Abstract
AML1-ETO is generated from t(8;21)(q22;q22), which is a common form of chromosomal translocation associated with development of acute myeloid leukemia (AML). Although full-length AML1-ETO alone fails to promote leukemia because of its detrimental effects on cell proliferation, an alternatively spliced isoform, AML1-ETO9a, without its C-terminal NHR3/NHR4 domains, strongly induces leukemia. However, full-length AML1-ETO is a major form of fusion product in many t(8;21) AML patients, suggesting additional molecular mechanisms of t(8;21)-related leukemogenesis. Here, we report that disruption of the zinc-chelating structure in the NHR4 domain of AML1-ETO by replacing only one critical amino acid leads to rapid onset of leukemia, demonstrating that the NHR4 domain with the intact structure generates inhibitory effects on leukemogenesis. Furthermore, we identified SON, a DNA/RNA-binding domain containing protein, as a novel NHR4-interacting protein. Knock-down of SON by siRNA resulted in significant growth arrest, and disruption of the interaction between AML1-ETO and endogenous SON rescued cells from AML1-ETO-induced growth arrest, suggesting that SON is an indispensable factor for cell growth, and AML1-ETO binding to SON may trigger signals inhibiting leukemogenesis. In t(8;21) AML patient-derived primary leukemic cells and cell lines, abnormal cytoplasmic localization of SON was detected, which may keep cells proliferating in the presence of full-length AML1-ETO. These results uncovered the crucial role of the NHR4 domain in determination of cellular fate during AML1-ETO-associated leukemogenesis.
Keywords: AML1-ETO, Leukemia, NHR4, SON, t(8;21)
Chromosomal translocation is one of the most common genetic abnormalities in acute myeloid leukemia (AML) (1). AML1-ETO is a fusion protein transcription factor generated from t(8;21)(q22;q22). This translocation is identified in >10% of all cases and up to 40% of the French-American-British M2 subtype of AML (2–5). However, expression of AML1-ETO by itself fails to cause leukemia (6–11), which suggests the requirement of “additional hits” for AML1-ETO-positive cells to become leukemogenic.
Previously, our group reported that the C-terminally truncated form of AML1-ETO (AML1-ETOtr) is strongly leukemogenic (12). Interestingly, a short form of AML1-ETO, AML1-ETO9a, which is produced by alternative splicing, was identified in t(8;21) leukemia patient samples, and expression of AML1-ETO9a induced rapid leukemia development in mice (13). AML1-ETOtr and AML1-ETO9a encode 556 and 575 aa (a.a.), respectively, and both lack the NHR3 and NHR4 regions. These results suggest that the N-terminal portion of ETO (NHR1 and NHR2) fused with AML1, is sufficient to cause leukemia. In addition, the C-terminal domains of AML1-ETO (NHR3 and NHR4) may actually play a role in suppressing disease development.
In this report, we demonstrate that the zinc (Zn)-chelating structure of the NHR4 is responsible for generating inhibitory effects on leukemia development, and that this region interacts with SON, a potential DNA/RNA-binding protein, to cause growth arrest in AML1-ETO-expressing cells. Furthermore, we demonstrate a crucial role of SON in cell proliferation, and abnormal localization of SON in AML1-ETO-expressing leukemia patient samples.
Results
Deletion or Disruption of the Zn-Chelating Structure of the NHR4 Domain of AML1-ETO Leads to leukemogenesis.
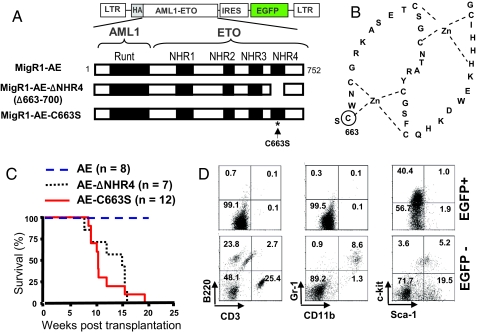
The NHR4 domain of AML1-ETO contains two Zn-chelating motifs including cysteines 663, 666, 674, 677, 683, 687, 699, and histidine 695 (Fig. 1B) (14). To investigate the role of the NHR4 domain in AML1-ETO-mediated leukemogenesis, we performed transplantation experiments using murine hematopoietic cells infected with the retroviral vector MigR1 containing full-length AML1-ETO (MigR1-AE), an NHR4-deleted form of AML1-ETO (MigR1-AE-ΔNHR4), or AML1-ETO with a single amino acid mutation at one of the zinc-chelating residues, cysteine 663 (MigR1-AE-C663S) (Fig. 1A). The point mutation C663S has been shown to disrupt the Zn-chelating structure (14, 15). As expected, expression of AML1-ETO alone (MigR1-AE) did not induce leukemogenesis. However, MigR1-AE-ΔNHR4 and MigR1-AE-C663S rapidly induced leukemia (Fig. 1C). Western blot analysis of spleen extracts from the leukemic mice showed the expression of AE-ΔNHR4 and AE-C663S in splenocytes [supporting information (SI) Fig. S1A]. Analysis of hematopoietic cells from mice transplanted with AE-ΔNHR4 showed the presence of myeloid blasts in blood, bone marrow, and spleen (Fig. S1B). Histological examination revealed extensive infiltrations of leukemic cells in the spleen and the liver from the AE-ΔNHR4 leukemic mice (Fig. S1C). Flow-cytometrric analysis of peripheral blood from leukemic mice with AE-ΔNHR4 showed that EGFP+ cells are negative for lymphoid and myeloid differentiation markers, such as CD3, B220, CD11b, and Gr-1. The proportion of c-Kit+ cells was greatly increased in the EGFP+ population, indicating the AE-ΔNHR4 protein causes an increase in immature myeloid progenitor-like cells (Fig. 1D). AE-C663S leukemic mice showed the similar phenotypes (data not shown), and the disease latency and the survival curve from the mice with NHR4-deleted or -disrupted AML1-ETO were very similar to those of AML1-ETOtr or AML1-ETO9a-tranplanted mice (12, 13).
