Abstract
The neuropeptide arginine vasopressin (AVP) is arguably among the most potent regulators of social behaviors in mammals identified to date. However, only the related neuropeptide oxytocin (OXT) has been shown to promote maternal behavior. Here, we assess the role of AVP in maternal care, in particular in arched back nursing, pup retrieval, and pup contact by using complementary pharmacological and genetic approaches. Also, experiments were performed in rat dams with differences in trait anxiety, i.e., rats bred for either high (HAB) or low (LAB) anxiety-related behavior as well as nonselected (NAB) dams. Viral vector-mediated up-regulation of AVP V1a receptors (AVP-Rs) within the medial preoptic area of lactating NAB rats and chronic central AVP treatment of NAB and LAB dams improved, whereas local blockade of AVP-R expression by means of antisense oligodeoxynucleotides or central AVP-R antagonism impaired, maternal care in NAB dams. Also, in HAB rats with a genetically determined elevated brain AVP activity, intrinsically high levels of maternal care were reversed by blockade of AVP-R actions. Treatment-induced impairment of AVP-mediated maternal behavior increased adult emotionality and impaired social interactions in male offspring of NAB dams. These findings provide direct evidence for an essential and highly potent role of brain AVP in promoting maternal behavior, which seems to be independent of the dam's trait anxiety.
Keywords: adenoviral vector, arched back nursing, medial preoptic area, antisense oligodeoxynucleotide, pup retrieval
The neuropeptide arginine vasopressin (AVP) is a key player in the regulation of complex social behaviors in mammals (1). Investigations have mainly focused on males and shown that AVP-dependent social behaviors include social recognition and interaction (2–5), intermale aggression (6, 7), pair-bonding (8–10), and paternal care in monogamous biparental rodents (11, 12). In addition, the related ancestral neuropeptide vasotocin, which is found in nonmammalian vertebrates, influences a wide range of reproductive behaviors in fish (13) and other vertebrates (14). So far, any direct involvement of AVP in regulating maternal behavior, one of the most important prosocial behaviors found in mammals, is largely unknown (1, 15). However, there is substantial evidence for significant activation of the brain AVP system around parturition and in lactation (16–20), which has mainly been discussed, to date, in the context of osmotic homeostasis maintenance (1, 16, 18). Interestingly, central infusion of AVP (21) or of an AVP receptor anta-gonist (AVP-A) altered the onset of maternal behavior (22), interpreted to be due to actions on oxytocin (OXT) receptors (OXT-Rs) (22), and affected maternal memory (23), respectively.
In the present experiments, we aimed to reveal AVP effects on maternal behavior mediated by the AVP V1a receptor (AVP-R). Therefore, we repeatedly treated lactating rat dams with either a selective AVP-A or OXT-R antagonist (OXT-A), or chronically infused either synthetic AVP or OXT during the first days of lactation. We assessed several aspects of maternal behavior, including arched back nursing (ABN; defined as a quiescent kyphotic nursing posture) [for a representative picture see supporting information (SI) Fig. S1a] (24), licking and grooming the pups (LG), the time dams spend in direct pup contact (dam on), and pup retrieval. To demonstrate the specific involvement of AVP-Rs and to localize the AVP-R-mediated effects on maternal behavior, we manipulated AVP-Rs within the medial preoptic area (MPOA), an important area for the onset and maintenance of maternal care (15, 22, 25–27). To reach this goal, we used either an AVP-R antisense oligodeoxynucleotide for down-regulation (5) or an adeno-associated viral vector for up-regulation of AVP-R expression (4, 10).
We extended our studies to rats selectively bred for either high (HAB) or low (LAB) anxiety-related behavior. The hyperanxiety is due to a single nucleotide polymorphism in the promoter region of the AVP gene (28), which results in elevated hypothalamic AVP activity (29). Because HAB dams display an enhanced level of maternal care (30), we hypothesized the causal involvement of the activated brain AVP system.
Results
Blockade of AVP-R Impaired Maternal Care.
In an initial experiment, repeated daily i.c.v. infusion of either AVP-A or OXT-A was performed at 9 AM between lactation day (LD) 1 and LD 5. Shown in Fig. 1A are the frequencies of ABN from 8 to 9 AM on each day between LD 1 and LD 5, and the overall mean frequency of ABN and dam on from 8 to 9 AM over LD 2 to LD 5 (Fig. 1B). The antagonists affected the frequency of ABN from 8 to 9 AM between LD 1 and LD 5 (F8,15 4.59, P < 0.0001; Fig. 1A). Beginning 23 h after the first i.c.v. infusion (i.e., on LD 2), ABN was reduced in both AVP-A- (P < 0.01) and OXT-A- (P < 0.05) treated dams compared with vehicle (VEH). In support, the mean frequency of ABN from 8 to 9 AM between LD 2 and LD 5 was lower in both AVP-A- and OXT-A-treated dams (F2,29 32.7, P < 0.0001; P < 0.01 vs. VEH; Fig. 1B). Interestingly, treatment effects were mainly seen in the morning hours (Fig. S1 for full-day observation on LD 4).
Fig. 1.
