Abstract
Urokinase-type plasminogen activator (uPA) is expressed at elevated levels in atherosclerotic human arteries, primarily in macrophages. Plasminogen (Plg), the primary physiologic substrate of uPA, is present at significant levels in blood and interstitial fluid. Both uPA and Plg have activities that could affect atherosclerosis progression. Moreover, correlations between increased Plg activation and accelerated atherosclerosis are reported in several human studies. However, a coherent picture of the role of the uPA/Plg system in atherogenesis has not yet emerged, with at least one animal study suggesting that Plg is atheroprotective. We used a transgenic mouse model of macrophage-targeted uPA overexpression in apolipoprotein E-deficient mice to investigate the roles of uPA and Plg in atherosclerosis. We found that macrophage-expressed uPA accelerated atherosclerotic plaque growth and promoted aortic root dilation through Plg-dependent pathways. These pathways appeared to affect lesion progression rather than initiation and to include actions that disproportionately increase lipid accumulation in the artery wall. In addition, loss of Plg was protective against atherosclerosis both in the presence and absence of uPA overexpression. Transgenic mice with macrophage-targeted uPA overexpression reveal atherogenic roles for both uPA and Plg and are a useful experimental setting for investigating the molecular mechanisms that underlie clinically established relationships between uPA expression, Plg activation, and atherosclerosis progression.
Keywords: proteolysis, aorta, aneurysm
The urokinase-type plasminogen activator (uPA)/plasminogen (Plg) system mediates numerous important biological processes including fibrinolysis, ECM and growth factor metabolism, cell migration, and inflammation (1, 2). Because all of these processes affect the development of atherosclerosis and because the critical components of the uPA/Plg system [i.e., uPA, the uPA receptor (uPAR), and Plg] are present in the artery wall, several groups have investigated the role of the uPA/Plg system in atherogenesis (1, 3). Despite much work, a coherent picture of this role has not yet emerged.
Human correlational studies support an atherogenic role for the uPA/Plg system. Both uPA (expressed predominantly by lesion macrophages; ref. 4) and uPAR (expressed by multiple cell types; ref. 5) are present at increased levels in atherosclerotic versus nondiseased human arteries, and expression levels of both molecules correlated directly with disease severity (6, 7). Plg is present in plasma and interstitial fluid at concentrations that would allow efficient plasmin generation by uPA bound to uPAR in the artery wall (8, 9). Moreover, increased plasmin generation in humans (detected as circulating plasmin-α2-antiplasmin complexes) predicted myocardial infarction in two large clinical studies (10, 11) and was associated with increased subclinical atherosclerosis in a third study (12). Data from in vitro systems and animal models also suggest that Plg is atherogenic. Plasmin(ogen) facilitated macrophage migration (13), modified LDL particles to increase both their complement-activating capacity and uptake by macrophages (14), enhanced release of inflammatory mediators, and stimulated platelet degranulation and monocyte chemotaxis (2).
Because these data support atherogenic roles for the uPA/Plg system, it was surprising that (i) mice deficient in both uPA and apolipoprotein E (Plau−/− Apoe−/− mice) had the same atherosclerotic plaque area as Plau+/+ Apoe−/− mice (15); and ii) Plg−/− Apoe−/− mice had more atherosclerosis than Plg+/+ Apoe−/− mice (16). However, it is not clear that mice with systemic deletions of uPA or Plg are—by themselves—optimal experimental settings for investigating whether macrophage-expressed uPA in human plaques contributes to the progression of atherosclerosis. To more directly investigate the role of uPA expression by plaque macrophages, we generated transgenic mice with macrophage-targeted overexpression of uPA and bred the “SR-uPA” (scavenger receptor-driven uPA) transgene into the Apoe−/− background. SR-uPA Apoe−/− mice had elevated aortic uPA activity and significant two- to threefold increases in aortic atherosclerosis (17). This result supported an atherogenic role for uPA but did not identify the atherogenic mechanisms. Specifically, a role for Plg in SR-uPA-accelerated atherosclerosis remained conjectural because macrophage overexpression of uPA could have unanticipated, Plg-independent effects including proteolysis of a nonphysiologic substrate, cleavage of a physiologic non-Plg substrate, or nonproteolytic Plg-independent effects including those mediated by binding to uPAR (18). The latter two activities are particularly plausible roles for uPA because the related molecule tissue plasminogen activator (tPA) cleaves a non-Plg substrate and has important nonproteolytic, Plg-independent effects in the brain (19, 20).
To investigate the mechanisms of SR-uPA-accelerated atherosclerosis and determine whether uPA increases atherosclerosis by activation of Plg, we compared atherosclerosis in SR-uPA and nontransgenic Apoe−/− littermates, both deficient in Plg. Next, to further unmask effects of elevated artery wall uPA expression, we bred the SR-uPA transgene into Apoe−/− mice that lack the uPA inhibitor plasminogen activator inhibitor-1 (PAI-1) and compared atherosclerosis in SR-uPA versus nontransgenic PAI-1 KO (i.e., Serpine1−/−) mice. Finally, because our data showed that uPA-accelerated atherosclerosis required Plg, we tested whether absence of Plg retarded atherosclerosis.
Results
SR-uPA+/0 Macrophages Have Increased uPA Expression.
