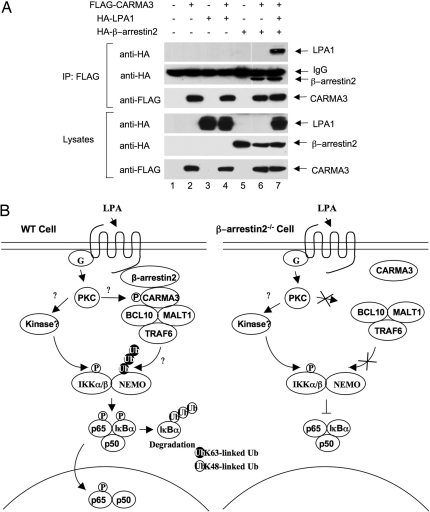
Because previous studies indicate that β-arrestin 2 is associated with GPCR after the ligand stimulation, we hypothesized that β-arrestin 2 might recruit CARMA3 to LPA receptors. To test this hypothesis, we coexpressed CARMA3 and LPA receptor LPA1, with or without β-arrestin 2 (Fig. 6A). Although the association of CARMA3 with LPA1 was not detectable when they were coexpressed (Fig. 6A, lane 4), this association was significantly enhanced in the presence of β-arrestin 2 (Fig. 6A, lane 6). Together, these results suggest that the role of β-arrestin 2 in GPCR-induced NF-κB activation is to recruit CARMA3 into GPCR complexes.
Fig. 6.
β-Arrestin 2 links CARMA3 to LPA receptor. (A) Expression vectors encoding HA-β-arrestin 2, HA-LPA1, and FLAG-CARMA3 at different combinations were transfected into HEK293T cells. Twenty hours after transfection, cell lysates were subjected to immunoprecipitation (IP) with anti-FLAG antibody-conjugated beads, and immunoprecipitated proteins and cell lysates were subjected to SDS/PAGE and analyzed by immunoblotting with the indicated antibodies. (B) Working model for β-arrestin 2-mediated NF-κB activation. GPCR (LPA receptor)-induced NF-κB activation involves in the β-arrestin-dependent recruitment of CARMA3 to the receptor. The recruitment of CARMA3 leads to the formation of a complex containing Bcl10, MALT1, and TRAF6. This complex may regulate polyubiquitination or unknown modification of the IKK complex, whereas a β-arrestin 2- and CARMA3-independent, but PKC-dependent signal induces IKK phosphorylation by an unknown kinase in the GPCR signaling pathway. In the absence of β-arrestin 2, CARMA3 is not recruited into the LPA receptor complex. Therefore, CARMA3-dependent regulation of IKK complex is impaired, which results in the defect of IKK and NF-κB activation.
Discussion
LPA binds to its receptors, a family of GPCRs, leading to activation of NF-κB. Although the signaling pathways induced by LPA and other GPCRs have been intensively investigated, the mechanism by which LPA and other GPCRs activates NF-κB has not been fully defined. Recent studies from our group and others have demonstrated that CARMA3 (6) and its downstream components, Bcl10 and MALT1, are required for LPA- and GPCR-induced NF-κB activation (7–9). However, how CARMA3 is linked to the GPCRs and mediates this signaling pathway has not been defined. In this work, we demonstrate that β-arrestin 2, but not β-arrestin 1, mediates LPA-induced NF-κB activation likely by recruiting CARMA3 to LPA receptors (Fig. 6B). Therefore, our results reveal a pathway that links GPCRs to NF-κB.
Previous studies suggest that β-arrestin 1 and β-arrestin 2 can bind to IκBα, the inhibitor of NF-κB (22, 23). The association of β-arrestins with IκBα may play negative roles in inhibiting TNFα-, UV light-, and TLR/IL-1 receptor-induced NF-κB activation (20–23). However, the role of β-arrestins in GPCR-induced NF-κB activation has not been investigated. Our findings that LPA-induced NF-κB activation is inhibited in cells deficient in β-arrestin 2 indicates that β-arrestin 2 plays a positive role in GPCR-induced NF-κB activation. Of note, a recent proteomic study suggests that β-arrestin 2 is associated with complexes containing TAK1 and IKKα, two positive regulators of NF-κB activation, after AngII stimulation (24). These data are consistent with our findings because AngII, like LPA, can induce NF-κB activation (7, 9).
