Abstract
The multiple memory systems framework proposes that distinct circuits process and store different sorts of information; for example, spatial information is processed by a circuit that includes the hippocampus, whereas certain forms of instrumental conditioning depend on the striatum. Disruption of hippocampal function can enhance striatum-dependent learning in some paradigms, which has been interpreted as evidence that these systems can compete with one another in an intact animal. However, it remains unclear whether such competition can occur in the opposite direction, as suggested by the multiple memory systems framework, or is unidirectional. We addressed this question using lesions and genetic manipulations in mice. Impairment of dorsal striatal function with either excitotoxic lesions or transgenic inhibition of the transcription factor cAMP response element-binding protein, which disrupts striatal synaptic plasticity, impaired striatum-dependent cued learning but enhanced hippocampus-dependent spatial learning. Conversely, excitotoxic lesions of the dorsal hippocampus disrupted spatial learning and enhanced cued learning. This double dissociation demonstrates bidirectional competition that constitutes strong evidence for the parallel operation of distinct memory systems.
Keywords: basal ganglia, cAMP response element-binding protein, habit, learning and memory, procedural learning
Navigating a complex environment requires processing different configurations of salient information (1). The multiple memory systems model proposes that distinct brain circuits are adapted to store different sorts of information (2–4). For example, spatial learning requires the dorsal hippocampus (5), and lesions or more subtle disruptions of the dorsal hippocampus impair performance in spatial tasks (6–8). In contrast, the dorsal striatum is involved in stimulus-response learning (9–11), and lesions or more subtle functional disruptions of the dorsal striatum impair performance in cue-response and cue-driven navigation tasks (12–16). Similarly, in humans, hippocampal pathology can disrupt spatial learning and memory (along with declarative memory more generally), but leave striatum-dependent procedural learning intact (17, 18). Conversely, striatal pathology can produce the opposite pattern of effects (17, 19).
The nature of interactions between these systems during learning remains unclear (20, 21). When a task can be learned in different ways, a spatial strategy is often acquired first, with a more stereotyped cue-response strategy coming to dominate after repeated training (14, 16). The medial temporal lobe system can partially compensate for striatal dysfunction in some instances of basal ganglia pathology (22, 23). In circumstances in which the two systems produce different behavioral outputs, it has been proposed that they may interfere with one another, or compete, during learning (4, 24). A clear demonstration of such competition would constitute perhaps the strongest argument for the existence of parallel memory systems. Human neuroimaging studies have revealed an inverse relationship between activation of striatum and hippocampus during certain learning tasks (25, 26). In rodents, hippocampal lesions can enhance acquisition of a striatum-dependent win-stay behavioral strategy in a radial arm maze task (12, 27), perhaps by removing competitive interference from spatial information.
To date, however, we are aware of no studies that have provided clear evidence for interference by striatum-dependent processes on hippocampus-dependent learning; as a result, it remains unclear whether there is true bi-directional competition between these learning systems. We addressed this question in WT and transgenic mice by using a water maze navigation task that permits parallel assessment of spatial and cued learning (13). We find that pre-training excitotoxic lesions of the dorsal striatum disrupt striatum-dependent learning and enhance hippocampus-dependent spatial learning. Similarly, interference with hypothesized mechanisms of striatal synaptic plasticity through inhibition of the transcription factor cAMP response element-binding protein (CREB) in transgenic mice (8, 16) impairs cued learning and enhances spatial learning. Conversely, disruption of the dorsal hippocampus impairs spatial learning but enhances striatum-dependent cued learning. This double dissociation reveals bidirectional competition between striatum and hippocampus and constitutes strong evidence for the parallel operation of distinct memory systems.
Results
Dorsal Striatal Lesions Impair Cued Learning and Enhance Spatial Learning.