Effects of manipulation of brain AVP or OXT systems on maternal behavior between LD 1 and LD 5 (A and B) or LD 2 and LD 6 (C and D). Dams were daily injected i.c.v. with VEH, AVP-A, or OXT-A at 9 AM (A and B), or chronically infused with VEH, AVP or OXT i.c.v. by means of osmotic minipumps starting on LD 1 (C and D) and monitored every second minute between 8 and 9 AM. Shown are the frequency of ABN (A and C) and the mean frequency of ABN and the time the dams spent in direct pup contact (dam on; B and D) over LD 2 to LD 5 (B) or over LD 2 to LD 5 (D). Numbers in parentheses indicate group size. Data represent mean plus SEM. **, P < 0.01; *, P < 0.05 vs. VEH; ##, P < 0.01; #, P < 0.05 vs. OXT-A and vs. OXT, respectively.
A first indication for a peptide-specific effect of AVP on maternal behavior comes from the finding that the mean frequency of dam on between LD 2 and LD 5 (F2,29 6.85, P = 0.004) was reduced in AVP-A (P < 0.01), but not OXT-A, dams (Fig. 1B). The frequency of LG was not altered by any antagonist treatment (data not shown).
Chronic i.c.v. Infusion of AVP Improved Maternal Care.
To assess the consequences of up-regulation of brain AVP availability on maternal behavior, AVP (or OXT) was chronically infused i.c.v. by means of an osmotic minipump after surgery on LD 1. Chronic i.c.v. treatment altered ABN from LD 2 to LD 6 (F2,10 15.7, P = 0.0001; Fig. 1C). Specifically, AVP increased ABN compared with both VEH and OXT (P < 0.01), whereas OXT had no effect. In support, between LD 2 and LD 5, the mean frequency of ABN (F2,20 15.6, P = 0.0001) was higher in AVP-treated compared with VEH- and OXT-treated dams (P < 0.01), whereas the mean frequency of LG (data not shown) or dam on (Fig. 1D) did not differ. Thus, intracerebral AVP availability seems to essentially regulate the performance of maternal behavior, specifically ABN, whereas further increasing brain OXT availability has a minor role.
Down-Regulation of AVP-R in the MPOA Decreased Maternal Care.
The MPOA is importantly involved in the onset and maintenance of maternal care (15, 22, 25–27), and local AVP-Rs have been described (31). Here, we found increased AVP-R binding within the MPOA in lactating compared with virgin rats (P < 0.05; Fig. 2D). Consequently, we aimed to reveal the functional involvement of local AVP-Rs in the context of maternal care. However, cross-interactions between AVP and OXT and their receptors are likely (32). Therefore, AVP-R antisense oligodeoxynucleotides (5) were chronically infused by means of osmotic minipumps into the MPOA, resulting in reduced AVP-Rs (P < 0.01; Fig. 2 D and F), but not OXT-Rs (Fig. 2E).
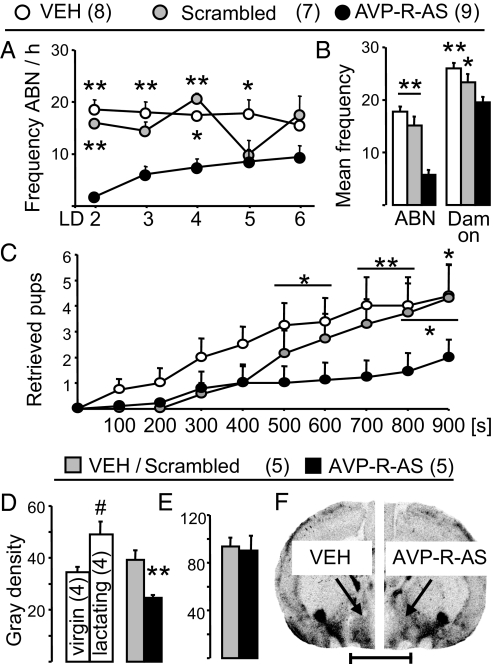
Fig. 2.
Effects of antisense-induced down-regulation of AVP-R expression within the MPOA on maternal behavior and local AVP-R and OXT-R binding. Dams were chronically infused with VEH, scrambled sequence, or AVP-R antisense (AVP-R-AS) into the left and right MPOA starting on LD 1. Frequency of ABN between LD 2 and LD 6 (A) and the mean frequency of ABN and dam on over LD 2 to LD 5 (B) is indicated. Also, the number of retrieved pups in a novel cage during a 900-s observation period was monitored (C). AVP-R binding in untreated virgin (V) and lactating (L) rats and after respective treatment (D and F) as well as OXT-R binding (E) within the MPOA was quantified as gray density on coronal sections by receptor autoradiography. (Scale bar, 500 μm.) Numbers in parentheses indicate group size. Data represent mean plus SEM. **, P < 0.01; *, P < 0.05 vs. AVP-R-AS; #, P < 0.05 vs. virgin.
AVP-R antisense decreased ABN from LD 2 to LD 6 (F8,119 2.50, P = 0.02; Fig. 2A) compared with VEH or scrambled sequence control (P < 0.05). Also, between LD 2 and LD 5, the mean frequency of ABN (F2,23 54.6, P < 0.0001) and of dam on (F2,23 12.8, P = 0.0002) were lower in AVP-R antisense-treated dams (Fig. 2B); LG was not altered by the treatment (data not shown). Pup retrieval (33) on LD 3 differed among treatment groups over the 900-s observation period (F18,239 1.84, P = 0.02; Fig. 2C). AVP-R antisense decreased the number of collected pups compared with controls (P < 0.05). These data provide evidence for the selective involvement of AVP-Rs within the MPOA in the regulation of established maternal behavior.