Fig. 1.
Deletion of the NHR4 domain or a point mutation at the Zn-chelating residue of the NHR4 domain of AML1-ETO causes rapid onset of leukemia. (A) Schematic representation of AML1-ETO fusion protein and the retroviral construct MigR1. Three constructs were used in the retroviral infection and transplantation experiments: full-length AML-ETO (MigR1-AE), NHR4 domain-deleted AML1-ETO (MigR1-AE-ΔNHR4), and AML1-ETO with serine substitution of cysteine at a.a. 663 (MigR1-AE-C663S). (B) Schematic representation of the Zn-chelating structures in NHR4 of AML1-ETO. One of the Zn-chelating residues, cysteine residue at position 663 (marked with a circle), was chosen for a point mutation that would disrupt the Zn-chelating structure of NHR4 domain. (C) Kaplan-Meier survival curves of MF-1 mice transplanted with fetal liver cells retrovirally-transduced with MigR1-AE, MigR1-AE-ΔNHR4, and MigR1-AE-C663S. (D) Flow-cytometric analysis of lineage markers expressed in EGFP-positive (EGFP+) and EGFP-negative (EGFP-) populations of peripheral blood from the leukemic mice with MigR1-AE-ΔNHR4. The numbers in each quadrant represent the percentage of cells.
SON Is an NHR4-Interacting Protein.
Because our transplantation experiments above indicate that NHR4 of AML1-ETO attenuates the effect of AML1-ETO on leukemogenesis, we hypothesized that NHR4 may interact with proteins, which abrogate the ability of AML1-ETO to induce leukemia. To search for NHR4-interacting proteins, we performed yeast two hybrid screening using three baits, including full-length wild-type ETO (ETO-wt), ETO with a point mutation in NHR4 (ETO-C663S), and the C-terminal region of ETO (NHR3-NHR4) (Fig. 2A). We isolated one clone that interacts with ETO-wt and more strongly with NHR3-NHR4 but not with ETO-C663S. Sequence analysis revealed that this clone contained a 0.31-kb insert cDNA that encodes the first 102 a.a. of the mouse SON protein (GenBank accession NM_178880). SON was reported to have a DNA-binding ability (16–18), and contains RNA-binding motifs (19, 20) (details in Fig. S2), suggesting that SON may have a dual ability to interact with both DNA and RNA. However, no profound studies on the function of SON have been performed.
Fig. 2.
Identification of SON as an NHR4 domain-interacting protein. (A) Schematic representation of three baits used for yeast two hybrid screening and their interaction with the N-terminal 102 a.a. of SON in yeast; full-length wild-type ETO (ETO-wt), full-length ETO with a point mutation at the NHR4 domain (ETO-C663S), and the C-terminal region of ETO (NHR3-NHR4). Binding activities were determined (-, +, and ++) by expression of β-galactosidase reporter gene and the growth of yeast colonies in URA- media. (B) Interaction of the SON N-terminal fragments with ETO in mammalian cells. The N-terminal region of mouse SON encoding a.a 1–25, 1–81, or 1–102 were fused with GST, co-expressed with Flag-tagged ETO in 293T cells, and GST pull-down complexes were immunoblotted with GST antibody or Flag antibody. Note that the GST control shows a similar molecular weight to GST-SON-1–25, because the translational termination site for GST alone is located further downstream of the multiple cloning sites, resulting in the additional 20 a.a. to the C terminus of GST. (C) Interaction of endogenous SON and AML1-ETO in t(8;21) leukemia patient-derived cell line, SKNO-1. The SON antibody pulled down AML1-ETO in SKNO-1 cell lysates. SON was detected as several bands, although its calculated molecular weight is ≈267 kDa. A band marked with an asterisk is nonspecific. (D) Colocalization of SON and AML1-ETO. HeLa cells were transfected with HA-AML1-ETO or Flag-ETO and immunostained with HA- or Flag-antibody and SON antibody followed by confocal microscopy.
GST pull-down experiments showed that SON a.a 1–81 fragment, which is encoded by exons 1 and 2, was sufficient to interact with the ETO protein (Fig. 2B). Furthermore, in AML1-ETO-transfected HeLa cells, endogenous SON bound to the full-length AML1-ETO (Fig. S3). The interaction between endogenous proteins was further confirmed in a t(8;21) leukemia patient-derived cell line, SKNO-1. Immunoprecipitation with SON antibody pulled down AML1-ETO in SKNO-1 cell lysates (Fig. 2C), demonstrating that SON is an AML1-ETO-interacting protein.