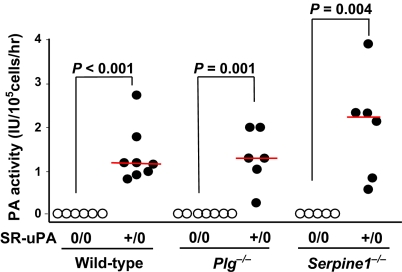
Because documentation of SR-uPA transgene expression is essential to the interpretation of experiments with SR-uPA+/0 mice, we tested whether elevated macrophage PA activity in SR-uPA+/0 Apoe−/− mice (17) persisted in the Plg−/− and Serpine1−/− backgrounds (all mice in this study were Apoe−/−; therefore, we use the term “WT” for Apoe−/− Plg+/+ Serpine1+/+ Plau+/+ mice). We first verified the specificity of the plasminogen activator (PA) activity assay by measuring PA activity in medium conditioned by macrophages of Plau−/− mice. No PA activity was detectable (n = 4; data not shown). In contrast, SR-uPA+/0 macrophages from both Plg−/− mice and Serpine1−/− mice had substantial PA activity, significantly greater than nontransgenic Plg−/− and Serpine1−/− macrophages (P ≤ 0.004; Fig. 1). The PA activity of SR-uPA+/0 Plg−/− and SR-uPA+/0 Serpine1−/− macrophages did not differ significantly from PA activity of SR-uPA+/0 WT macrophages (P ≥ 0.2; Fig. 1).
Fig. 1.
Macrophage Plg activator (PA) activity. Bone marrow-derived macrophages were cultured from wild-type, Plg KO (Plg−/−), or PAI-1 KO (Serpine1−/−) mice. Mice were either hemizygous for the SR-uPA transgene (+/0) or nontransgenic (0/0); all mice were Plau+/+. Culture media were assayed for PA activity using Plg and S-2251. Dots represent macrophages from a single mouse; bars are group medians.
Elevated uPA Activity in SR-uPA+/0 Aortae.
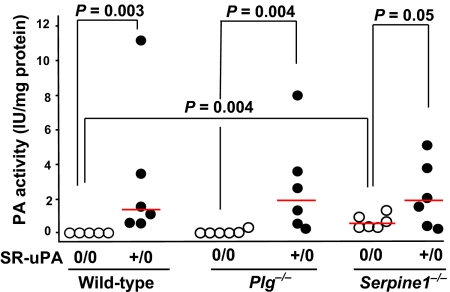
Essentially no PA activity was secreted by explanted aortae of nontransgenic WT, Plg−/−, or Plau−/− mice (Fig. 2 and data not shown). However, medium conditioned by aortae of nontransgenic Serpine1−/− mice contained increased PA activity (P = 0.004 versus nontransgenic WT aortae; Fig. 2). Medium conditioned by aortae of SR-uPA+/0 mice from WT, Plg−/−, and Serpine1−/− backgrounds had elevated PA activity (Fig. 2). Therefore, absence of PAI-1 in nontransgenic Apoe−/− mice is sufficient to modestly increase aortic PA activity. The SR-uPA transgene increased secreted aortic PA activity substantially (and to similar levels) in WT, Plg−/−, and Serpine1−/− mice.
Fig. 2.
Aortic Plg activator (PA) activity. Thoracic aortae of wild-type, Plg KO (Plg−/−), or PAI-1 KO (Serpine1−/−) mice were placed in explant culture. Mice were either hemizygous for the SR-uPA transgene (+/0) or nontransgenic (0/0); all mice were Plau+/+. Culture media were assayed for PA activity using Plg and S-2251. Dots represent individual aortae; bars are group medians.
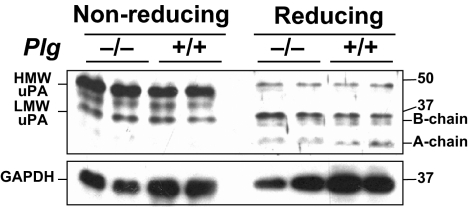
Because the aortic PA activity assay is performed with added Plg, plasmin generated during the assay would convert inactive single-chain uPA (sc-uPA) to active two-chain uPA (tc-uPA) (8). Therefore, this assay does not differentiate sc-uPA from tc-uPA, leaving the possibility that SR-uPA+/0 Plg−/− aortae contain primarily sc-uPA, not tc-uPA. If this were true, then any loss of SR-uPA+/0 mouse phenotypes in the Plg−/− background could be because of absence of tc-uPA rather than absence of Plg. To address this, we performed western analysis of nonreduced and reduced extracts of SR-uPA+/0 Plg−/− and SR-uPA+/0 Plg+/+ aortae. Total uPA protein and tc-uPA levels were equivalent in all aortae (Fig. 3). Therefore, breeding the SR-uPA transgene into Plg−/− mice does not affect the abundance of aortic tc-uPA.
Fig. 3.
Aortic uPA expression. Western analysis was performed on extracts of aortae from SR-uPA+/0 Plg−/− and SR-uPA+/0 Plg+/+ mice (all Plau+/+). (Upper Left lanes) Aortic extracts were analyzed under nonreducing conditions. Major bands correspond to high molecular weight (HMW) and low molecular weight (LMW) uPA. (Upper Right lanes) The same extracts analyzed under reducing conditions. The dissociated B and A chains of two-chain uPA are indicated. The blot was stripped and reprobed to detect GAPDH (Lower). Size markers are in kilodaltons. Analysis of three other aortae from each group revealed similar results.
Lack of Systemic Effects of the SR-uPA Transgene.