Up-Regulation of AVP-Rs Within the MPOA Increased ABN.
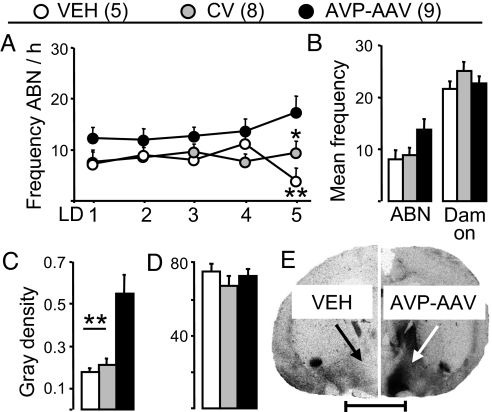
To provide further evidence of local AVP-R-specific regulation of maternal behavior an AVP-R adenoviral vector was infused bilaterally into the MPOA on pregnancy day 10 to selectively up-regulate local AVP-R expression and binding (F2,21 9.82, P = 0.001; Fig. 3 C and E). OXT-R binding was not affected by this treatment (Fig. 3D). AVP-R up-regulation increased ABN in lactation (F2,109 3.59, P = 0.047; Fig. 3A), reaching statistical significance on LD 5 (P < 0.01 vs. VEH; P < 0.05 vs. control virus). The mean frequency of ABN between LD 2 and LD 5 also tended to be higher in viral vector-treated dams (F2,21 3.25, P = 0.06; Fig. 3B). There was no treatment effect on dam on (Fig. 3B), LG (data not shown), and pup retrieval (Fig. S2).
Fig. 3.
Effects of up-regulation of AVP-R expression within the MPOA on maternal behavior and local AVP-R and OXT-R binding. Dams received a single, bilateral infusion of VEH, control virus (CV), or an adeno-associated viral vector for overexpression of the AVP-R (AVP-AAV) on pregnancy day 10. Frequency of ABN between LD 1 and LD 5 (A) and the mean frequency of ABN and dam on over LD 2 to LD 5 (B) is indicated. AVP-R (C and E) and OXT-R (D) binding within the MPOA was quantified as gray density on coronal sections by receptor autoradiography. (Scale bar, 500 μm.) Data represent mean plus SEM. **, P < 0.01; *, P < 0.05 vs. AVP-AAV.
Blocking of AVP-Rs Reduced the High Level of Maternal Care in HAB Dams.
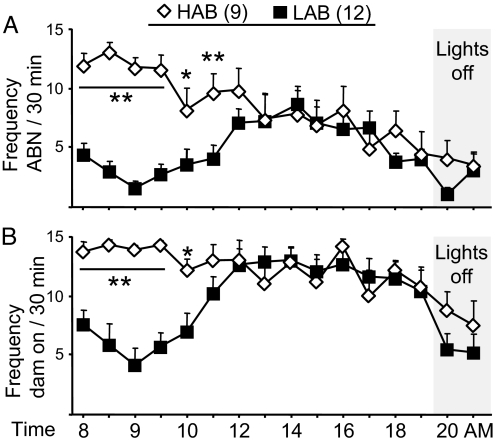
Genetic differences in brain AVP activity underlie the behavioral phenotype of HAB and LAB Wistar rats with respect to anxiety- and depression-related behavior (28, 29). Here, untreated HAB and LAB dams were observed on LD 4 between 8 and 21 AM. As shown before (30), HAB dams displayed more ABN (F15,335 4.70, P < 0.0001; Fig. 4A) and dam on (F15,335 3.78, P < 0.0001; Fig. 4B) between 8 and 11 AM compared with LAB dams. In the following hours, maternal behavior did not differ. The frequency of LG was similar between HAB and LAB dams (data not shown) (30).
Fig. 4.
ABN (A) and time spent in direct contact with the pups (dam on) (B) of HAB and LAB dams on LD 4. Data represent mean plus SEM. **, P < 0.01; *, P < 0.05 vs. LAB.
To reveal the role of brain AVP in the high level of maternal care in HAB rats, AVP-A (or OXT-A) was infused daily i.c.v. at 9 AM. AVP-A reduced ABN in HAB dams in the hour before acute treatment (F8,129 3.08, P = 0.004; Fig. 5A) beginning 23 h after the first i.c.v. infusion (P < 0.01). Between LD 2 and LD 5, the mean frequency of ABN (F2,25 41.2, P < 0.0001) and of dam on (F2,25 4.37, P = 0.03) were reduced by AVP-A compared with VEH and OXT-A, which did not alter any of these parameters (Fig. 5B). Thus, the genetically determined high activity of the brain AVP system in HAB rats (28, 29) is likely to be causally involved in their high level of maternal care. Also, these results show that AVP is significantly more potent than OXT in regulating ongoing maternal care in HAB rats.
Fig. 5.
Effects of manipulation of brain AVP and OXT systems on maternal behavior of HAB (A and B) and LAB (C and D) dams. HAB dams received a daily i.c.v. infusion of VEH, AVP-A, or OXT-A at 9 AM; LAB dams were chronically i.c.v. infused with VEH, synthetic AVP, or OXT i.c.v. starting on LD 1. For details, see Fig. 1. **, P < 0.01; *, P < 0.05 vs. VEH; ##, P < 0.01; # P < 0.05 vs. OXT-A; +, P < 0.05 vs. VEH and LAB AVP.