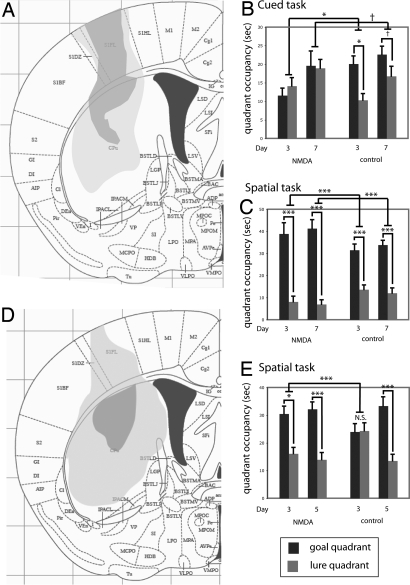
Cued and spatial learning were assayed in mice by using a water-maze task modified from that described previously in rats by Packard and McGaugh (13) (see Materials and Methods). We hypothesized that pre-training excitotoxic lesions would disrupt cued but not spatial learning. Lesioned and control mice (Fig. 1A) were trained in either a cued or a spatial task for 7 days [supporting information (SI) Figs. S1 and S2] (n = 8 control, cued; n = 8 lesioned, cued; n = 9 control, spatial; n = 7 lesioned, spatial). Learning was assayed by probe trials, in which we quantified bias toward the goal cue (or quadrant) relative to the lure. As hypothesized, control animals trained in the cued task showed a goal-quadrant bias on both day 3 (Fig. 1B; two-tailed paired t test, t = 3.21; P = 0.016) and day 7 (t = 3.65; P = 0.08; Fig. 1). Striatal lesioned animals showed no similar bias on any trial (all P > 0.3). The difference between groups was significant on day 3 (two-tailed unpaired t test of goal-lure difference, t = 3.02; P = 0.007), although only at trend level on day 7 (P > 0.1), because some of the lesioned animals had begun to show some goal-quadrant bias and the group therefore showed a greater variance. Similar effects were seen when data were analyzed by target zone occupancy or cue proximity (not shown).
Fig. 1.
Dorsal striatal lesions impair cued learning and enhance spatial learning. (A) Minimum and maximum extent of lesions used in this experiment (adapted from ref. 46). No lesions extended into ventral striatum or affected amygdala, hippocampus, or thalamus; one animal was excluded because of unilateral extension of the lesion into the ventral striatum. (B) Cued learning, shown by occupancy in goal quadrant (black bars) and lure quadrant (gray bars) during probe trials. A significant bias toward the goal quadrant was seen on day 3 of training in control mice (n = 8) but not lesioned mice (n = 8). The same trends were apparent on day 7 (mean ± SEM; see text for statistics). (C) Spatial learning in day-3 and day-7 probe trials showing quadrant occupancy. Both lesioned and control mice showed clear spatial learning on all probe trials, with lesioned animals (n = 7) consistently showing greater spatial bias than controls (n = 9); this was significant on all days. (D) Somewhat larger dorsal striatal lesions were produced by stereotactic injection of NMDA; minimum and maximum lesion extent are shown. No lesions extended into ventral striatum, amygdala, hippocampus, or thalamus. (E) Striatal lesions accelerated spatial learning, shown by differential goal (black) and lure (gray) quadrant occupancy during day-3 and day-5 probe trials (n = 9 lesioned, n = 7 control; mean ± SEM; see text for details of statistical analysis). †, P < 0.1; *, P < 0.05; **, P < 0.01; ***, P < 0.005.
To test effects and interactions beyond these predicted effects, we analyzed all probe trials in a linear mixed model, with task (i.e., cued vs. spatial) and condition (i.e., lesion vs. control) as between-subject factors and probe trial day and quadrant (i.e., goal vs. lure) as within-subject factors. This analysis revealed a significant lesion-task-quadrant interaction (F[1,145] = 5.35; P = 0.02), indicating that lesions differentially affected quadrant bias in the two tasks. It also confirmed the effect of striatal lesions on the cued task: control animals showed a quadrant bias across probe trials (P = 0.035), on day 3 (P = 0.005), and on day 7 (P = 0.06); whereas lesioned animals showed no significant bias (all P > 0.2). The differential cue bias between groups was again significant on day 3 (P = 0.045).
We hypothesized that the effect of striatal lesions would be specific to the cued task—that is, that the spatial task would not be impaired by striatal dysfunction. The spatial task was learned more quickly than the cued task and resulted in more robust goal-cue biases; this recapitulates the pattern seen in rats (13) and in our own extensive pilot experiments (not shown). In the spatial task, both groups showed a clear goal bias on all probe trials (Fig. 1C; both groups, P < 0.0005 across probe trials). There was a highly significant effect of lesion on performance in the spatial task (P < 0.0005 across probe trials): lesions enhanced goal bias in the spatial task (Fig. 1). Similar effects were seen when data were analyzed by target zone occupancy or cue proximity (not shown).
This combination of impaired cued learning and enhanced spatial learning after a striatal lesion suggests alleviation of “normal” competition between memory systems during learning. Because an enhancement of spatial learning by striatal impairment has been hypothesized (4) but, to our knowledge, never observed, we replicated the effect in an independent cohort of mice after the creation of slightly larger excitotoxic dorsal striatal lesions (Fig. 1D). Animals were trained in the spatial task for 5 days (Fig. S3); training was conducted for only 5 days because pilot experiments showed control animals to always learn the spatial task in this time (whereas 7 days are sometimes required for acquisition of the cued task). On day 3, lesioned animals (n = 9) showed clear learning (Fig. 1E; two-tailed paired t test of goal vs. lure, t = 2.99, P = 0.017). Control animals learned more slowly in this experiment than in the first experiment (Fig. 1C) and had not yet achieved a significant goal-quadrant bias by day 3 (n = 7; t = −0.062; P > 0.9); this represents inter-experiment variability in the rate of learning as a result of environmental variables, but it enables us to see the enhancement of learning after the lesion with particular clarity in this experiment. After 5 days of training, both groups showed a clear goal bias (lesioned, t = 4.16, P = 0.003; control, t = 4.69, P = 0.003). When all data were analyzed by linear mixed model, the group-day-quadrant interaction was significant (F[1,42] = 4.65; P = 0.037). The effect of lesion was significant on day 3 (P < 0.005). Therefore, as in the first experiment, the striatal lesion enhanced (and/or accelerated) spatial learning in this task.