We also analyzed the cellular localization of AML1-ETO and SON by immunofluorescence microscopy. First, we examined the localization of endogenous SON. SON localized within the nuclei in a speckled distribution, excluded from the DAPI-stained DNA-rich region (Fig. S4). These results are consistent with previous reports showing localization of SON at the nuclear speckles, called interchromatin granule clusters (21). To determine whether SON colocalizes with AML1-ETO, HA-tagged AML1-ETO was transfected in HeLa cells and the cells were immunostained with HA antibody and SON antibody. We detected colocalization of endogenous SON and HA-tagged AML1-ETO (Fig. 2D), although not all AML1-ETO colocalized with SON. In addition, SON was found to colocalize with the transfected ETO protein (Fig. 2D).
ETO-C663S Mutation Eliminates SON Interaction, but Not N-CoR Interaction.
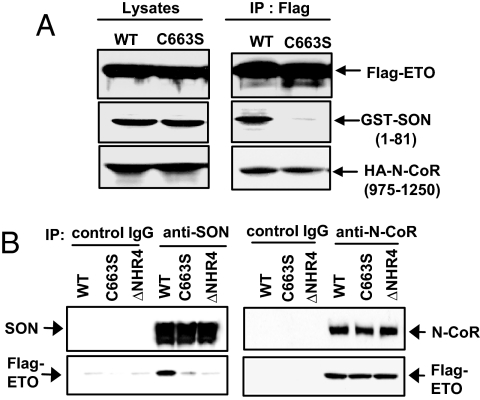
Because the NHR4 domain of ETO has been known to interact with N-CoR/SMRT, we tested whether the cysteine 663 mutant is also defective in N-CoR binding. SON N-terminal fragment (a.a. 1–81) and the N-CoR fragment containing RIII domain (a.a. 975-1250) were transfected with wt-ETO or ETO-C663S. The PPPLIP sequence present in the RIII domain of N-CoR has been reported to interact with the region encompassing NHR3 and NHR4 of ETO (14, 22). However, it had not been clearly demonstrated whether NHR4 alone interacts with RIII domain of N-CoR in mammalian cells. Interestingly, ETO-C663S completely lost its interaction with the SON fragment, while retaining its ability to interact with the N-CoR RIII domain (Fig. 3A).
Fig. 3.
Deletion of NHR4 or C663S mutation abolishes SON interaction but not N-CoR interaction. (A) Effects of the C663S mutation on interactions with the SON N-terminal fragment and the N-CoR fragment containing the RIII domain. GST-tagged SON (1–81 a.a.) and HA-tagged N-CoR (975–1250 a.a.) were transfected into 293T cells with either Flag-tagged wild-type ETO (WT) or ETO with C663S mutation, and immunoprecipited with Flag antibody. (B) Effects of NHR4 deletion or C663S mutation on interactions with full-length SON and N-CoR. SON antibody or N-CoR antibody was used for immunoprecipitation to pull down endogenous SON or N-CoR, and immunoblotted to detect Flag-tagged ETO; wild-type ETO (WT), ETO with a C663S mutation (C663S), and NHR4-deleted ETO (ΔNHR4).
To compare the effect of NHR4 mutation on interactions with full-length SON and full-length N-CoR, wild-type ETO, ETO-C663S, and ETO-ΔNHR4 were expressed in 293T cells, and the SON antibody or N-CoR antibody was used for immunoprecipitation. Both SON and wild-type ETO was detected in the complex precipitated with SON antibody (Fig. 3B). However, neither ETO-C663 nor ETO-ΔNHR4 was significantly detected in the immunoprecipitates (Fig. 3B). In contrast, N-CoR interaction was not affected by deletion or C663S mutation in the NHR4 region of ETO (Fig. 3B). It is likely that N-CoR interaction is still mediated by other domains of ETO whereas SON interaction absolutely requires Zn-chelating topology of ETO.
Knock-Down of SON by siRNA Induces Significant Growth Arrest in the Cells.
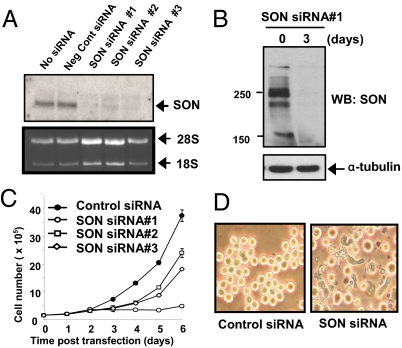
It has been demonstrated that full-length AML1-ETO fails to induce leukemia (6–11), possibly because of AML1-ETO-induced growth arrest and cell death (23). This observation suggests that the preleukemic AML1-ETO-positive cells need to overcome these cell cycle and apoptotic defects to realize their leukemogenic potential. We found that deletion or mutations of NHR4 promotes leukemogenic ability of AML1-ETO, indicating that disruption of the interaction between AML1-ETO and proteins that interact with NHR4 alleviate the hindrance to leukemogenesis. Therefore, we hypothesize that the interaction of SON with NHR4 of AML1-ETO may cause growth arrest. To block SON binding to AML1-ETO, we used siRNA to knock down the SON protein (Figs. 4A and 4B). Interestingly, when SON siRNA was transfected into cells, substantial growth arrest was induced in several cell lines, including K562 (Fig. 4C), HeLa, and BJ primary fibroblasts (data not shown). Significant morphological changes, such as elongation of K562 cells, were also observed after introducing SON siRNA (Fig. 4D). As knock-down of SON alone was able to induce growth arrest, we could not test the specific effect of disrupting the interaction of SON with AML1-ETO. However, these results indicate that SON is essential for normal cell growth, and knock-down of SON results in impaired cell proliferation that is also observed in cells with AML1-ETO expression.