Chronic i.c.v. Infusion of AVP Increased ABN in LAB Dams.
To provide further evidence that high brain AVP availability is important for maternal behavior, the less maternal LAB dams (Fig. 4) were chronically infused i.c.v. with VEH, synthetic AVP, or OXT starting on LD 1 after implantation of the osmotic minipump. Both AVP and OXT increased ABN from LD 2 to LD 6 (F2,89 36.9, P < 0.0001; Fig. 5C), but the effect of AVP was more pronounced (P < 0.01 vs. VEH; P < 0.05 vs. OXT). In support, the mean frequency of ABN between LD 2 and LD 5 was highest in AVP-treated dams (F2,17 37.8, P < 0.0001; P < 0.01 vs. VEH and OXT). dam on did not differ between the groups (F2,17 2.23, P = 0.14; Fig. 5D). Thus, and in confirmation of chronic AVP-infusion effects in nonselected dams, increased intracerebral availability of AVP clearly improved maternal care also in LAB dams. The finding that chronic AVP treatment was, again, more effective than OXT treatment further emphasizes the potent role of AVP in the regulation of maternal behavior.
AVP and OXT Exert Opposing Effects on Anxiety.
With respect to anxiety-related behavior, opposite effects of AVP (anxiogenic) (5, 29, 34) and OXT (anxiolytic) (35–37) have been shown, a phenomenon that was used to confirm peptide-specific behavioral consequences of manipulating the brain AVP or OXT system. Lactating dams were tested on the elevated plus-maze (EPM) (38) on day 3 of respective treatment.
Repeated i.c.v. infusions of AVP-A or OXT-A altered the anxiety level in opposing manners (F2,26 15.9, P < 0.0001; Fig. 6A). AVP-A increased (P < 0.05 vs. VEH, P < 0.01 vs. OXT-A), whereas OXT-A reduced (P < 0.01 vs. VEH) the percentage time spent on the open arms of the EPM. In support, in HAB dams, i.c.v. AVP-A also increased the percentage time (F2,24 5.58, P = 0.01; P < 0.05 vs. VEH, OXT-A), whereas OXT-A had no effect.
Fig. 6.
Differential effects of manipulation of the brain AVP and OXT systems on anxiety-related behavior on the plus-maze of NAB, HAB, and LAB dams. Lactating NAB (from Fig. 1 A and B) and HAB (from Fig. 5 A and B) rats received a daily i.c.v. infusion of VEH, AVP-A, or OXT-A (A). NAB (from Fig. 1 C and D) and LAB (from Fig. 5 C and D) dams were chronically treated with VEH, synthetic AVP, or OXT (B). Anxiety is reflected by the percentage time spent on the open arms. Data represent mean plus SEM. **, P < 0.01; *, P < 0.05 vs. VEH; ##, P < 0.01; #, P < 0.05 vs. OXT-A (A) or OXT (B).
Further, chronic i.c.v. infusion of synthetic AVP or OXT exerted opposite effects on anxiety on the EPM (F2,20 19.3, P < 0.0001; Fig. 6B). AVP reduced (P < 0.01), whereas OXT increased (P < 0.05) the percentage time spent on the open arms. Similarly, i.c.v. infusion of AVP increased the percentage time in LAB dams (F2,17 23.9, P < 0.0001; P < 0.01 vs. VEH).
The locomotor activity was not altered by any treatment, confirming the specificity of the manipulations on anxiety-related behavior (data not shown).
Manipulation of local AVP-R expression within the MPOA did not affect anxiety-related behavior of the dams as assessed by the percentage time on open arms (down-regulation of AVP-R: VEH, 35% ± 4%; scrambled sequence control, 38% ± 6%; AVP-R antisense, 33% ± 4%; F2,23 0.35; up-regulation of AVP-R: VEH, 30% ± 4%; control virus, 32% ± 6%; viral vector, 37% ± 5%; F2,21 0.55). These data indicate that AVP-Rs within the MPOA are significantly involved in the regulation of maternal behavior, but not of anxiety.
Effects of Manipulation of Maternal Care on Offspring Parameters.
Given the capacity of brain AVP in regulating maternal care, the question arises as to the importance of these behaviors for the development of the offspring. However, treatment-induced alterations in maternal behavior did not result in differences in pup weight gain during the first 6 days of life (data not shown). Importantly, reduced maternal behavior by means of selective down-regulation of AVP-Rs within the MPOA (Fig. 2) altered the behavior of adult male offspring, namely increased anxiety at age of 8 weeks (percentage time: F2,17 3.51, P = 0.05; Fig. S3a). Also, separate statistics performed on AVP-R antisense and VEH offspring revealed a reduced time spent with social interaction in AVP-R antisense offspring (U test, P < 0.05; Fig. S3b).