A degree of extinction is likely to occur over the course of a probe trial as an animal learns that the platform is not present; indeed, in pilot experiments we found repeated probe trials to result in reduced quadrant bias in later trials, especially in the cued task (not shown). Differential performance on later probe trials may therefore derive from differential extinction on earlier trials. However, the enhancement of spatial learning seen after striatal lesions is apparent in the first probe trial in both experiments (Fig. 1 C and E). Therefore, although we cannot exclude the possibility that striatal lesions affect extinction during probe trials, any such effect cannot explain the enhancement of spatial learning observed. Likewise, a series of control experiments ruled out the possibility that an effect of lesions on anxiety explained the differential impact on the spatial and cued tasks (Fig. S4). Swim speed did not differ between the groups (Fig. S4 C and F).
Disruption of Striatal Synaptic Plasticity in Transgenic Mice Also Impairs Cued Learning and Enhances Spatial Learning.
An advantage of investigating striatum-dependent learning tasks in mice is the opportunity to probe the underlying cellular and molecular mechanisms by using genetic manipulations (16). In many cases, such manipulations allow a greater degree of cellular and molecular specificity than is possible in other experimental systems. We have previously described transgenic mice expressing a dominant-negative mutant of the transcription factor CREB, KCREB, exclusively in the striatum, and predominantly in the dorsal striatum. CREB has been shown to have a role in long-lasting synaptic plasticity and in learning in numerous systems (28, 29); KCREB-expressing transgenic mice have a marked deficit in cortico-striatal synaptic plasticity (16). We tested these transgenic mice in cued and spatial learning in our water maze task to determine whether this disruption of cortico-striatal plasticity would be accompanied by an impairment in cued learning and an enhancement in spatial learning.
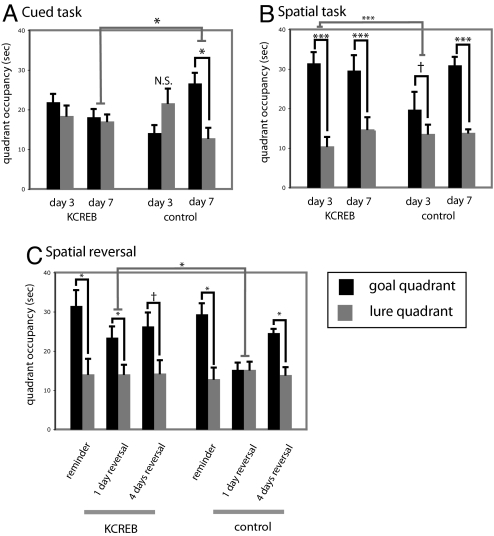
KCREB transgenic mice and litter-mate controls were trained in either the cued or the spatial task for 7 days, as before (Fig. S5; n = 8 KCREB-spatial, n = 10 KCREB-cued; n = 8 control-spatial; n = 7 control-cued). In the cued task, control animals showed a clear bias toward the goal by day 7 (Fig. 2A; two-tailed paired t test, t = 3.25; P = 0.023), whereas transgenic animals did not (t = .356; P > 0.5). This effect of genotype was significant (two-tailed t test of goal-lure difference, t = 2.57; P = 0.02), confirming that impairment of striatal CREB function, which disrupts cortico-striatal synaptic plasticity, impairs cued learning in this water maze task.
Fig. 2.
Impairment of striatal plasticity through inhibition of the transcription factor CREB impairs cued learning and enhances spatial learning. (A) Transgenic animals with impaired CREB activity in the dorsal striatum (16) (n = 10) showed no learning of the cued task after 7 days of training, whereas control animals (n = 7) showed significant bias toward the goal quadrant (black) relative to the lure quadrant (gray) by day 7 (mean ± SEM; see text for statistics). (B) Transgenic animals (n = 8) showed robust spatial learning by the third day of training in the spatial task, whereas controls (n = 8) required further training to develop a spatial bias; this enhancement in spatial learning recapitulates that seen after dorsal striatal lesions (Fig. 1). (C) Transgenic animals again show accelerated spatial learning after a spatial reversal. Spatial bias toward the original training quadrant persisted after 2 days of reminder training in both groups. After 1 day of reversal, learning of the new spatial location was exhibited by transgenic animals but not controls; both groups exhibited spatial learning after more extensive reversal training (all graphs display mean ± SEM; see text for details of statistical analysis). †, P < 0.1; *, P < 0.05; **, P < 0.01; ***, P < 0.005.