Fig. 4.
SON siRNA completely knocks down endogenous SON and induces growth arrest. (A) Knock-down of SON by SON siRNAs in 293T cells confirmed by Northern blotting. (B) Knock-down of SON confirmed by Western blotting. K562 cells were transfected with SON siRNA no. 1. Whole cell lysates were prepared after 3 days and immunoblotted with SON antibody. (C) SON siRNA induces growth arrest. K562 cells were transfected with 1.3 μM negative control siRNA or three different SON siRNAs by nucleofection in 100 μl solution, and cells were counted every day. The graph is representative of four experiments. (D) Morphological changes in K562 cells 3 days after SON siRNA (#1) transfection.
Expression of the SON N-Terminal Fragment Rescues AML1-ETO-Induced Growth Arrest.
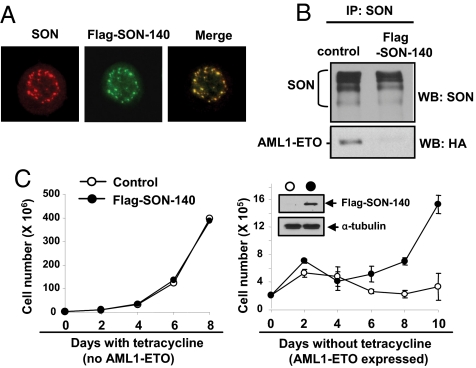
Knock-down of SON by siRNA alone causes growth arrest. Therefore, we chose another approach to block the interaction between AML1-ETO and SON. Since the N-terminal region of SON corresponding to a.a. 1–81 was sufficient to interact with NHR4 of AML1-ETO (Fig. 2B), we overexpressed the N-terminal fragment of SON to interfere with the interaction between AML1-ETO and endogenous SON. To ensure the proper nuclear localization, we expressed the first 140 a.a. of SON (SON-140) that has a putative nuclear localization signal at a.a. 117–128 (based on a GenBank search). Immunostaining revealed that SON-140 perfectly colocalized with endogenous SON (Fig. 5A). Expression of this SON-140 fragment efficiently inhibited the interaction between AML1-ETO and endogenous SON (Fig. 5B).
Fig. 5.
Expression of SON N-terminal fragment inhibits AML1-ETO binding to endogenous SON, and rescues cells from AML1-ETO-induced growth arrest. (A) The SON N-terminal fragment encoding a.a. 1–140 (Flag-SON-140) colocalizes with endogenous SON. K562 cells expressing MigR1-Flag-SON-140 were immunostained with SON antibody (recognizing C terminus of SON) for endogenous SON, and with Flag antibody for the SON-140 fragment. (B) Expression of the SON-140 fragment interferes with the interaction between endogenous SON and AML1-ETO. K562 cells stably expressing control vector or Flag-SON-140 were transfected with HA-tagged AML1-ETO, and immunoprecipitation was performed with SON antibody, and the immunocomplexes were subjected to Western blotting with the indicated antibodies. (C) Expression of the SON-140 fragment alone does not affect cell growth and rescues cells from AML1-ETO-induced growth arrest. U937T cells with inducible AML1-ETO expression were infected with control vector or Flag-SON-140. The GFP-positive populations were sorted, cultured with or without tetracycline, and cells were counted every other day. Western blot shows expression of Flag-tagged SON-140 in sorted cells. Growth curve shown represents four independent experiments. Each circle and error bar represents mean ± SD, from the triplicate cell culture.
To test whether expression of the SON N-terminal fragment could overcome AML1-ET-induced growth arrest, U937 cells with inducible AML1-ETO expression (23) were infected with MigR1 (control vector) or MigR1-Flag-SON-140. In the presence of tetracycline (without AML1-ETO), control cells and Flag-SON-140-expressing cells showed identical growth (Fig. 5C), confirming that, unlike SON siRNA, SON-140 itself does not affect cell growth. Then we cultured these two populations in the absence of tetracycline to induce AML1-ETO expression. As reported previously, induction of AML1-ETO in the MigR1 control line caused growth arrest. The Flag-SON-140-expressing line also underwent growth arrest during the first 2–3 days after tetracycline removal when the induced AML1-ETO expression exceeded SON-140 expression (Fig. S5). However, unlike MigR1 control cells, these cells started to expand after days 4–5, when AML1-ETO induction is at a moderate level (Fig. S5), and recovered a normal growth rate (Fig. 5C). These results demonstrate that inhibition of the interaction between AML1-ETO and endogenous SON can rescue cells from AML1-ETO-induced growth arrest, suggesting that AML1-ETO and SON interaction generates negative effects on cell growth.
Abnormal Localization of SON in AML1-ETO-Positive Cell Lines and t(8;21) AML Patient Samples.