Discussion
The present study provides complementary evidence demonstrating that AVP is a highly potent brain regulator of social behavior in female rats, in particular of maternal behavior. Blockade of brain AVP-R either by AVP-A or by AVP-R antisense applied i.c.v. and directly into the MPOA, respectively, decreased maternal care. The effects of AVP-A were particularly prominent in HAB dams, which display a high innate level of anxiety and maternal behavior based on a genetically determined activation of the brain AVP system (28, 29). In support, chronic elevation of brain AVP levels increased maternal care-related parameters not only in nonselected dams, but also in LABs, which show a rather low innate level of maternal behavior. Importantly, the comparably subtle increase in ABN in LAB dams after chronic OXT, and the lack of OXT effects on maternal care in nonselected dams, indicate peptide-specific AVP effects. Peptide specificity could be confirmed by sequence-specific manipulation of intracerebral AVP-R gene expression and binding specifically within the MPOA; down-regulation of AVP-R binding impaired maternal behavior, whereas up-regulation of AVP-R binding increased the frequency of ABN.
To date, established functions of AVP in social behaviors have almost exclusively been described in males (see above), whereas the regulation of complex social behaviors in females has mainly been linked to the related neuropeptide OXT (39). Interestingly, in the monogamous and alloparental prairie vole AVP was shown to facilitate paternal behavior (11, 12). Also, in some other nonmammalian vertebrate species, the related nonapeptide vasotocin has been linked to complex parental behaviors (13, 14). Therefore, it seems likely that the high level of neuropeptide conservation found in the neurohypophysial systems throughout species is linked to a stable conservation of behavioral functions, in particular of parental behavior.
The demonstration of neuropeptide-specific effects of AVP in the context of maternal behavior is undoubtedly an important issue, because AVP and OXT show structural similarities, and cross-interactions between these neuropeptides and their receptors are likely (32). OXT is an established regulator of the onset and fine-tuned maintenance of maternal behavior (15, 21, 40). Therefore, our findings of peptide-specific and, especially in the context of anxiety, also opposing effects are crucial for the overall confirmation of our hypothesis that brain AVP significantly promotes maternal behavior. Thus, the opposite effects of AVP and OXT on emotionality underline their unidirectional effects on several aspects of maternal behavior due to receptor-specific actions.
The MPOA is important for the onset and maintenance of maternal care, including retrieval of pups (15, 22, 25–27). Within the MPOA, local AVP-Rs were found to be up-regulated during lactation (Fig. 2D). Importantly, sequence-specific manipulation of AVP-Rs revealed their potent involvement in maternal behavior; chronic blockade of local AVP-R expression by means of antisense reduced ABN as well as the number of retrieved pups in early lactation, whereas up-regulation of AVP-Rs by a selective AVP-R viral vector increased ABN. Because AVP-dependent effects on ABN and dam on were found mainly in the morning hours, it is of interest to note that the MPOA receives vasopressinergic input from the suprachiasmatic nucleus, and diurnal changes in local AVP release have been described (41). The result of altered maternal behavior after manipulation of AVP-Rs within the MPOA provides further support of our hypothesis of neuropeptide-specific and OXT-R-independent effects of AVP on maternal behavior, the more as local OXT-R binding was not affected (Figs. 2E and 3D). However, the viral vector-induced increase in AVP-R binding was localized within an area exceeding the boundaries of the MPOA (Fig. 3C) (42). Regions such as the lateral septum and the bed nucleus of the stria terminalis are in close anatomical attendance of the MPOA and have a role in the regulation of maternal behavior (15, 22, 27). Therefore, increased levels of AVP-R binding outside the MPOA have also to be considered to contribute to the behavioral effects of the AVP-R viral vector on maternal behavior.
HAB and LAB dams were used to provide additional evidence for the involvement of the brain AVP system in the regulation of maternal behavior. The high innate level of anxiety of HAB is accompanied by a more protective mothering style (Fig. 4), including maternal aggression (30, 43). Importantly, their genetically determined high activity of the brain AVP system (28, 29) seems to be causally involved in the remarkable differences in maternal behavior found between HAB and LAB dams. Blockade of central AVP-Rs in HAB dams reduced the level of maternal behavior, whereas daily infusion with OXT-A was without effect (Fig. 5A). In further support, daily i.c.v. infusions of AVP increased maternal behavior in LAB dams, an effect that was even more pronounced than that of chronic OXT. These studies utilizing the inherent genetic difference between HAB and LAB rats provide additional evidence for the peptide-selective effect of AVP on maternal behavior, which seems to be independent of the dams' trait anxiety.
However, a possible link between the level of anxiety and maternal behavior has been suggested, raising the possibility that the effects of AVP on maternal behavior are indirectly mediated by means of its anxiogenic actions. For example, in human mothers, a high state anxiety has been related to a high level of child-directed protective behavior (15, 44, 45). A link between increased maternal anxiety and an elevated level of maternal care has also been found in mouse dams after chronic pregnancy stress (46). In our study, local down-regulation of AVP-R expression within the MPOA decreased ABN on one hand, but did not affect the dams' anxiety level on the other. Both i.c.v. AVP and OXT promote maternal care but exert opposite effects on anxiety (Fig. 6), which rather suggests dissociation between anxiety and maternal behavior in rats.
If AVP is crucial for the maintenance of maternal behavior, adaptations of the brain AVP system in the peripartum period would be likely. In fact, the brain AVP system is activated around birth; AVP mRNA expression (16, 17, 19) and immunoreactivity (20) within the hypothalamus is elevated in lactation, the release of AVP within the dorsal hippocampus is stimulated during parturition (18), and AVP-R binding is increased within the MPOA in lactation (Fig. 2D). Until now, these adaptations were mainly interpreted as being essential for balancing the osmotic challenges the dam has to meet peripartum (1, 16, 18). However, our results provide strong evidence that the increased vasopressinergic drive in lactation is also importantly involved in maternal care.