Previous studies show that stimulation of β2-adrenergic receptor (β2AR), another GPCR family member, inhibits TNFα-induced NF-κB activation (23). This work suggests that β2AR induces IκBα stabilization through β-arrestin 2, which is opposite to its role in LPA signaling pathway. Currently, it is not clear why β-arrestin 2 functions differently for NF-κB activation in these two GPCR pathways. However, it should be pointed out that different GPCRs activate different Gα subunits and downstream effectors (2, 3). β2AR signals through a Gαs–PKA signaling cascade, which may inhibit NF-κB activation (2), whereas LPA receptors trigger a Gαq–PKC-mediated signaling cascade, leading to activation of NF-κB (6). One possibility is that these pathways may induce different posttranslational modifications of β-arrestin 2. Therefore, β-arrestin 2 serves as a positive regulator in LPA-induced NF-κB activation, whereas in other pathways, it serves as a negative regulator. Future investigation of signal-dependent posttranslational modification of β-arrestin 2 may provide the molecular mechanism of β-arrestin 2-mediated functions.
It has been shown that PKC is involved in LPA-induced NF-κB activation (25, 26). Our results, showing that NF-κB activation is defective in β-arrestin 2-deficient cells with stimulation of PMA plus Ionomycin, the pharmacological agonist of PKC, indicate that β-arrestin 2 is also required for PKC-induced NF-κB activation (6). Earlier studies indicate that PKC-mediated phosphorylation of CARMA1 (27, 28) is required for antigen receptor-induced NF-κB activation. Because CARMA3, the nonhematopoietic homolog of CARMA1, associates with β-arrestin 2 and is required for PKC-induced NF-κB activation (6), we hypothesize that β-arrestin 2 may recruit CARMA3 to the proximity of PKC in GPCR signaling pathways and facilitate PKC-mediated phosphorylation of CARMA3, leading to NF-κB activation. This hypothesis will be tested in the future studies.
The current model for IKK activation is that a signal-induced IKKα/β phosphorylation is required for the activation of the IKK complex. However, our recent studies indicate that the deficiency of either CARMA1 or CARMA3 impairs IKK activation without affecting the signal-induced IKKα/β phosphorylation after TCR, LPA, or PMA/Iono stimulation, suggesting that the signal-induced phosphorylation of IKKα/β is not sufficient to activate the IKK complex, and other modifications such as ubiquitination may be required to do so (6, 29). Therefore, we have proposed that GPCR-induced IKK activation is controlled through two signaling events: a CARMA3-independent IKK phosphorylation and CARMA3-dependent IKK ubiquitination (6). Consistent with our model that β-arrestin 2 recruits CARMA3 in the GPCR signaling pathway, we find that, although β-arrestin 2 is required for LPA-induced IKK activation, it is not required for the LPA-induced IKK phosphorylation.
Although β-arrestin 1 and β-arrestin 2 share 70% of homologous sequence with similar expression pattern and are involved in desensitizing GPCR-induced signaling pathways, recent studies suggest that they have different functions (10–16, 20, 21, 23, 30–35). Our data further support that these two proteins play different roles in GPCR-induced signaling pathways. Of note, although only β-arrestin 2 is functionally required for LPA-induced NF-κB activation, both β-arrestin 1 and β-arrestin 2 associate with CARMA3. One possible explanation is that β-arrestin 1 and β-arrestin 2 can form heterodimeric complexes in cells (24), whereas only β-arrestin 2 is required for association with other key components for activation of NF-κB. Another possibility is that β-arrestin 1 associates with CARMA3 and mediates other unknown functions, which remains to be investigated further.
In summary, our studies reveal a signaling pathway in which β-arrestin 2 recruits CARMA3 to LPA receptors (Fig. 6B), thereby functioning as a positive regulator, leading to NF-κB activation. Because the signaling pathways around the membrane-proximal region of LPA receptor and GPCRs are quite conserved, our results not only provide the genetic evidence that β-arrestin 2 is required for LPA-induced NF-κB activation, but also provide a possible link between other GPCRs and CARMA3-mediated NF-κB activation. However, it remains to be determined whether PKC phosphorylates CARMA3 and how IKKα/β is phosphorylated in the β-arrestin 2/CARMA3-independent pathway (Fig. 6B).
Materials and Methods
Cell Lines.
Wild-type, βarr1 KO, βarr2 KO, and βarr1/2 dKO MEF cells were provided by Robert Lefkowitz (Duke University, Durham, NC) and maintained in DMEM containing 10% FCS at 37°C with 5% CO2. HEK293T cells were cultured in the same condition.
Antibodies, Plasmids, and Reagents.