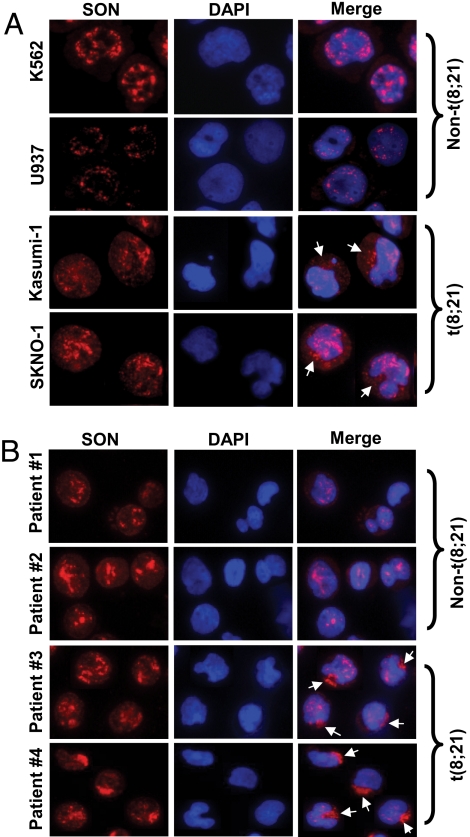
Because the function of SON appears to be critical for normal cell proliferation, we further investigated a possible involvement of abnormal SON expression in leukemia development. First we checked to see whether the SON expression level is altered in t(8;21)-positive cell lines. Four leukemic cell lines were used to compare the SON mRNA level; K562 and U937 cells as non-t(8;21) cells, and Kasumi-1 and SKNO-1 as t(8;21)-positive cells. Quantitative real-time polymerase chain reaction analysis showed that there is no apparent correlation between the presence of t(8;21) and the level of SON mRNA (data not shown). Then we analyzed cellular localization of the SON protein in these cell lines by immunostaining using SON antibody. In addition to the nuclear SON, cytoplasmic localization of SON was detected in t(8;21)-positive cell lines, Kasumi-1 and SKNO-1, whereas SON was perfectly localized within the nucleus in non-t(8;21) leukemic cells K562 and U937 (Fig. 6A). Quantification of density of SON staining from image analysis revealed that cytoplasmic SON accounts for 33 ± 6% and 29 ± 5% of the total SON protein in Kasumi-1 and SKNO-1 cells, respectively. However, expression of AML1-ETO in other hematopoietic cells, K562 and U937, did not cause cytoplasmic export of SON (data not shown), suggesting that t(8;21) AML cells, such as Kasumi-1 and SKNO-1, may have acquired abnormalities in SON localization, so that they overcome the growth arrest effect generated by the interaction between SON and AML1-ETO.
Fig. 6.
Abnormal cytoplasmic localization of SON in t(8;21)-positive leukemic cells. (A) Two leukemic cell lines without t(8;21), K562 and U937, and two t(8;21) leukemic cell lines, Kasumi- 1 and SKNO-1, were stained with SON antibody and DAPI (for DNA). (B) Blood cells from four different AML patients were stained with SON antibody and DAPI, and analyzed by fluorescence microscopy. Patient 1, FAB M2 subtype, non-t(8;21); patient 2, FAB M4 subtype, non-t(8;21); patients 3 and 4, FAB M2 subtype, t(8;21) -positive. Arrows indicate cytoplasm-localized SON. Pictures are of representative features of each cell line and patient samples from >1000 cells analyzed by fluorescence microscopy.
Next we analyzed SON localization in primary AML cells from patients. Leukemic blasts from the blood of non-t(8;21) AML patients and t(8;21) AML patients were collected and immunostained with SON antibody. Strikingly, t(8;21) AML cells showed remarkable cytoplasmic localization of SON (Fig. 6B), which is even more obvious than that observed in t(8;21)-positive cell lines. Approximately 55 ± 7% of the total amount of SON was located in the cytoplasm, based on image analysis. In contrast, non-t(8;21) AML cells did not show any SON proteins present in the cytoplasm (Fig. 6B). These results strongly suggest that abnormal cytoplasmic localization of SON is involved in t(8;21)-associated AML.
Discussion
Although the abnormal fusion protein AML1-ETO generated by t(8;21) is necessary to cause leukemia, this fusion protein alone has failed to cause leukemia (6–11), indicating the secondary mutation may cooperate with AML1-ETO for leukemogenesis. Here we demonstrated that the NHR4 domain of AML1-ETO with the intact Zn-chelating structure generates growth inhibitory effects to suppress leukemia development. Furthermore, SON is identified as an NHR4-interacting protein that regulates AML1-ETO-associated growth arrest. Although SON was shown to be ubiquitously expressed (18) and evolutionarily conserved (24), its functions are largely unknown. In the early 1990s, Chumakov et al. reported that expression of a SON fragment corresponding to a.a.1267–2409 of human full-length SON in transformed NIH 3T3 cells inhibited tumorigenesis, suggesting that SON may have antioncogenic potential (25). In contrast, the 1940–2256 a.a. fragment has been shown to inhibit Bax-mediated apoptosis in yeast (26). Because, in both experiments, only partial fragments of SON were expressed, we cannot rule out the possibility that some expressed proteins may dominantly inhibit the function of the endogenous full-length SON protein, rather than revealing the authentic function of SON. However, these data strongly suggest that SON is involved in cell proliferation and/or apoptosis, which regulate tumorigenesis. In this study, we demonstrated that knock-down of endogenous SON by siRNA leads to growth arrest of cells, indicating that SON is an indispensable factor for cell proliferation. We have observed that SON knockdown causes cell cycle arrest and subsequent apoptosis in multiple cell types (Ahn and Zhang, data in preparation for publication). Because full-length AML1-ETO expression also induces growth arrest/apoptosis, and disruption of AML1-ETO and SON interaction alleviates this adverse effect on cell growth, it is likely that the interaction of AML1-ETO with SON inhibits or alters the normal function of SON.