We again analyzed all data using a linear mixed model. This revealed a main effect of task (F[1,140] = 4.72; P = 0.03) as well as a nearly significant genotype-task-quadrant interaction (F[1,140] = 3.49; P = 0.064), suggesting that striatal KCREB expression, like striatal excitotoxic lesions, affected the two tasks differentially. The mixed-model analysis recapitulated effects in the cued task described in the primary analysis: control animals showed a clear goal bias on day 7 (P = 0.001), whereas transgenic animals showed no goal bias on any day (all P > 0.2). The effect of genotype on goal bias was significant on day 7 (P = 0.04).
In the spatial task, the effect of genotype on quadrant bias was highly significant (Fig. 2B; P < 0.0001). Control animals showed a trend toward spatial learning on day 3 (P = 0.094) and a clear spatial bias on the day 7 probe trial (P < 0.0001), whereas transgenic animals showed a highly significant spatial bias on all probe days (all P < 0.0001). The effect of transgene on quadrant bias in the spatial task was significant both across probe trials and specifically on day 3 (both P < 0.0001). Therefore, impairment of striatal CREB function and plasticity enhances spatial learning similarly to more profound striatal disruption by excitotoxic lesion.
KCREB Transgenic Mice Continue to Show Accelerated Learning upon Spatial Reversal.
If this enhancement of spatial learning derives from alleviation of competition, learning of a new spatial location should likewise be accelerated. To test this hypothesis, we conducted a spatial reversal on these KCREB animals. After 2 days of reminder training, animals trained in the spatial task continued to show clear bias toward the goal quadrant (Fig. 2C; transgenic, n = 9; paired t test, t = 2.45, P = 0.04; WT, n = 7; paired t test, t = 3.21, P = 0.02), with no statistically significant difference between genotypes.
We then continued training but switched the goal to the diametrically opposite quadrant. This manipulation dramatically increased the latency to escape (Fig. S5D). In a probe trial at the end of the first day of reversal training (i.e., after three training trials), transgenic animals showed clear spatial bias (Fig. 2C; paired t test, t = 2.314; P = 0.049), whereas controls did not (t = −0.27; P > 0.7); this predicted that enhancement of spatial learning in transgenic mice was significant (one-tailed t test of goal-lure occupancy difference, t = 1.99; P = 0.033). In contrast, after 4 days of reversal training, both groups showed spatial bias, with no significant difference between groups (Fig. 2C). Therefore, disruption of striatal information processing in these transgenic mice accelerates spatial learning on original learning and reversal and does not result in cognitive inflexibility or perseverative search, at least as assayed by this measure.
As in the case of striatal lesions, we speculated that alterations in anxiety might influence the balance between cued and spatial learning and thus confound our results. However, analysis in the elevated plus maze did not reveal any substantial effect of genotype on anxiety, and there was no difference in thigmotaxis between KCREB animals and controls. Likewise, total activity as measured in an open field was unchanged by the KCREB transgene. There was no significant difference between transgenic animals and controls in swim speed (Fig. S6).
Lesions of Dorsal Hippocampus Impair Spatial Learning and Potentiate Cued Learning.
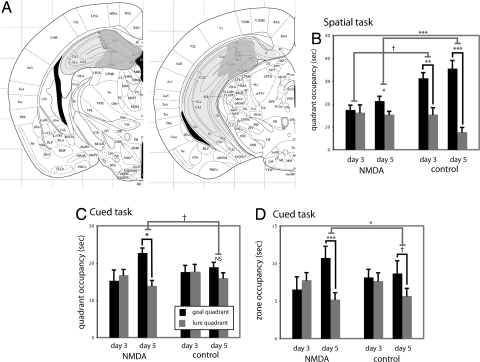
Because of the well established role of the dorsal hippocampus in spatial learning (5, 6), we hypothesized that dorsal hippocampal lesions would specifically disrupt learning in the spatial task. We further hypothesized that such lesions would also potentiate cued learning, as has been seen in certain other striatum-dependent tasks (12, 27). Excitotoxic lesions of dorsal hippocampus were produced by stereotaxic infusion of NMDA (30) (Fig. 3A). Lesioned and sham-operated control animals were trained in either the spatial or cued task, with 5 days of two-cue training (Fig. S7; n = 13 lesioned, spatial; n = 12 control, spatial; n = 15 lesioned, cued; n = 13 control, cued).