Antibodies to IKKα/β (H470), IKKα (H744), IKKγ (FL419), IκBα (C21), ERK2 (C14), HA (7392), FLAG (M2), phospho-ERK1/2 (9101S), phospho-IκBα (9246L), phospho-JNK1/2 (9251L), and JNK1/2 (9252) were purchased from Santa Cruz Biotechnology, Sigma, or Cell Signaling. Rabbit IgG TrueBlot was purchased from Bioscience (18-8816-31). β-Arrestin antibodies were provided by Robert Lefkowitz. CARMA3 antibody was generated as described in ref. 6.
FLAG-tagged β-arrestin 1 and β-arrestin 2 expression vectors were kindly provided by Robert Lefkowitz. HA-tagged LPA1 was provided by Shuang Huang (Medical College of Georgia, Augusta, GA). HA-tagged full-length β-arrestin 2 and FLAG-tagged deletion mutants of β-arrestin 2 were generated by PCR and subcloned into pCMVtag4 in-frame with an epitope tag at the C terminus. Full-length HA-CARMA3, FLAG-CARMA3, and FLAG-CARMA3 mutants with C-terminal tags were similarly constructed.
LPA (18:1) was from Avanti Polar Lipids. PMA and Ionomycin were from Sigma. TNFα was from Endogen.
Immunoprecipitation and Western Blotting.
Various epitope-tagged constructs were transfected into HEK293T cells by calcium phosphate precipitation. After a 16-h incubation, HEK293T cells were detached by manual scraping, collected by centrifugation, and lysed in lysis buffer [250 mM NaCl, 50 mM Hepes (pH 7.4), 1 mM EDTA, 1% Nonidet P-40, protease inhibitors]. Each sample was incubated with anti-HA antibodies and protein A–agarose beads (Roche Applied Science) or anti-FLAG beads (Sigma) at 4°C overnight. Agarose beads were washed three times in cold lysis buffer. Then, the precipitates were boiled for 5 min in SDS loading buffer, loaded onto 10% SDS/polyacrylamide gels, transferred to nitrocellulose membrane, and immunoblotted with corresponding antibodies.
EMSA.
NF-κB and OCT-1 oligonucleotide probes were purchased from Promega and labeled with [γ-32P]ATP. MEF cells (1 × 106) were starved for 4 h and stimulated for 30–60 min. Nuclear extracts were prepared from these cells and then incubated with 32P-labeled probes in 10 mM Hepes (pH 7.9), 40 mM NaCl, 1 mM EDTA, 4% glycerol, 3 μg of poly(dI·dC), and 0.5 mM DTT for 15 min at room temperature. The samples were then run on a nondenaturing polyacrylamide gel and exposed to x-ray film at −80°C.
In Vitro Kinase Assay.
IKK proteins were immunoprecipitated from cells by using IKKα and IKKγ antibodies and protein A–agarose for 4 h. The immunoprecipitates were washed and mixed with kinase buffer [10 mM Hepes (pH 7.4), 1 mM MnCl2, 5 mM MgCl2, 12.5 mM glycero-2-phosphate, 0.05 mM Na3VO4, 2 mM NaF, 0.5 mM DTT, 10 μM ATP] plus 0.5 μCi of [γ-32P]ATP in the presence of 1 μg of recombinant GST-tagged IκBα. Samples were incubated for 30 min at 30°C. The kinase reaction was terminated by adding 20 μl of 2× SDS loading buffer. The reaction mixtures were then subjected to SDS/PAGE and autoradiography.
ELISA.
Wild-type or β-arrestin 2-deficient MEF cells were serum-starved for 4 h and then stimulated with LPA (10 μM), PMA (40 ng/ml) plus Ionomycin (100 ng/ml), or TNFα for different time points. The media from these cultures were collected and subjected to IL-6 ELISA analysis according to the manufacturer's instructions (Quantikine kit; R&D Systems).
β-Arrestin 1- or 2-Reconstituted MEF Cells.
Phoenix cells were transfected with pLPC vector encoding either FLAG-tagged β-arrestin 1 or 2 by using calcium phosphate precipitation. After 2 days, supernatants were collected to infect βarr1/2 dKO MEF cells for 1 day. Then, the infected cells were selected under puromycin for 2 days. The puromycin-resistant cells were used for EMSA or Western blot analysis.
Supplementary Material
Acknowledgments.
We thank Drs. Robert Lefkowitz and Shuang Huang for reagents. This work was supported by National Institutes of Health Grants GM079451 and GM065899 (to X.L.). X.L. is a Scholar of the Leukemia and Lymphoma Society and a recipient of the Investigator Award of the Cancer Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. V.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802701105/DCSupplemental.