Although it has been reported that deletion of the NHR4 domain eliminates the ability of ETO to interact with N-CoR (27, 28), these interactions were observed in yeast using Gal4-fused ETO fragments. In fact, Lutterbach et al. observed that the ETO fragment with NHR3/NHR4 deleted still interacted with full-length N-CoR in Cos-7 cell lysates (27). Recently, Liu et al. also reported that a C-terminal truncation of AML1-ETO that removed NHR3/NHR4 domains did not affect N-CoR or SMRT binding in transfected Cos7 cell lysates (29). We also demonstrated here that N-CoR binding is not disrupted when the NHR4 domain is deleted or mutated at C663 in mammalian cells. These observations from several groups indicate that N-CoR interaction is not significantly disrupted in mammalian cells when NHR4 is deleted. This is possibly due to other regions of ETO providing N-CoR binding sites. In contrast, NHR4 deletion or C663S mutation significantly disrupted ETO-SON interaction. Therefore, it is highly possible that disruption of AML1-ETO interaction with SON, rather than AML1-ETO interaction with N-CoR, contributes to progression of AML1-ETO-associated leukemia, although we still cannot totally exclude the possibility that the slight reduction in N-CoR recruitment to AML1-ETO, which is not detectable by immunoprecipitation and Western blot, also contributes to leukemogenesis.
Because AML1-ETO with the C663S mutation, which specifically disrupts the interaction with SON, is leukemogenic, and expression of the N-terminal fragment of SON (SON-140) that binds to AML1-ETO keeps cells proliferating in the presence of AML1-ETO, it is highly predictable that expression of AML1-ETO together with SON-140 would be leukemogenic in mice. However, when AML1-ETO expression exceeds the amount of SON-140, cells still undergo growth arrest (Fig. S5), suggesting that the relative amount of AML1-ETO and SON-140 would be an important factor to consider for the mouse model of coexpression of AML1-ETO and SON-140. We are currently seeking a method to coexpress these two proteins at the right level in primary hematopoietic cells.
We confirmed that AML1-ETO is indeed localized in the nucleus in the t(8;21)-positive cell lines (data not shown) and patient samples (Fig. S6). Therefore, cytoplasmic SON observed in t(8;21)-positive cell lines and t(8;21) AML patient samples does not interact with AML1-ETO and may help cells keep proliferating in the presence of AML1-ETO. As both Kasumi-1 and SKNO-1 cells have relatively low levels of AML1-ETO9a isoform expression (13), and as the patient samples that we tested also contained full-length AML1-ETO as a dominant form (data not shown), dislocation of SON might be one of the common “additional mutations” for leukemogenesis of t(8;21)q (22, 22)-positive cells that express mainly full-length AML1-ETO. Although we observed cytoplasmic localization of SON in t(8;21)-positive patient samples, exogenous expression of AML1-ETO, AML1-ETO9a isoform, ETO, or ETO-C663S in HeLa cells did not cause cytoplasmic localization of SON (Fig. S7A). In addition, when AML1-ETO and AML1-ETO9a isoform were coexpressed (Fig. S7B), which was the case in t(8;21)-positive patients (13), SON was still localized as speckles within the nucleus (Fig. S7C). These results suggest that AML1-ETO or its isoform do not directly cause cytoplasmic localization of SON. It is likely that expression of AML1-ETO and abnormal localization of SON are independent events that cooperate for leukemia development.
It is of interest to determine the exact localization of cytoplasmic SON. We performed experiments to determine whether cytoplasmic SON in t(8;21)-positive cells is localized in particular cytoplasmic organelles, such as the endoplasmic reticulum (ER), the Golgi, and lysosomes/endosomes. SON did not colocalize with any of these organelles (Fig. S8), indicating that cytoplasmic SON is not present as a component of the ER, the Golgi, or lysosomes/endosomes, and the SON protein detected in the cytoplasm does not merely represent a degraded form of SON. Interestingly, costaining of SON with a Golgi marker, GM130, showed that although sequestered SON protein is not exactly colocalized with the Golgi, it is adjacent to the Golgi. We found that the N-terminal fragment of SON (1–140 aa) is sufficient to localize at the nuclear speckles (Fig. 5A). Therefore, it is possible that the SON protein detected in cytoplasm has mutations at the N-terminal region, or is an isoform that does not contain nuclear localization signals. Because SON contains potential RNA-binding motifs at its C-terminal end, it is likely that SON is associated with RNAs in the cytoplasm. The function of nuclear/cytoplasmic SON is currently under investigation.
Taken together, our study demonstrates that the molecular events associated with disruption of normal function of NHR4 could be a secondary mutation that cooperates with AML1-ETO to promote leukemogenesis. Furthermore, we identified a NHR4-interacting partner SON, a protein with potential DNA-RNA dual binding ability, providing the a link between SON and a disease-causing factor. The interaction between NHR4 of AML1-ETO and SON may be one of the factors generating inhibitory effects on leukemia development by blocking cell proliferation. Abnormalities of SON localization in blood cells from t(8;21) AML patients were first demonstrated in our study, which reveals a novel drug target for leukemia. Further studies to elucidate the biological function of SON may provide valuable insight into the control of cell transformation and cancer development.