In conclusion, we now have to consider AVP as an additional potent regulator of complex social behaviors in females, in particular of the fine-tuned maintenance of maternal behavior. These AVP-dependent aspects of maternal care were found to be independent of the dam's trait anxiety. Also, they are likely to have a significant role in the healthy behavioral, in particular emotional and social, development of the offspring. Further studies are needed to reveal whether maladaptations of the AVP system in the peripartum period are linked to disturbances of mother–child interactions. These results implicate the exciting possibility of manipulation of the brain AVP system for treating mothers suffering from postpartum depression or psychosis with diminished interest and enjoyment in (47), or even inflicting harm on their babies (48).
Materials and Methods
Animals.
The studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication no. 80-23) and of the local government of Bavaria.
Females Wistar rats purchased from Charles River (nonselected) or female HAB and LAB Wistar rats from the own breeding colony (SI Materials and Methods) were used (28, 29). Rats were kept in the animal facilities under standard laboratory conditions (12:12 light–dark cycle, lights on at 7 AM, 22 °C, 55% relative humidity; water and standard rat chow ad libitum). Virgin rats were mated with sexually-experienced males at the age of 14 weeks and were housed in groups of 3 in standard rat cages (40 × 60 × 20 cm) until day 19 of pregnancy, when they were singly housed in observation cages (38 × 22 × 35 cm) until the end of the experiments.
Surgeries and Intracerebral Infusions.
Surgical procedures were performed under isoflurane anesthesia and temperature-controlled conditions by using semisterile procedures. After surgery, rats received a 30-μL s.c. injection of antibiotic (enrofloxacin 3 mg/0.03 mL; Baytril; Bayer).
Implantation of an i.c.v. Guide Cannula.
For repeated i.c.v. infusions of AVP-A or OXT-A rats were stereotaxically implanted with a guide cannula targeting the right ventricle (stereotaxic coordinates relative to bregma: caudal 1.0 mm, lateral 1.6 mm, depth 1.8 mm) (42) on day 19 of pregnancy as described earlier (35). Animals were repeatedly handled during pregnancy to habituate them to the i.c.v. infusion procedure.
Acute i.c.v. Infusions.
Between LD 1 and LD 5, lactating dams received daily an acute i.c.v. injection of VEH (Ringer's solution; pH adjusted to 7.4; Fresenius), AVP-A [0.75 μg/5 μL; d(CH2)5[Tyr(Me)2]AVP] (32), or OXT-A [0.75 μg/5 μL; des-Gly-NH2,d(CH2)5[Tyr(Me)2,Thr4]OVT] (49) at 9 AM. The substances were slowly infused by using a 27-gauge i.c.v. cannula, which extended 2 mm beyond the guide cannula (35).
Implantation of Osmotic Minipumps.
To chronically up-regulate brain AVP (or OXT) levels postpartum, an osmotic minipump (0.5 μL/h for 7 days; model 1007 D; Alzet Osmotic Pumps) was filled with VEH, synthetic AVP (20 ng/μL), or OXT (20 ng/μL; Sigma–Aldrich) and implanted s.c. (50), with the attached infusion cannula targeting the right ventricle (23-gauge; caudal 1.0 mm, lateral 1.6 mm, depth 4.0 mm) (42). For sequence-specific down-regulation of AVP-R expression within the MPOA, 2 osmotic minipumps were filled with VEH, scrambled sequence oligo control (5′-CAT GTT CCA AGT GGT AAT CCG-3′), or AVP-R antisense (5′-GGA AAC TCA TGC TGT CCG TAC-3′; Entelechon) (5) and implanted on LD 1 with the infusion cannulas targeting the left and right MPOA (27-gauge; caudal 0.3 mm, lateral 0.8 mm, depth 8.8 mm) (42). The implantations were performed on LD 1 to prevent treatment effects on the birth process; behavioral observations started on the day after and lasted until LD 6.
Up-Regulation of AVP-R in the MPOA.
To specifically elevate AVP-R expression and binding in the MPOA, an AVP-R adeno-associated viral vector (0.5 μL; titer 5 × 107) was acutely infused into the left and right MPOA on pregnancy day 10. The AVP-R vector (10), which was free of contaminating helper virus and contained only the gene of interest with no viral genes, has been shown to selectively increase AVP-R binding in rats (4) and voles (10).
Behavioral Tests.
Maternal care.
Undisturbed maternal behavior in the home cage was observed for 5–10 s every second minute between 8 and 9 AM daily, and additionally on the first and fourth day of the experiments over 1 h at 9 and 12 AM and 2, 4, 6, and 8 PM (dark phase under red light). Treatment or line differences were especially prominent in the morning hour, which is presented in Figs. 1–5. ABN was defined as quiescent kyphotic nursing including high- and low-crouch, during which mothers maintain a fixed position and do not engage in any other behaviors (24). Further, dam on reflects the time the dam spent in direct pup contact, which includes all nursing postures (ABN, blanket postures, and lying on side or back), LG as well as pup carrying. The frequency of the recorded behaviors has been calculated as the total number of observations per time interval with a maximum frequency of 15 (30-min time interval) or 30 (60-min time interval) events. It is interesting to note that, in contrast to Long–Evans rats (51), Wistar dams generally spend little time on LG (frequency ≈1 per 30-min interval; data not shown) (30).