Peripheral blood monocyte counts, percentage of monocytes, plasma triglycerides, plasma cholesterol, and body weight did not differ significantly between SR-uPA+/0 Plg−/− or SR-uPA+/0 Serpine1−/− mice and their nontransgenic controls (Table 1). In a separate cohort of SR-uPA+/0 and nontransgenic Plg+/+ Serpine1+/+ mice (see below) the SR-uPA transgene did not affect serum glucose or other markers of liver and kidney function (Table 2).
Table 1.
SR-uPA transgene effects in Serpine1−/− and Plg−/− mice (all Plau+/+)
|
Serpine1−/− |
Plg−/− |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SR-uPA+/0 | (n) | SR-uPA0/0 | (n) | P | SR-uPA+/0 | (n) | SR-uPA0/0 | (n) | P | |
| Peripheral blood monocytes, cells/mm3 | 52 (32–68) | (7) | 28 (0–67) | (5) | 0.3 | 130 (55–350) | (7) | 220 (140–430) | (5) | 0.4 |
| Monocytes, % of total leukocytes | 2 (2–3) | (7) | 1 (0–2) | (5) | 0.3 | 3 (1–6) | (7) | 8 (5–12) | (5) | 0.07 |
| Plasma cholesterol, mg/dl | 840 ± 120 | (9) | 740 ± 140 | (9) | 0.1 | 830 ± 130 | (5) | 760 ± 150 | (6) | 0.5 |
| Plasma triglycerides, mg/dl | 123 ± 44 | (9) | 118 ± 33 | (9) | 0.8 | 52 (48–99) | (5) | 96 (74–180) | (6) | 0.2 |
| Total pinned aortic surface area, mm2 | 80 ± 6.3 | (9) | 80 ± 7.2 | (11) | 1.0 | 72 ± 5.5 | (11) | 74 ± 4.1 | (8) | 0.3 |
| Aortic root ORO positive area, mm2 | 0.53 ± 0.17 | (9) | 0.17 ± 0.10 | (9) | <0.001 | 0.18 ± 0.08 | (9) | 0.14 ± 0.08 | (7) | 0.4 |
| Aortic root ORO positive area, % of intimal lesion area | 69 ± 4.4 | (9) | 59 ± 7.0 | (9) | 0.001 | 60 ± 13 | (9) | 58 ± 5.3 | (7) | 0.6 |
| Aortic root MOMA-2 positive area, mm2 | 0.20 ± 0.13 | (8) | 0.066 ± 0.02 | (9) | 0.005 | 0.073 ± 0.01 | (9) | 0.069 ± 0.012 | (6) | 0.6 |
| Aortic root MOMA-2 positive area, % of intimal lesion area | 19 ± 9.8 | (8) | 22 ± 7.2 | (9) | 0.6 | 17 ± 3.9 | (9) | 21 ± 5.4 | (6) | 0.1 |
| Aortic root IEL circumference, mm | 6.5 ± 0.56 | (8) | 4.6 ± 0.43 | (9) | <0.001 | 4.8 ± 0.5 | (10) | 4.4 ± 0.33 | (6) | 0.1 |
| Maximal coronary artery stenosis, % | 27 (0–66) | (12) | 0 (0–19) | (17) | 0.07 | 0 (0–5.9) | (15) | 0 (–0) | (14) | 0.9 |
| Body weight, g | 21 ± 3.0 | (8) | 22 ± 3.5 | (10) | 0.7 | 20 ± 2.2 | (11) | 21 ± 0.9 | (9) | 0.2 |
Values are reported as mean ± SD or median (25–75%) range. (n) = number of mice for all except coronary stenosis, for which (n) = number of arteries. ORO = Oil red O; IEL = internal elastic lamina.
Table 2.
Serum chemistries in SR-uPA mice and nontransgenic littermates (all Plg+/+ Serpine1+/+ Plau+/+)
| SR-uPA+/0 | SR-uPA0/0 | P | |
|---|---|---|---|
| Cholesterol, mg/dl | 1190 ± 220 | 1290 ± 370 | 0.7 |
| Blood urea nitrogen, mg/dl | 20.0 ± 4.5 | 22.0 ± 4.0 | 0.5 |
| Creatinine, mg/dl | 0.40 (0.38–0.58) | 0.40 (0.40–0.40) | 0.7 |
| Glucose, mg/dl | 310 ± 150 | 320 ± 100 | 0.9 |
| AST, U/L | 109 ± 27 | 106 ± 14 | 0.8 |
| Alkaline phosphatase, U/L | 170 ± 102 | 196 ± 96 | 0.7 |
| Total protein, g/dl | 5.0 ± 0.5 | 4.5 ± 0.4 | 0.2 |
| Albumin, g/dl | 2.6 ± 0.3 | 2.3 ± 0.2 | 0.1 |
| Calcium, mg/dl | 8.8 (6.1–8.9) | 9.0 (4.8–9.2) | 0.6 |
| Phosphorus, mg/dl | 7.5 ± 1.1 | 7.5 ± 1.1 | 1.0 |
| Magnesium, mEq/L | 1.4 ± 1.1 | 1.8 ± 1.0 | 0.6 |
Values are reported as mean ± SD or median (25–75%) range of 4 SR-uPA+/0 and 5 SR-uPA0/0 mice.
The SR-uPA Transgene Does Not Accelerate Atherosclerosis in Plg−/− Mice.
We reported previously that the SR-uPA transgene increased aortic atherosclerosis, promoted lesion lipid and macrophage accumulation and increased coronary artery stenoses in Plg+/+ mice (17). In contrast, in the present study the SR-uPA transgene did not affect aortic root intimal lesion area, percent aortic surface lesion area, or median coronary stenosis in Plg−/− mice (P ≥ 0.2; Figs. 4 and 5, Table 1). Similarly, neither lipid content (total or percentage oil red O-positive area) nor macrophage content (total or percentage MOMA-2 positive area) differed between SR-uPA+/0 Plg−/− and nontransgenic Plg−/− mice (P ≥ 0.1 for all; Table 1).