Methods
Cell Lines and AML Patient Samples.
Kasumi-1, SKNO-1, K562, U937, and U937T-AML1-ETO tet-inducible cell lines were grown in RPMI MEDIUM 1640, and 293T and HeLa cells in Dulbecco's modified Eagle's medium, supplemented with 10% (vol/vol) FBS. Blood samples were collected from patients with newly diagnosed AML at Aarhus University Hospital (Aarhus, Denmark). All sampling was performed as part of the diagnostic process and according to protocols approved by the ethical committee for the County of Aarhus.
Fetal Liver Cell Isolation, Retroviral Transduction, Transplantation, Hematological Analysis, and Flow Cytometry.
Fetal liver cells were harvest from E14.5 mouse embryos (MF-1), infected with retroviruses containing MigR1 empty vector, MigR1-AE-ΔNHR4, or MigR1-AE-C663S and transplanted into the recipient MF-1 mice. Procedures were performed as described previously (12, 13).
Transfection, Infection, and Nucleofection.
Transfection of 293T and HeLa cell was performed using Polyfect (Qiagen) as described previously (30). Infection of K562 and U937T cells was performed as described previously (31). Nucleofection of K562 cells was performed according to the manufacturer's protocol (Amaxa). Negative control siRNA (catalog no. 4621) and SON siRNA (catalog no. 16708, siRNA ID no. 143161, 143162, 143163) were purchased from Ambion and transfected into cells using Lipofectamine 2000 (Invitrogen) for adherent cells, and nucleofection for suspension cells.
Yeast Two Hybrid Screening.
Yeast two hybrid screening was performed by the TetR-based Two-hybrid system (Proteinlinks Inc.) to screen a cDNA library of the murine multipotential hematopoietic precursor cell line, EML (a gift from Dr. Schickwann Tsai, University of Utah, Salt Lake City, UT).
Production of SON Antibody.
Polyclonal antibody was produced in rabbits for human SON a.a. 2090–2107 (EEKVAKKSGGATIEELTE), and purified by affinity chromatography (Open Biosystems).
Further Details.
Additional information about the experimental methods may be found in SI Methods.
Supplementary Material
Acknowledgments.
We are grateful to Dr. N. Speck for her valuable suggestions on the manuscript. We thank Drs. S. Hiebert (Vanderbilt University) and B. Meyer (Brown University) for providing plasmids, Dr. S. Tsai (University of Utah) for the murine EML cell line cDNA library, and Kasusa DNA Research Institute, Kisarazu, Chica, Japan, for the KIAA1019 cDNA. We also thank Dr. J. Biggs for critical editing of the manuscript. This work was supported by National Institutes of Health grants CA096735 and CA104509 (to D.E.Z.), by a Lady TATA Memorial Trust Award fellowship (to E.Y.A.), and by grants from the the Danish Cancer Society and the Danish Medical Research Council (to P.H.). This is manuscript 18734 from The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.D.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802696105/DCSupplemental.
References
- 1.Rowley JD. Chromosome translocations: Dangerous liaisons revisited. Nat Rev Cancer. 2001;1:245–250. doi: 10.1038/35106108. [DOI] [PubMed] [Google Scholar]
- 2.Langabeer SE, et al. Incidence of AML1/ETO fusion transcripts in patients entered into the MRC AML trials. MRC Adult Leukaemia Working Party. Br J Haematol. 1997;99:925–928. doi: 10.1046/j.1365-2141.1997.4663270.x. [DOI] [PubMed] [Google Scholar]
- 3.Nucifora G, Rowley JD. AML1 and the 8;21 and 3;21 translocations in acute and chronic myeloid leukemia. Blood. 1995;86:1–14. [PubMed] [Google Scholar]
- 4.Rege K, et al. Disease features in acute myeloid leukemia with t(8;21)(q22;q22). Influence of age, secondary karyotype abnormalities, CD19 status, and extramedullary leukemia on survival. Leuk Lymphoma. 2000;40:67–77. doi: 10.3109/10428190009054882. [DOI] [PubMed] [Google Scholar]
- 5.Rowe D, et al. Cytogenetically cryptic AML1-ETO and CBF beta-MYH11 gene rearrangements: Incidence in 412 cases of acute myeloid leukaemia. Br J Haematol. 2000;111:1051–1056. doi: 10.1046/j.1365-2141.2000.02474.x. [DOI] [PubMed] [Google Scholar]
- 6.de Guzman CG, et al. Hematopoietic stem cell expansion and distinct myeloid developmental abnormalities in a murine model of the AML1-ETO translocation. Mol Cell Biol. 2002;22:5506–5517. doi: 10.1128/MCB.22.15.5506-5517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higuchi M, et al. Expression of a conditional AML1-ETO oncogene bypasses embryonic lethality and establishes a murine model of human t(8;21) acute myeloid leukemia. Cancer Cell. 2002;1:63–74. doi: 10.1016/s1535-6108(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 8.Mulloy JC, et al. Maintaining the self-renewal and differentiation potential of human CD34+ hematopoietic cells using a single genetic element. Blood. 2003;102:4369–4376. doi: 10.1182/blood-2003-05-1762. [DOI] [PubMed] [Google Scholar]
- 9.Rhoades KL, et al. Analysis of the role of AML1-ETO in leukemogenesis, using an inducible transgenic mouse model. Blood. 2000;96:2108–2115. [PubMed] [Google Scholar]