Fig. 3.
Hippocampal lesions impair spatial learning and enhance cued learning. (A) Minimum and maximum hippocampal lesions at two rostro-caudal levels (adapted from ref. 46). (B) Hippocampal lesions impaired spatial learning, as assayed in probe trials after 3 and 5 days of training (n = 13 lesioned, spatial; n = 12 control, spatial; n = 15 lesioned, cued; n = 13 control, cued; see text for statistical analysis). (C) Hippocampal lesions enhanced cued learning. Lesioned animals showed significant learning on day 5, whereas controls showed only a trend goal bias that did not reach significance at this time point. (D) Similar effects in the cued task were seen in an alternate analysis, wherein probe trial data were analyzed by occupancy in circular zones (25 cm diameter) around goal and lure cues. See text for statistical analysis. All data are mean ± SEM; †, P < 0.1; *, P < 0.05; **, P < 0.01; ***, P < 0.005.
In the spatial task, control animals showed clear learning on both day 3 (Fig. 3B; two-tailed paired t test, t = 3.05; P = 0.01) and day 5 (t = 5.36; P < 0.001), whereas lesioned animals showed no evidence of learning on day 3 (t = 0.54; P > 0.6) and only modestly significant learning on day 5 (t = 2.29; P = 0.043). The difference between groups was at trend level on day 3 (two-tailed t test of goal-lure difference, t = 1.736; P = 0.097) and robustly significant on day 5 (t = 3.79; P = 0.002). Therefore, as predicted, hippocampal lesions impair learning in the spatial task.
To examine all comparisons and interactions, we again analyzed all probe trial data by using a linear mixed model. This analysis revealed a significant lesion-task-quadrant interaction (F[1,147] = 14.85; P = 0.0002), indicating that the hippocampal lesions influenced goal bias in the two tasks differentially, and showed the same significant effects in the spatial task as our primary analysis. In the cued task, control animals developed only a weak bias toward the goal quadrant, which did not reach significance (Fig. 3C). This is consistent with pilot experiments in which cued learning was often not learned until day 7 (not shown; see also Fig. 2). In contrast, the lesioned animals developed a bias toward the goal quadrant after 5 days of training (P = 0.01). The effect of lesion on performance in the cued task was of borderline significance (Fig. 3C; P = 0.058). The effect of lesion was more clearly apparent when probe trial data were analyzed by occupancy in a 25-cm-diameter zone around the goal and lure cues (Fig. 3D); linear mixed model analysis of these data similarly showed a significant lesion-task-quadrant interaction (F[1,147] = 13.52; P = 0.0003). Lesioned animals showed clear learning on day 5 by this metric (P = 0.002) whereas control animals showed a trend (P = 0.065), and the difference between the two was significant (P = 0.013). This indicates that hippocampal lesions, which disrupt spatial learning, can enhance cued learning in this water maze task.
Control experiments showed that these hippocampal lesions did not lead to significant changes in anxiety or basal activity; the two groups showed no differences in swim speed (Fig. S8).
Discussion
The multiple memory systems theory suggests that dissociable neural circuits process and store information in distinct ways (2, 3). In many circumstances, these are likely to complement each other, but under some conditions they may compete for control of behavioral output during learning or performance (4). Enhancement of striatum-dependent learning after hippocampal disruption in a radial arm maze paradigm (12, 27) suggests that the hippocampus can inhibit striatum-dependent processing. However, we are aware of no evidence that these parallel systems compete bidirectionally, as predicted by the multiple memory systems model (3). The absence of a clear demonstration of bidirectional competition has left open several questions about the relationship between these two memory systems, such as whether they are recruited in parallel or in series and whether their interactions are more likely to be mediated by direct projections or through the participation of a downstream structure.
We describe a double dissociation between hippocampus-dependent and striatum-dependent systems that demonstrates such bidirectional competition. In a water maze task in which cued and spatial learning can be assayed in parallel, we find that dorsal striatal lesions both disrupt cued learning and enhance spatial learning. Similarly, disruption of dorsal striatal plasticity through expression of a dominant-interfering mutant of CREB (16) disrupts cued learning but enhances spatial learning. Finally, excitotoxic lesions of the dorsal hippocampus produce the converse patterns of effects, impairing spatial learning while accelerating cued learning. The results demonstrate that hippocampus-dependent spatial learning in mice occurs more quickly and more robustly than striatum-dependent cued learning, recapitulating what has previously been described in the rat (13). As a consequence, enhanced spatial learning after striatal manipulations is particularly striking at early time points (Fig. 1 C and E).