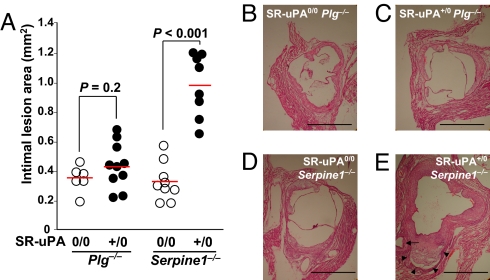
Fig. 4.
Aortic root lesions. Aortic root sections from Plg KO (Plg−/−), or PAI-1 KO (Serpine1−/−) mice were sectioned and intimal lesion areas were measured. Mice were either hemizygous for the SR-uPA transgene (+/0) or nontransgenic (0/0); all mice were Plau+/+. (A) Mean intimal lesion area. Dots represent values for individual mice; bars are group means. (B–E) Sections from the 4 genotypes. (E) Arrow indicates plaque in coronary artery; arrowheads indicate area of medial destruction and outward remodeling; H&E stain. (Scale bars, 1 mm.)
Fig. 5.
Aortic surface lesions. Aortae from Plg KO (Plg−/−) or PAI-1 KO (Serpine1−/−) mice were pinned and stained with Sudan IV. Mice were either hemizygous for the SR-uPA transgene (+/0) or nontransgenic (0/0) all mice were Plau+/+. (A) Percent aortic surface area stained with Sudan IV. Dots represent values for individual mice; bars are group means (Plg−/−) or medians (Serpine1−/−). (B–E) Aortae from the four genotypes.
The SR-uPA Transgene Accelerates Atherosclerosis and Increases Plaque Lipid in Serpine1−/− Mice.
Aortic root intimal area and percent aortic surface lesion area were both significantly higher in SR-uPA+/0 Serpine1−/− mice than in nontransgenic Serpine1−/− controls (P < 0.001 and P = 0.03, respectively; Figs. 4 and 5). Aortic root lesions in SR-uPA+/0 Serpine1−/− mice had more lipid as well as a significantly higher percentage of lipid than lesions in nontransgenic Serpine1−/− mice (P ≤ 0.001; Table 1). There was a trend toward more severe coronary stenoses in SR-uPA+/0 Serpine1−/− mice versus nontransgenic Serpine1−/− mice [27 (0–66) versus 0 (0–19)%; P = 0.07; Table 1].
The SR-uPA Transgene Causes Aortic Medial Destruction and Aortic Root Dilation in Serpine1−/− but Not Plg−/− Mice.
Aortic medial wall destruction (assessed by loss of medial smooth muscle cells and ECM on Movat-stained sections) appeared more severe in SR-uPA+/0 Serpine1−/− mice than nontransgenic Serpine1−/− mice. To test this objectively, a blinded observer was given a single Movat-stained aortic root section from each of 12 Serpine1−/− mice (6 SR-uPA+/0 and 6 nontransgenic) and asked to assign a genotype (SR-uPA+/0 or nontransgenic) based only on the extent of medial destruction. All 12 mice were correctly genotyped (P = 0.002). In contrast, when the same observer was given sections from 12 Plg−/− mice (6 SR-uPA+/0 and 6 nontransgenic), the observer was unable to genotype any of the mice with confidence because severe medial destruction was not seen. Similarly, the SR-uPA transgene substantially increased aortic root IEL circumference in Serpine1−/− mice but not in Plg−/− mice (41% increase; P < 0.001; Table 1 and Fig. 4E).
uPA-Generated Plasmin Accelerates Atherosclerosis and Plg Is Atherogenic.
SR-uPA Plg−/− mice did not have accelerated atherosclerosis (Figs. 4 and 5 and Table 1). These results, compared with our previous finding of accelerated atherosclerosis in SR-uPA+/0 Plg+/+ mice (17), suggested that generation of plasmin by tc-uPA is atherogenic. A finding that plasmin is atherogenic would conflict with a report of accelerated aortic atherosclerosis in nontransgenic Apoe−/− mice deficient in Plg (16). We therefore set up an experiment both to confirm that accelerated atherosclerosis in SR-uPA mice depends on Plg and to examine the role of Plg in atherosclerosis. For this experiment, we bred SR-uPA+/0 and nontransgenic Plg+/− mice to generate littermates with four experimental genotypes: SR-uPA+/0 and nontransgenic mice that were either Plg−/− or Plg+/+. Among these 4 genotypes, lipid values were similar between SR-uPA and nontransgenic mice (Table 3) but aortic root atherosclerosis was increased in SR-uPA+/0 Plg+/+ mice (Fig. 6A; P < 0.001 by t test versus both nontransgenic Plg+/+ mice and SR-uPA+/0 Plg−/− mice). Lesions in SR-uPA+/0 Plg+/+ mice were histologically similar to lesions in the other 3 groups, with macrophage and collagen area increased in proportion to the increase in total lesion size (Table 3). Smooth muscle cells contributed only a small percentage of lesion area (well below 5%) in all 4 groups (data not shown). Aortic root IEL circumference was also greater in SR-uPA+/0 Plg+/+ mice than in nontransgenic Plg+/+ mice (P = 0.001; Table 3). As before (Figs. 4 and 5, Table 1), the SR-uPA transgene did not accelerate aortic root atherosclerosis or cause aortic root dilation in Plg−/− mice (Fig. 6A, Table 3). Comparison of nontransgenic Plg+/+ and Plg−/− mice revealed that Plg−/− mice had smaller aortic root lesions (25% decrease; P = 0.04; Fig. 6A). Two-way ANOVA of the aortic root lesion data confirmed both that absence of Plg decreased atherosclerosis irrespective of transgene status (P < 1 × 10−9) and that the atherogenicity of the SR-uPA transgene required Plg (P < 0.001). Analysis of Sudan-IV-stained aortae showed that SR-uPA+/0 Plg+/+ and SR-uPA+/0 Plg−/− mice had similar percentages of aortic lesion area (Fig. 6B). However, among the nontransgenic mice, Plg−/− mice had less atherosclerosis than Plg+/+ mice (45% decrease; P = 0.04; Fig. 6B). Two-way ANOVA of the whole aorta data confirmed both an overall effect of Plg genotype on atherosclerosis (less atherosclerosis in Plg−/− mice; P = 0.03) and a significant decrease in atherosclerosis in nontransgenic Plg−/− versus Plg+/+ mice (P = 0.02).