Pup retrieval test.
On LD 3 dams with AVP-R manipulations within the MPOA were separated from their pups from 9 to 10 AM. Eight pups of each litter were then spread into the corners of a larger cage (34 × 53 × 32 cm) and the time of collection of pups was monitored during a 900-s observation period (30).
EPM.
To monitor peptide-specific effects on anxiety-related behavior, dams were tested on the EPM (30, 52) between 10 and 12 AM on the third day of treatment. Dams were transferred to the test room in their home cage 1 h before the test. In the case of acute treatment (AVP-A or OXT-A), dams were infused 5 min before testing started. During the 5-min exposure, the percentage time spent on the open arms was recorded as anxiety-related parameter. The number of entries into closed arms was interpreted as indicative of locomotor activity (53).
Offspring Parameters.
On the day of birth, each litter was culled to 8 pups. Each day at 5 PM, the litters were weighed until LD 5 in acutely or until LD 6 in chronically treated dams (SI Materials and Methods).
Histology.
At the end of the experiment, dams were killed with an overdose of isoflurane and blue ink was injected by means of infusion system. The correct placement of the infusion site in the ventricles and the MPOA was verified macro- or microscopically. All infusion sites were found to be within the targeted brain areas.
Receptor Autoradiography.
AVP-R and OXT-R binding in the MPOA region was analyzed by receptor autoradiography using the linear AVP-A 125I-d(CH2)5[Tyr(Me)]-AVP (Perkin–Elmer) and the ornithine vasotocin analog 125I-d(CH2)5[Tyr(Me)2-Tyr-NH2]9-OVT (Perkin–Elmer), respectively, as described previously (54). Receptor density on autoradiographic films was quantified as gray density with a computerized image program (ImageJ 1.31, National Institutes of Health, http://rsb.info.nih.gov/ij/). Unilateral measurements from the right hemisphere from 3 sections per animal were taken and the mean density measurement was calculated by subtracting the background activity.
Statistical Analysis.
Statistical analyses were performed by means of statistical software (GB-Stat V6.0, Dynamic Microsystems). One-way (factor treatment; breeding line) or 2-way (factors time × treatment; time × breeding line) analyses of variance (ANOVA) followed by the Newman–Keuls post hoc test or Mann–Whitney U test for nonparametric comparisons were used where appropriate. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank Dr. M. Manning (University of Toledo, Toledo, OH) for kindly providing the AVP and OXT receptor antagonists, Dr. L. Young (Emory University, Atlanta, GA) for generously providing the adeno-associated viral vector, Drs. M. Numan (Boston College, Boston, MA), J. Goodson (Indiana University, Bloomington, IN), R. Landgraf (Max Planck Institute for Psychiatry, Munich, Germany), and C. Pedersen (University of North Carolina, Chapel Hill, NC) for critical reading of the manuscript, Ms. B. Halser and Ms. C. Limmer for their support of extensive behavioral observations, and Mrs. G. Schindler, Ms. L. Traulsen, and Mr. R. Bredewold for excellent technical help.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807412105/DCSupplemental.
References
- 1.Caldwell HK, Lee HJ, Macbeth AH, Young WS., III Vasopressin: Behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 3.Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Landgraf R, et al. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: Improved social discrimination and active social behaviour. Eur J Neurosci. 2003;18:403–411. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- 5.Landgraf R, et al. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris CF. Vasopressin/oxytocin and aggression. Novartis Found Symp. 2005;268:190–198. discussion 198–200, 242–53. [PubMed] [Google Scholar]
- 7.Veenema AH, Neumann ID. Neurobiological mechanisms of aggression and stress coping: A comparative study in mouse and rat selection lines. Brain Behav Evol. 2007;70:274–285. doi: 10.1159/000105491. [DOI] [PubMed] [Google Scholar]
- 8.Lim MM, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 9.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 10.Pitkow LJ, et al. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci USA. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wynne-Edwards KE, Timonin ME. Paternal care in rodents: Weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 14.Moore FL, Wood RE, Boyd SK. Sex steroids and vasotocin interact in a female amphibian (Taricha granulosa) to elicit female-like egg-laying behavior or male-like courtship. Horm Behav. 1992;26:156–166. doi: 10.1016/0018-506x(92)90039-x. [DOI] [PubMed] [Google Scholar]
- 15.Numan M, Insel TR. In: The Neurobiology of Parental Behavior. Ball GF, Balthazart J, Nelson RJ, editors. New York: Springer; 2003. Hormones, Brain, and Behavior Series. [Google Scholar]
- 16.Walker CD, Toufexis DJ, Burlet A. Hypothalamic and limbic expression of CRF and vasopressin during lactation: Implications for the control of ACTH secretion and stress hyporesponsiveness. Prog Brain Res. 2001;133:99–110. doi: 10.1016/s0079-6123(01)33008-x. [DOI] [PubMed] [Google Scholar]
- 17.Lightman SL, et al. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- 18.Landgraf R, Neumann I, Pittman QJ. Septal and hippocampal release of vasopressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinology. 1991;54:378–383. doi: 10.1159/000125917. [DOI] [PubMed] [Google Scholar]