These results substantially clarify the nature of the interactions between these two systems during learning of a task in which both can be recruited. Interference by the hippocampus and associated medial temporal lobe structures with striatum-dependent processes may be mediated by direct projections (31) that appear to have an inhibitory effect on striatal function (32). However, there is no known similarly direct projection from the striatum, or the basal ganglia system more generally, to the medial temporal lobe. Therefore, although unidirectional competition—interference by hippocampal activity with striatum-dependent learning processes—may result from direct projections, the bidirectional competition we document is likely to require the involvement of other brain areas, such as medial frontal cortex (33–35) or amygdala (4, 36, 37).
Our results also shed light on the temporal relationship between hippocampus- and striatum-dependent processes during learning. Numerous studies suggest that, when both strategies can lead to successful navigation of a task, a hippocampus-dependent search strategy is acquired more quickly whereas more automatic striatum-dependent processing comes to dominate after more extensive training (14, 16, 38). Unidirectional competition, in which hippocampal function competes with the initial use of a striatum-dependent strategy, is consistent with this sequential model, as is the observation of increased reliance on a spatial strategy when the striatal system is perturbed (13, 39).
An alternative is the notion that both hippocampus and striatum are recruited early in a task and operate in parallel (3, 40); one may then come to dominate behavior if it is differentially reinforced by the contingencies of the task. This parallel-process model has been supported by human neuroimaging findings showing negatively correlated activation of hippocampus and striatum cognitive tasks (25) and by simultaneous processing by these two regions of different features of a virtual reality environment during navigation (41). The finding of bidirectional competition between memory systems is consistent with a parallel process model. Specifically, enhancement of hippocampus-dependent learning by striatal lesions even early during learning (i.e., before performance in control animals has reached asymptotic levels) indicates that the striatum is engaged, and therefore capable of competing with the hippocampus-dependent system, even during early phases of learning in intact animals.
Major advantages of performing such studies in mice include the ability to use sophisticated genetic tools to explore the underlying cellular and molecular mechanisms (16) and the opportunity to investigate striatum-dependent learning in mouse disease models (42). We take advantage of the unique strengths of this model system by analyzing the effect of functional disruption of striatal CREB in transgenic mice, and find that this manipulation both impairs cued learning and enhances spatial learning. Previous work has demonstrated that interference with CREB in the hippocampus can similarly disrupt spatial learning (8, 43); in conjunction with our current and past results (16), this clearly demonstrates that overlapping molecular mechanisms are used by these distinct memory systems. Further work will be needed to characterize the extent to which downstream, CREB-regulated genes overlap or are distinct in these different circuits.
In sum, we provide strong evidence for bidirectional competition between hippocampus- and striatum-dependent learning, in the form of a double dissociation. Striatal lesions impair cued learning while enhancing spatial learning, as does disruption of processes associated with striatal synaptic plasticity through expression of a dominant-negative CREB mutant in transgenic mice. In contrast, lesions of the dorsal hippocampus impair spatial learning and enhance cued learning. This study represents a substantial step toward understanding the interactions between these two memory systems during learning in a complex environment.
Materials and Methods
Animals.
These experiments were conducted under the supervision of Yale University's Institutional Animal Care and Use Committee (Animal Welfare Assurance Number A3230–1). Food (standard laboratory chow) and water were available ad libitum. All experiments examined adult male mice 2.5 to 6 months of age.
For lesion experiments, stereotaxic surgery was performed following standard procedures, under sterile conditions. Anesthesia was induced by i.p. injection of tribromoethanol (Sigma) dissolved in 2-methyl-2-butanol (Sigma) and then diluted 1:40 in normal saline solution (total dose, 275 mg tribromoethanol per kg). Excitotoxic lesions were performed by manual infusion of 0.1 to 0.2 μl NMDA (Sigma; 20 mg/ml in sterile saline solution) through a 0.5-μl Hamilton syringe over the course of 2 to 3 min. Targeting coordinates were determined from the work of Paxinos (44) and refined empirically. For dorsal striatal lesions, in the first experiment (Fig. 1A), 0.15 μl NMDA per side was infused (anterior-posterior [AP], +0.74 mm; medial-lateral [ML], ±2.2 mm; dorsal-ventral [DV], −3.0 mm); one animal was excluded because of extension of the lesion into ventral striatum unilaterally. In the second experiment (Fig. 1D), 0.2 μl NMDA per side was infused (AP, +0.74 mm; ML ±2.3 mm; DV, −3.5 mm); three animals lacked histologically demonstrable bilateral dorsal striatal lesions and were excluded. Hippocampal lesions (Fig. 3A) were produced as previously described (30) with 0.1 μl NMDA infused into each of two sites per side (coordinates: AP, −1.3 mm; ML ±1.0 mm; DV, −2.0 mm; and AP, −2.1 mm; ML, ±1.5 mm; DV, −2.2 mm); three animals were excluded with unilateral lesions.