Table 3.
SR-uPA Transgene Effects in Wild-type and Plg KO Mice (all Plau+/+)
|
Plg+/+ |
Plg−/− |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SR-uPA+/0 | (n) | SR-uPA0/0 | (n) | P | SR-uPA+/0 | (n) | SR-uPA0/0 | (n) | P | |
| Plasma cholesterol, mg/dl | 1090 ± 260 | (9) | 1170 ± 220 | (10) | 0.5 | 820 ± 290 | (6) | 970 ± 170 | (6) | 0.3 |
| Plasma triglycerides, mg/dl | 55 (38–74) | (9) | 44 (40–59) | (10) | 0.9 | 59 ± 27 | (6) | 49 ± 20 | (5) | 0.5 |
| Aortic root IEL circumference, mm | 6.4 ± 0.3 | (10) | 5.6 ± 0.5 | (9) | 0.001 | 5.2 ± 0.5 | (6) | 5.2 ± 0.2 | (7) | 1.0 |
| Aortic root Mac-2 area, mm2 | 0.26 ± 0.12 | (10) | 0.13 ± 0.075 | (8) | 0.02 | 0.10 ± 0.061 | (6) | 0.11 ± 0.05 | (6) | 0.7 |
| Aortic root Mac-2 area, % of intimal lesion area | 33 ± 12 | (10) | 31 ± 13 | (8) | 0.8 | 40 ± 15 | (6) | 30 ± 7.8 | (6) | 0.2 |
| Aortic root collagen, mm2 × 10−2 | 6.0 ± 0.68 | (10) | 3.3 ± 0.71 | (9) | 0.02 | 2.4 ± 0.96 | (5) | 2.7 ± 0.81 | (7) | 0.8 |
| Aortic root collagen, % of intimal lesion area | 5.8 ± 0.94 | (10) | 5.7 ± 1.0 | (9) | 0.5 | 6.0 ± 1.3 | (5) | 4.9 ± 1.1 | (7) | 0.6 |
| Whole aorta surface area, mm2 | 71 ± 5 | (12) | 74 ± 6 | (10) | 0.2 | 71 ± 8 | (5) | 69 ± 6 | (7) | 0.8 |
| Body weight, g | 22 (20–23) | (15) | 24 (22–24) | (15) | 0.02 | 21 ± 3.4 | (8) | 22 ± 2.6 | (7) | 0.5 |
Values are reported as mean ± SD or median (25–75%) range. (n) = number of mice. IEL = internal elastic lamina.
Fig. 6.
Four-genotype atherosclerosis study. Atherosclerosis was measured in aortic roots (A) and pinned aortae (B) of littermates generated by mating SR-uPA+/0 and nontransgenic Plg+/− mice; all mice were Plau+/+. Dots represent individual mice; bars are group means except for Plg−/− mice in B, for which bars are medians. P values are for t tests; for 2-way ANOVA results, see text.
Discussion
To begin to elucidate the mechanisms through which macrophage-expressed uPA accelerates atherosclerosis, we generated Plg−/− and Serpine1−/− mice with macrophage-targeted uPA expression and compared atherosclerosis in these mice and their nontransgenic littermates. Our major findings were i) Plg is not required for conversion of sc-uPA to tc-uPA in the artery wall; ii) absence of PAI-1 in Serpine1−/− mice increases aortic PA activity; iii) in Serpine1−/− mice, macrophage-expressed uPA accelerates atherosclerosis, causes aneurysmal aortic root dilation, and increases the percentage of lesion lipid; iv) in Plg−/− mice macrophage-expressed uPA has no effect on atherosclerosis, aortic root circumference, or lesion histology; and v) loss of Plg is atheroprotective. These data strongly support an atherogenic role for the uPA/Plg system.
uPA has two well-described physiologic roles: activation of Plg and binding to uPAR (8). The experiments in Plg−/− mice show conclusively that Plg is required both for the atherogenicity of macrophage-expressed uPA and for the destructive effect of uPA on the aortic root. Because both uPA (18, 21) and tPA (22) have non-Plg substrates and nonproteolytic activities and because a transgenic overexpression model such as the SR-uPA mouse could acquire a phenotype by activation of a nonphysiologic substrate or by a random effect of transgene insertion, Plg dependence of the SR-uPA phenotypes could not be assumed. Here we show unambiguously that uPA is not acting through a non-Plg substrate and that the vascular phenotypes of SR-uPA mice are caused by enzymatic activity of uPA. Because SR-uPA+/0 Plg−/− mice have uPAR but do not have uPA-mediated phenotypes, our data eliminate uPAR as the sole mediator of uPA activity in SR-uPA mice. This is significant because uPAR is expressed on macrophages and uPA-uPAR interactions could contribute to atherogenesis (23). We cannot yet eliminate the possibility that uPAR contributes to the SR-uPA phenotype through its ability to enhance cell surface Plg activation by uPA (9). Exploration of this possibility in Apoe−/− mice that also lack uPAR was not possible because uPAR and apo E are linked, precluding generation of doubly deficient mice (data not shown).