- 19.Van Tol HH, Bolwerk EL, Liu B, Burbach JP. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology. 1988;122:945–951. doi: 10.1210/endo-122-3-945. [DOI] [PubMed] [Google Scholar]
- 20.Caldwell JD, Greer ER, Johnson MF, Prange AJ, Jr, Pedersen CA. Oxytocin and vasopressin immunoreactivity in hypothalamic and extrahypothalamic sites in late pregnant and postpartum rats. Neuroendocrinology. 1987;46:39–47. doi: 10.1159/000124794. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 23.Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiol Behav. 2008;95(1-2):182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stern JM, Johnson SK. Ventral somatosensory determinants of nursing behavior in Norway rats. I. Effects of variations in the quality and quantity of pup stimuli. Physiol Behav. 1990;47:993–1011. doi: 10.1016/0031-9384(90)90026-z. [DOI] [PubMed] [Google Scholar]
- 25.Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area and onset of maternal behavior in the rat. J Comp Physiol Psychol. 1977;91:146–164. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda KO, et al. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Mol Cell Neurosci. 2007;36:121–131. doi: 10.1016/j.mcn.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Murgatroyd C, et al. Impaired repression at a vasopressin promoter polymorphism underlies overexpression of vasopressin in a rat model of trait anxiety. J Neurosci. 2004;24:7762–7770. doi: 10.1523/JNEUROSCI.1614-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wigger A, et al. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: Critical role of vasopressin. Neuropsychopharmacology. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- 30.Neumann ID, Kromer SA, Bosch OJ. Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology. 2005;30:791–806. doi: 10.1016/j.psyneuen.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Clerget-Froidevaux MS, Pittman QJ. AVP V1a-R expression in the rat hypothalamus around parturition: Relevance to antipyresis at term. Exp Neurol. 2003;183:338–345. doi: 10.1016/s0014-4886(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 32.Kruszynski M, et al. [1-beta-Mercapto-beta,beta-cyclopentamethylenepropionic acid),2-(O-methyl)tyrosine]argine-vasopressin and [1-beta-mercapto-beta,beta-cyclopentamethylenepropionic acid)]argine-vasopressin, two highly potent antagonists of the vasopressor response to arginine-vasopressine. J Med Chem. 1980;23:364–368. doi: 10.1021/jm00178a003. [DOI] [PubMed] [Google Scholar]
- 33.van Leengoed E, Kerker E, Swanson HH. Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- 34.Ring RH. The central vasopressinergic system: Examining the opportunities for psychiatric drug development. Curr Pharm Des. 2005;11:205–225. doi: 10.2174/1381612053382241. [DOI] [PubMed] [Google Scholar]
- 35.Neumann ID, Torner L, Wigger A. Brain oxytocin: Differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 37.Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- 38.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 39.Caldwell HK, Young WS. Oxytocin and vasopressin: Genetic and behavioral implications. In: Lim R, editor. Handbook of Neurochemistry and Molecular Neurobiology. 3rd Ed. New York: Springer; 2006. pp. 573–607. [Google Scholar]
- 40.Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams' oral grooming and upright posturing over pups. Physiol Behav. 2003;80:233–241. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: Coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. Sydney: Academic; 1998. [Google Scholar]
- 43.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: Link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin K, Burgess K. Parents of aggressive and withdrawn children. In: Bornstein M, editor. Handbook of Parenting. 2nd Ed. Vol 1. Hillsdale, NJ: Erlbaum; 2002. pp. 383–418. [Google Scholar]
- 45.Coplan RJ, Arbeau KA, Armer M. Don't fret, be supportive! Maternal characteristics linking child shyness to psychosocial and school adjustment in kindergarten. J Abnorm Child Psychol. 2008;36:359–371. doi: 10.1007/s10802-007-9183-7. [DOI] [PubMed] [Google Scholar]
- 46.Maestripieri D, Badiani A, Puglisi-Allegra S. Prepartal chronic stress increases anxiety and decreases aggression in lactating female mice. Behav Neurosci. 1991;105:663–668. doi: 10.1037//0735-7044.105.5.663. [DOI] [PubMed] [Google Scholar]
- 47.Jameson PB, Gelfand DM, Kulcsar E, Teti DM. Mother-toddler interaction patterns associated with maternal depression. Dev Psychopathol. 1997;9:537–550. [PubMed] [Google Scholar]
- 48.Resnick PJ. Child murder by parents: A psychiatric review of filicide. Am J Psychiatry. 1969;126:325–334. doi: 10.1176/ajp.126.3.325. [DOI] [PubMed] [Google Scholar]
- 49.Manning M, et al. Solid-phase synthesis of 16 potent (selective and nonselective) in vivo antagonists of oxytocin. J Med Chem. 1989;32:382–391. doi: 10.1021/jm00122a016. [DOI] [PubMed] [Google Scholar]
- 50.Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID. Anxiolytic and anti-stress effects of brain prolactin: Improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J Neurosci. 2001;21:3207–3214. doi: 10.1523/JNEUROSCI.21-09-03207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 52.Bosch OJ, Musch W, Bredewold R, Slattery DA, Neumann ID. Prenatal stress increases HPA axis activity and impairs maternal care in lactating female offspring: Implications for postpartum mood disorder. Psychoneuroendocrinology. 2007;32:267–278. doi: 10.1016/j.psyneuen.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 53.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 54.Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.