Transgenic animals expressing a dominant-negative mutant form of human CREB, termed KCREB, have previously been described (8, 16). Animals hemizygous for the tetO-KCREB transgene (line str-KCREB) were bred to animals hemizygous for the calcium/calmodulin-dependent protein kinase II tTA transgene (ref. 45, line B); both these transgenic lines have been back-crossed to C57/Bl6 to greater than N12. Progeny were genotyped by PCR as previously described (16). Double-positive mice and litter-mate controls were used in all experiments; control animals were double-negative or KCREB-positive/tTA-negative. These two groups of control animals did not differ from one another in any analysis and were pooled.
Behavioral Testing.
Water maze.
Our water maze task is adapted from that described by Packard and McGaugh in rats (13). Modification of the protocol for mice was achieved through extensive pilot experiments. (See SI Methods and Fig. S1 for a detailed description of the procedure.)
Briefly, animals learned to escape a pool of opaque water (similar to that used in the Morris water maze [46, 47]) by swimming to one of two visually distinct cues. The round pool was 164 cm in diameter; the escape platform was 12 cm square and located 1 cm below the surface of the water. Three distinct visible cues were used; cues consisted of plastic cylinders, 11 cm high and 2.5 cm in diameter, painted either uniform gray or with sharp black-and-white stripes, 1 cm in width, oriented either horizontally or vertically.
The first 5 days consisted of shaping to the task. On day −5, animals were placed on the platform four times (20-min inter-trial interval). On days −4 through −1, the escape platform was marked with the uniform gray cue; animals were placed in the pool and allowed 120 seconds to swim to it.
Following shaping, animals were trained in the two-cue task for 5 or 7 days; each animal was trained in either the cued or spatial task, never in both. All experiments consisted of four trials per day with a 20-min inter-trial interval. In the cued task, the escape platform was moved on each trial but was reliably marked by one of the two cues (i.e., either horizontal or vertical stripes, held constant throughout training for each animal but counterbalanced across animals within each group). In the spatial task, the escape platform was always in the same location but was variably associated with the two striped cues. In both tasks, the second visible cue (i.e., the lure) was present in a quadrant adjacent to the escape platform and its associated cue (i.e., the goal) on a stand that held it at an identical height in the water but did not permit escape. Latency to find the escape platform was measured for all training trials; search was recorded by an overhead digital camera.
Learning was assayed by using a probe trial, administered in place of the fourth training trial after 3, 5, and/or 7 days of training, as specified later for each experiment. In the probe trial, both goal and lure cues were placed on stands that did not allow escape; the animal's search was monitored by an overhead camera over 60 seconds. Extra-maze cues were identical to those present in a training trial. In both the cued and the spatial task, a systematic bias toward the goal cue relative to the lure cue (i.e., toward the location where the platform would have been on a regular training trial) was interpreted as evidence of learning. This was quantified by quadrant occupancy. Other measures (mean distance from the goal and lure cues during search and occupancy in circular zones centered on the goal and lure cues) gave similar results (not shown). Probe trial track analysis was performed using Ethovision (Noldus).
Other behavioral tests.
Anxiety was assayed in an elevated plus maze as previously described (48). Locomotor activity was quantified in an unfamiliar open field box (50 cm × 50 cm); exploratory activity over 10 min was monitored using an overhead camera. Time spent in the central zone (25 cm × 25 cm) was quantified.
Temperature Monitoring.
Temperature was monitored using a DAS-6007 s.c. probe (Bio-Medic Data Systems).
Documentation of Lesions.
Excitotoxic lesions were documented using immunohistochemistry for GFAP and NeuN; Nissl staining gave similar results but documented the lesions less clearly in striatum (not shown). See SI Methods for full details.
Statistical Analysis.
All analysis was performed in consultation with a staff statistician. In water maze experiments, a priori hypotheses were tested by t test. Subsequently, all data were subjected to linear mixed modeling, with task (cued vs. spatial) and condition (lesion vs. control or KCREB transgenic vs. control) as between-subject factors, and probe trial and quadrant (goal or lure) as within-subject factors. Data were modeled for each experiment to determine the best-fit variance-covariance matrix; in all cases, compound symmetry was found to provide the best fit. Lower-order effects were extracted from the model and corrected for multiple comparisons with Bonferroni correction. Latency data were analyzed by multivariate ANOVA, with condition as a between-subject factor and day and trial as nested within-subject factors. Control parameters were tested by t test or, where data were non-normal, by the Mann–Whitney U test.
Supplementary Material
Acknowledgments.