There are many reasons why we expected that increased activity of the uPA/Plg system would be atherogenic, including the roles of uPA and Plg in cell migration and inflammation, the Plg dependence of neointimal formation and transplant arteriosclerosis in animal models, and clinical studies (2, 10–13, 24, 25). Therefore, our finding that uPA accelerates atherosclerosis in a Plg-dependent manner fits with a large body of data. However, our results conflict with two closely related studies that show unaltered plaque size in Plau−/− mice (15) and increased atherosclerosis in Plg−/− mice (16). We suggest several possible explanations for these divergent results. The study in Plau−/− mice used a highly inflammatory cholate-containing diet, which may be an insensitive setting in which to test an intervention (i.e., loss of uPA) that decreases atherosclerosis. Also, germ-line deletion of uPA could prompt compensatory changes that prevent appearance of uPA-deficient phenotypes. Because this previous study focused primarily on pseudoaneurysm formation and data on plaque size were “not shown,” further comparison is not possible. Differences between our study and the report on Plg−/− mice include use of mice that were not specific-pathogen free (J. Degen, personal communication) and lesion analysis at a more advanced age (18–25 vs. 15 wks in our study). Because Plg−/− mice develop a wasting disease as they age, use of older mice might introduce systemic disease as a confounder. In addition, 20-wk aortic root lesion areas in this previous study (median 1 × 103 and 8 × 103 μm2 for Plg+/+ and Plg−/− mice, respectively; ref. 16) were only 1/100th as large as the lesions in our 15-wk-old mice (Figs. 4 and 6 and ref. 17), suggesting that lesions were measured at different locations along the ascending aorta. Perhaps more importantly, both the Plau−/− and Plg−/− studies were carried out in mixed genetic backgrounds and are therefore challenging to reproduce. Our studies were performed in extensively backcrossed C57BL/6 mice and are therefore easily replicated. In fact, we have now reproduced the central finding of significantly increased atherosclerosis in SR-uPA mice in four independent experiments (ref. 17, Figs. 4 and 6A, and data not shown), and Plg dependence was shown twice (Figs. 4 and 6). The atherogenicity of the uPA/Plg system seems well proven by these data and the most fruitful next experiments are likely to be those that elucidate the mechanisms of plasmin-accelerated atherosclerosis.
Separate analyses of aortic roots and whole aortae in our study provide a clue to the mechanisms through which the uPA/Plg system causes vascular disease. Both in this study and our original report (17), the fold increase in atherosclerosis was greater in SR-uPA aortic roots than on pinned aortae (compare Figs. 4 and 5). Moreover, SR-uPA mice in the 4-genotype study (Fig. 6) had significantly increased atherosclerosis only in their aortic roots and aortic dilation in SR-uPA mice has always been limited to the root (ref. 17 and Tables 1 and 3). These observations suggest that uPA/Plg accelerates lesion progression (measurable in larger, more established aortic root lesions) but has little or no effect on lesion initiation. If we are able to bypass the sudden death phenotype in SR-uPA mice (17) and measure aortic lesions at later time points, we anticipate finding a more pronounced effect of uPA overexpression on lesions along the entire aortic surface.
Others have attempted to draw a coherent picture of the uPA/Plg system and atherosclerosis that also accounts for the role of PA1–1 (3). This is a challenge because PAI-1 KO mice have had either the same, less, or more atherosclerosis than PAI-1-expressing controls (reviewed in ref. 3). Recently we showed that PAI-1 has important vascular effects (including on cell migration and regulation of TGF-β1) that are independent of Plg (26). Therefore, we do not attempt to reconcile our data on uPA/Plg with others' data on atherosclerosis in PAI-1 KO mice. Nevertheless, we used PAI-1 KO mice in the present study to help elucidate the mechanisms of SR-uPA-accelerated atherosclerosis. Because PAI-1 mediates the internalization of the uPA/uPAR complex by LDL receptor-related protein (LRP) and loss of LRP causes accelerated atherosclerosis and aneurysm formation (27), it was possible that PAI-1 and LRP mediated the SR-uPA phenotypes. However, our finding of accelerated atherosclerosis and aortic root dilation in SR-uPA Serpine1−/− mice excludes a role for PAI-1 in these phenotypes. Two other results in SR-uPA Serpine1−/− mice are noteworthy. First, SR-uPA Serpine1−/− mice had an increased percentage of lesion lipid compared to nontransgenic Serpine1−/− mice (Table 1). Second, SR-uPA Serpine1−/− mice had a greater % increase in aortic root dilation compared to littermate nontransgenic mice than did SR-uPA Serpine1+/+ mice [41% (Table 1) vs. 10–14% (Table 3 and ref. 17)]. The former result suggests that plasmin may increase lipid accumulation either in macrophages or ECM; the latter result shows that an important physiologic role of PAI-1 is to limit uPA/Plg-mediated proteolysis of the artery wall that leads to aneurysm formation.