References
- 1.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 2.Ye RD. Regulation of nuclear factor κB activation by G protein-coupled receptors. J Leukocyte Biol. 2001;70:839–848. [PubMed] [Google Scholar]
- 3.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 6.Grabiner BC, et al. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-κB activation. Genes Dev. 2007;21:984–996. doi: 10.1101/gad.1502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, et al. Bcl10 plays a critical role in NF-κB activation induced by G protein-coupled receptors. Proc Natl Acad Sci USA. 2007;104:145–150. doi: 10.1073/pnas.0601894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klemm S, Zimmermann S, Peschel C, Mak TW, Ruland J. Bcl10 and Malt1 control lysophosphatidic acid-induced NF-κB activation and cytokine production. Proc Natl Acad Sci USA. 2007;104:134–138. doi: 10.1073/pnas.0608388103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAllister-Lucas LM, et al. CARMA3/Bcl10/MALT1-dependent NF-κB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci USA. 2007;104:139–144. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through β-arrestin. Sci STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- 11.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for β-arrestins in cell signaling: Not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 12.McDonald PH, Lefkowitz RJ. β-Arrestins: New roles in regulating heptahelical receptors' functions. Cell Signal. 2001;13:683–689. doi: 10.1016/s0898-6568(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 13.Shenoy SK, Lefkowitz RJ. Multifaceted roles of β-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan FG, DuBois RN. Emerging roles of β-arrestins. Cell Cycle. 2006;5:2060–2063. doi: 10.4161/cc.5.18.3212. [DOI] [PubMed] [Google Scholar]
- 15.Lefkowitz RJ, Whalen EJ. β-Arrestins: Traffic cops of cell signaling. Curr Opin Cell Biol. 2004;16:162–168. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 17.Gesty-Palmer D, El Shewy H, Kohout TA, Luttrell LM. β-Arrestin 2 expression determines the transcriptional response to lysophosphatidic acid stimulation in murine embryo fibroblasts. J Biol Chem. 2005;280:32157–32167. doi: 10.1074/jbc.M507460200. [DOI] [PubMed] [Google Scholar]
- 18.McDonald PH, et al. β-Arrestin 2: A receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 19.Luttrell LM, et al. β-Arrestin-dependent formation of β2-adrenergic receptor–Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Association of β-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 21.Luan B, Zhang Z, Wu Y, Kang J, Pei G. β-Arrestin 2 functions as a phosphorylation-regulated suppressor of UV-induced NF-κB activation. EMBO J. 2005;24:4237–4246. doi: 10.1038/sj.emboj.7600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. β-Arrestin inhibits NF-κB activity by means of its interaction with the NF-κB inhibitor IκBα. Proc Natl Acad Sci USA. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao H, et al. Identification of β-arrestin 2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 24.Xiao K, et al. Functional specialization of β-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings R, et al. Protein kinase Cδ mediates lysophosphatidic acid-induced NF-κB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- 26.Mahanivong C, et al. Protein kinase Cα–CARMA3 signaling axis links Ras to NF-κB for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. Oncogene. 2008;27:1273–1280. doi: 10.1038/sj.onc.1210746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommer K, et al. Phosphorylation of the CARMA1 linker controls NF-κB activation. Immunity. 2005;23:561–574. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto R, et al. Phosphorylation of CARMA1 plays a critical role in T Cell receptor-mediated NF-κB activation. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Shambharkar PB, et al. Phosphorylation and ubiquitination of the IκB kinase complex by two distinct signaling pathways. EMBO J. 2007;26:1794–1805. doi: 10.1038/sj.emboj.7601622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, et al. Critical regulation of CD4+ T cell survival and autoimmunity by β-arrestin 1. Nat Immunol. 2007;8:817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Wu Y, Ge X, Ma L, Pei G. Subcellular localization of β-arrestins is determined by their intact N domain and the nuclear export signal at the C terminus. J Biol Chem. 2003;278:11648–11653. doi: 10.1074/jbc.M208109200. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, et al. β-Arrestin 2 functions as a G protein-coupled receptor-activated regulator of oncoprotein Mdm2. J Biol Chem. 2003;278:6363–6370. doi: 10.1074/jbc.M210350200. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Cheng Z, Ma L, Pei G. β-Arrestin 2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 34.Kang J, et al. A nuclear function of β-arrestin 1 in GPCR signaling: Regulation of histone acetylation and gene transcription. Cell. 2005;123:833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Beaulieu JM, et al. A β-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.