The authors thank J. Taylor, J. Quinn, and S. Gourley for valuable input during the development of this behavioral task and for useful comments on the manuscript; S. Wilber for genotyping; the Connecticut Department of Mental Health and Addiction Services for its support of the Ribicoff Research Facilities; and G. Williams, C. Williams, M. Mercier, and S. Burkowsky for assistance with animal care. This work was supported by National Institutes of Health (NIH) grant R01-45481 (Method to Extend Research in Time Award; to R.S.D.); and by NARSAD, an APA/Wyeth Pharmaceuticals MD/PhD research fellowship, the Tourette Syndrome Association of America, and National Institutes of Health Grant K08 MH081190 (to C.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807749105/DCSupplemental.
References
- 1.Tolman EC. There is more than one kind of learning. Psychol Rev. 1949;56:144–155. doi: 10.1037/h0055304. [DOI] [PubMed] [Google Scholar]
- 2.Warrington EK. Neuropsychological evidence for multiple memory systems. CIBA Found Symp. 1979;69:153–166. doi: 10.1002/9780470720523.ch9. [DOI] [PubMed] [Google Scholar]
- 3.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 4.Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford Univ Press; 1978. [Google Scholar]
- 6.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 7.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger C, et al. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- 9.Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Ann Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 10.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 11.Graybiel AM. Habits, rituals, and the evaluative brain. Ann Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 12.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition oft two water maze tasks: further evidence for multiple memory systems. Behav Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- 14.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 15.Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- 16.Pittenger C, et al. Impaired bidirectional synaptic plasticity and procedural memory formation in striatum-specific cAMP response element-binding protein-deficient mice. J Neurosci. 2006;26:2808–2813. doi: 10.1523/JNEUROSCI.5406-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 18.Eldridge LL, Masterman D, Knowlton BJ. Intact implicit habit learning in Alzheimer's disease. Behav Neurosci. 2002;116:722–726. [PubMed] [Google Scholar]
- 19.Marsh R, et al. Habit learning in Tourette syndrome: a translational neuroscience approach to a developmental psychopathology. Arch Gen Psych. 2004;61:1259–1268. doi: 10.1001/archpsyc.61.12.1259. [DOI] [PubMed] [Google Scholar]
- 20.DeCoteau WE, et al. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc Natl Acad Sci USA. 2007;104:5644–5649. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson A, van der Meer MA, Redish AD. Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol. 2007;17:692–697. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behav Neurosci. 2004;118:438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- 23.Rauch SL, et al. Probing striatal function in obsessive-compulsive disorder: a PET study of implicit sequence learning. J Neuropsych Clin Neurosci. 1997;9:568–573. doi: 10.1176/jnp.9.4.568. [DOI] [PubMed] [Google Scholar]
- 24.Sherry DF, Schacter DL. The evolution of multiple memory systems. Psychol Rev. 1987;94:439–454. [Google Scholar]
- 25.Poldrack RA, et al. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- 26.Hartley T, Maguire EA, Speirs HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 27.McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Pittenger C, Kandel E. A genetic switch for long-term memory. C R Acad Sci III. 1998;321:91–96. doi: 10.1016/s0764-4469(97)89807-1. [DOI] [PubMed] [Google Scholar]
- 29.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen KE, Witter MP. Entorhinal efferents reach the caudato-putamen. Neurosci Lett. 1983;35:259–264. doi: 10.1016/0304-3940(83)90327-0. [DOI] [PubMed] [Google Scholar]
- 32.Finch DM, Gigg J, Tan AM, Kosoyan OP. Neurophysiology and neuropharmacology of projections from entorhinal cortex to striatum in the rat. Brain Res. 1995;670:233–247. doi: 10.1016/0006-8993(94)01279-q. [DOI] [PubMed] [Google Scholar]
- 33.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- 34.Coutureau E, Killcross S. Inactivaiton of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res. 2003;146:167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb Cortex. 2007;17:2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- 36.Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packard MG, Teather LA. Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Behav Neurosci. 1998;111:543–551. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 38.Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald RJ, White NM. Parallel processing in the water maze: evidence for independent memory systems involving the dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 40.Martel G, et al . Dynamic interplays between memory systems depend on practice: the hippocampus is not always the first to provide solution. Neuroscience. 2007;150:743–753. doi: 10.1016/j.neuroscience.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci USA. 2008;105:5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Leonibus E, et al. Spatial deficits in a mouse model of Parkinson disease. Psychopharm (Berl) 2007;194:517–525. doi: 10.1007/s00213-007-0862-4. [DOI] [PubMed] [Google Scholar]
- 43.Bourtchouladze R, et al. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G. The Mouse Brain in Stereotaxic Coordinates, Second edition. San Diego: Academic; 2003. [Google Scholar]
- 45.Mayford M, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 46.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 47.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunsberger JG, et al. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.