In summary, mice with macrophage-targeted uPA expression reveal roles for both uPA and Plg in atherogenesis and provide a useful experimental setting for investigations of mechanisms that explain clinical correlations between uPA, Plg activation, and human vascular disease (4, 10–12).
Methods
An expanded Methods is available in supporting information (SI) Methods.
Animals.
Experimental and control mice were all Apoe−/−. Apoe−/− mice with macrophage-targeted overexpression of uPA (SR-uPA+/0 mice) were generated in our laboratory (17). Apoe+/+ mice deficient in plasminogen (Plg−/−) or PAI-1 (Serpine1−/−) were purchased (The Jackson Laboratory) and bred with Apoe−/− mice (either SR-uPA+/0 or nontransgenic) to yield both SR-uPA+/0 and nontransgenic mice that were either Plg−/− Apoe−/− or Serpine1−/− Apoe−/−. Apoe+/+ mice deficient in uPA (Plau−/−) were purchased and bred with Apoe−/− mice to generate Plau−/− Apoe−/− mice. Plau−/− mice were used only as a source of Plau−/− macrophages and aortae for PA activity studies; atherosclerosis studies were performed only with Plau+/+ mice (SR-uPA+/0 or nontransgenic). SR-uPA+/0 and nontransgenic Plg+/− Apoe−/− mice were bred to produce littermates with four experimental genotypes: SR-uPA0/0 Plg+/+, SR-uPA+/0 Plg+/+, SR-uPA0/0 Plg−/−, and SR-uPA+/0 Plg−/−. All experimental mice were progeny of C57BL/6 backcrosses for at least 11 generations. Mice were genotyped by Southern blotting or PCR. All animal protocols were approved by the University of Washington Office of Animal Welfare.
Experimental Design.
Mice were fed an atherogenic diet (21% fat and 0.15% cholesterol) starting at 5 wks of age and were killed at 15 wks of age. Only female mice were used for atherosclerosis studies, plasma lipid analyses, and serum chemistries. Both genders were used for uPA expression studies and peripheral blood cell counts.
Tissue Harvest and Processing.
Aortic roots were processed into OCT medium and aortae were pinned and stained as described (17).
Histology.
Aortic root sections were stained with H&E, Movat pentachrome, oil red O, picrosirius red, and the macrophage-specific antibodies MOMA-2 (Biosource) and Mac-2 (Cedarlane Labs) (17). For each stain, a total of five to seven sections per mouse (at 56 μm steps) were analyzed.
Lesion Characterization.
Digital images of sections and pinned aortae were analyzed by blinded observers using image analysis software. Atherosclerosis in aortic root cross-sections was quantified by measuring the total intimal area. Oil red O-, MOMA-2-, Mac-2-, and picrosirius red-positive areas (total and percentage of lesion area) were measured using color thresholding and planimetry. Total and percentage of Sudan IV-positive aortic surface area were measured similarly. Aortic root circumference were measured at the level of the internal elastic lamina (IEL) (17).
Quantification of Coronary Artery Stenosis.
Percent luminal stenoses of proximal coronary artery segments were calculated using measurements made on oil red O-stained sections (17).
Macrophage Plg Activator Activity.
We used bone marrow-derived macrophages to measure SR-uPA transgene expression. Marrow was harvested from femurs of fat-fed mice and adherent cells were cultured in GM-CSF-containing medium (28). On day 10, serum-free conditioned medium was collected and the macrophages were counted. Plg activator (PA) activity in the medium was measured with Plg and the plasmin substrate S-2251 (17).
Aortic Plg Activator Activity.
Thoracic aortae from the same mice used for the macrophage PA activity assay were incubated in M199 and PA activity in the conditioned media was measured with Plg and S-2251, as above.
Western Analysis of Aortic uPA.
SR-uPA+/0 mice (either Plg+/+ or Plg−/−; n = 5 each) were perfused with saline and whole aortae excised. Protein was extracted (29) and separated by SDS/PAGE under both nonreducing and reducing conditions. uPA and GAPDH were detected by Western blot.
Plasma Lipids.
Retro-orbital blood was drawn after a 4-hr fast for measurement of total cholesterol and triglycerides (17).
Peripheral Blood Counts and Serum Chemistries.
Peripheral blood samples from 15-wk-old (blood counts) or 8-wk-old (serum chemistries) fat-fed mice were analyzed by an outside laboratory (Phoenix Central Laboratory).
Statistical Methods.
Data are mean ± SEM or median (25–75% range). A priori hypotheses were tested with the unpaired t test or by Mann–Whitney rank-sum test for non-normally distributed data. Two-way ANOVA on ranks was used to discern separate as well as interdependent effects of the SR-uPA transgene and Plg on atherosclerosis. Fisher's exact test was used to assess accuracy of genotyping based on assessment of medial destruction.
Supplementary Material
Acknowledgments.
We thank Katharine Liang, Amy Buben, Talyn Chu, Steven Farris, and Mia Jaffe for technical assistance; Margo Weiss for help with manuscript preparation; Jay Degen for helpful discussions, and Bill Parks for insightful suggestions. This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL080597 (to D.A.D.), T32 HL07828 (to M.K. and R.K.), and K08 HL070941 (to A.S-O.). D.A.D. was also supported by the John L. Locke, Jr. Charitable Trust.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808650105/DCSupplemental.