- 10.Schwieger M, et al. AML1-ETO inhibits maturation of multiple lymphohematopoietic lineages and induces myeloblast transformation in synergy with ICSBP deficiency. J Exp Med. 2002;196:1227–1240. doi: 10.1084/jem.20020824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan Y, et al. AML1-ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc Natl Acad Sci USA. 2001;98:10398–10403. doi: 10.1073/pnas.171321298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan M, et al. Deletion of an AML1-ETO C-terminal NcoR/SMRT-interacting region strongly induces leukemia development. Proc Natl Acad Sci USA. 2004;101:17186–17191. doi: 10.1073/pnas.0406702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan M, et al. A previously unidentified alternatively spliced isoform of t(8;21) transcript promotes leukemogenesis. Nat Med. 2006;12:945–949. doi: 10.1038/nm1443. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, et al. Structural basis for recognition of SMRT/N-CoR by the MYND domain and its contribution to AML1/ETO's activity. Cancer Cell. 2007;11:483–497. doi: 10.1016/j.ccr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutterbach B, Sun D, Schuetz J, Hiebert SW. The MYND motif is required for repression of basal transcription from the multidrug resistance 1 promoter by the t(8;21) fusion protein. Mol Cell Biol. 1998;18:3604–3611. doi: 10.1128/mcb.18.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berdichevskii FB, Chumakov IM, Kiselev LL. [Decoding of the primary structure of the son3 region in human genome: Identification of a new protein with unusual structure and homology with DNA-binding proteins] Mol Biol (Mosk) 1988;22:794–801. [PubMed] [Google Scholar]
- 17.Mattioni T, et al. A cDNA clone for a novel nuclear protein with DNA binding activity. Chromosoma. 1992;101:618–624. doi: 10.1007/BF00360539. [DOI] [PubMed] [Google Scholar]
- 18.Sun CT, et al. Transcription repression of human hepatitis B virus genes by negative regulatory element-binding protein/SON. J Biol Chem. 2001;276:24059–24067. doi: 10.1074/jbc.M101330200. [DOI] [PubMed] [Google Scholar]
- 19.Aravind L, Koonin EV. G-patch: A new conserved domain in eukaryotic RNA-processing proteins and type D retroviral polyproteins. Trends Biochem Sci. 1999;24:342–344. doi: 10.1016/s0968-0004(99)01437-1. [DOI] [PubMed] [Google Scholar]
- 20.Saunders LR, Barber GN. The dsRNA binding protein family: Critical roles, diverse cellular functions. FASEB J. 2003;17:961–983. doi: 10.1096/fj.02-0958rev. [DOI] [PubMed] [Google Scholar]
- 21.Saitoh N, et al. Proteomic analysis of interchromatin granule clusters. Mol Biol Cell. 2004;15:3876–3890. doi: 10.1091/mbc.E04-03-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lausen J, Cho S, Liu S, Werner MH. The nuclear receptor co-repressor (N-CoR) utilizes repression domains I and III for interaction and co-repression with ETO. J Biol Chem. 2004;279:49281–49288. doi: 10.1074/jbc.M407239200. [DOI] [PubMed] [Google Scholar]
- 23.Burel SA, et al. Dichotomy of AML1-ETO functions: Growth arrest versus block of differentiation. Mol Cell Biol. 2001;21:5577–5590. doi: 10.1128/MCB.21.16.5577-5590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn SL, et al. Organization and conservation of the GART/SON/DONSON locus in mouse and human genomes. Genomics. 2000;68:57–62. doi: 10.1006/geno.2000.6254. [DOI] [PubMed] [Google Scholar]
- 25.Chumakov IM, et al. [Identification of a protein product of a novel human gene SON and the biological effect upon administering a changed form of this gene into mammalian cells] Mol Biol (Mosk) 1991;25:731–739. [PubMed] [Google Scholar]
- 26.Greenhalf W, Lee J, Chaudhuri B. A selection system for human apoptosis inhibitors using yeast. Yeast. 1999;15:1307–1321. doi: 10.1002/(SICI)1097-0061(19990930)15:13<1307::AID-YEA455>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Lutterbach B, et al. ETO, a target of t(8;21) in acute leukemia, interacts with the N-CoR and mSin3 corepressors. Mol Cell Biol. 1998;18:7176–7184. doi: 10.1128/mcb.18.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, et al. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc Natl Acad Sci USA. 1998;95:10860–10865. doi: 10.1073/pnas.95.18.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, et al. The tetramer structure of the Nervy homology two domain, NHR2, is critical for AML1/ETO's activity. Cancer Cell. 2006;9:249–260. doi: 10.1016/j.ccr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Peterson LF, et al. The hematopoietic transcription factor AML1 (RUNX1) is negatively regulated by the cell cycle protein cyclin D3. Mol Cell Biol. 2005;25:10205–10219. doi: 10.1128/MCB.25.23.10205-10219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyapati A, et al. A leukemia fusion protein attenuates the spindle checkpoint and promotes aneuploidy. Blood. 2007;109:3963–3971. doi: 10.1182/blood-2006-09-045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.