References
- 1.Plow EF, Hoover-Plow J. The functions of plasminogen in cardiovascular disease. Trends Cardiovasc Med. 2004;14:180–186. doi: 10.1016/j.tcm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Syrovets T, Simmet T. Novel aspects and new roles for the serine protease plasmin. Cell Mol Life Sci. 2004;61:873–885. doi: 10.1007/s00018-003-3348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fay WP, Garg N, Sunkar M. Vascular functions of the plasminogen activation system. Arterioscler Thromb Vasc Biol. 2007;27:1231–1237. doi: 10.1161/ATVBAHA.107.140046. [DOI] [PubMed] [Google Scholar]
- 4.Falkenberg M, Björnheden T, Lindnér P, Risberg B. Co-localization of fibrinolytic activators and inhibitors with macrophages in atherosclerotic vessels. Cardiovasc Pathol. 1998;7:223–231. doi: 10.1016/s1054-8807(97)00114-2. [DOI] [PubMed] [Google Scholar]
- 5.Raghunath PN, et al. Plasminogen activator system in human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1432–1443. doi: 10.1161/01.atv.15.9.1432. [DOI] [PubMed] [Google Scholar]
- 6.Kienast J, et al. Relation of urokinase-type plasminogen activator expression to presence and severity of atherosclerotic lesions in human coronary arteries. Thromb Haemost. 1998;79:579–586. [PubMed] [Google Scholar]
- 7.Steins MB, et al. Overexpression of urokinase receptor and cell surface urokinase-type plasminogen activator in the human vessel wall with different types of atherosclerotic lesions. Blood Coagul Fibrinolysis. 2004;15:383–391. doi: 10.1097/01.mbc.0000114441.59147.56. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann F. In: Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Colman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editors. Philadelphia: Lippincott, Williams, and Wilkins; 2001. pp. 275–320. [Google Scholar]
- 9.Ellis V, Behrendt N, Danø K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991;266:12752–12758. [PubMed] [Google Scholar]
- 10.Folsom AR, et al. Prospective study of fibrinolytic factors and incident coronary heart disease: The atherosclerosis risk in communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2001;21:611–617. doi: 10.1161/01.atv.21.4.611. [DOI] [PubMed] [Google Scholar]
- 11.Cushman M, et al. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:493–498. doi: 10.1161/01.atv.19.3.493. [DOI] [PubMed] [Google Scholar]
- 12.Sakkinen PA, et al. Relationship of plasmin generation to cardiovascular disease risk factors in elderly men and women. Arterioscler Thromb Vasc Biol. 1999;19:499–504. doi: 10.1161/01.atv.19.3.499. [DOI] [PubMed] [Google Scholar]
- 13.Ploplis VA, et al. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91:2005–2009. [PubMed] [Google Scholar]
- 14.Torzewski M, et al. Enzymatic modification of low-density lipoprotein in the arterial wall: A new role for plasmin and matrix metalloproteinases in atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24:2130–2136. doi: 10.1161/01.ATV.0000144016.85221.66. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P, et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17:439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Q, et al. Plasminogen deficiency accelerates vessel wall disease in mice predisposed to atherosclerosis. Proc Natl Acad Sci USA. 1997;94:10335–10340. doi: 10.1073/pnas.94.19.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cozen AE, et al. Macrophage-targeted overexpression of urokinase causes accelerated atherosclerosis, coronary artery occlusions, and premature death. Circulation. 2004;109:2129–2135. doi: 10.1161/01.CIR.0000127369.24127.03. [DOI] [PubMed] [Google Scholar]
- 18.Blasi F, Carmeliet P. uPAR: A versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 19.Tsirka SE, et al. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y-H, Park J-H, Hong SH, Koh J-Y. Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science. 1999;284:647–650. doi: 10.1126/science.284.5414.647. [DOI] [PubMed] [Google Scholar]
- 21.Naldini L, et al. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992;11:4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest. 2007:3821–3832. doi: 10.1172/JCI32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuhrman B, et al. Urokinase plasminogen activator (uPA) stimulates cholesterol biosynthesis in macrophages through activation of SREBP-1 in a PI3-kinase and MEK-dependent manner. Atherosclerosis. 2007;195:e108–116. doi: 10.1016/j.atherosclerosis.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P, et al. Impaired arterial neointima formation in mice with disruption of the plasminogen gene. J Clin Invest. 1997;99:200–208. doi: 10.1172/JCI119148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moons L, et al. Reduced transplant arteriosclerosis in plasminogen-deficient mice. J Clin Invest. 1998;102:1788–1797. doi: 10.1172/JCI3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otsuka G, Stempien-Otero A, Frutkin AD, Dichek DA. Mechanisms of TGF-beta1-induced intimal growth: Plasminogen-independent activities of plasminogen activator inhibitor-1 and heterogeneous origin of intimal cells. Circ Res. 2007;100:1300–1307. doi: 10.1161/01.RES.0000266970.34017.8d. [DOI] [PubMed] [Google Scholar]
- 27.Boucher P, et al. LRP: Role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 28.Hume DA, Gordon S. Optimal conditions for proliferation of bone marrow-derived mouse macrophages in culture: The roles of CSF-1, serum, Ca2+, and adherence. J Cell Physiol. 1983;117:189–194. doi: 10.1002/jcp.1041170209. [DOI] [PubMed] [Google Scholar]
- 29.Stempien-Otero A, et al. Mechanisms of cardiac fibrosis induced by urokinase plasminogen activator. J Biol Chem. 2006;281:15345–15351. doi: 10.1074/jbc.M512818200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.