Abstract
Signals received at distal synapses of neurons must be conveyed to the nucleus to initiate the changes in transcription that underlie long-lasting synaptic plasticity. The presence of importin nuclear transporters and of select transcription factors at synapses raises the possibility that importins directly transport transcription factors from synapse to nucleus to modulate gene expression. Here, we show that cyclic AMP response element binding protein 2 (CREB2)/activating transcription factor 4 (ATF4), a transcriptional repressor that modulates long-term synaptic plasticity and memory, localizes to distal dendrites of rodent hippocampal neurons and neurites of Aplysia sensory neurons (SNs) and binds to specific importin α isoforms. Binding of CREB2 to importin α is required for its transport from distal dendrites to the soma and for its translocation into the nucleus. CREB2 accumulates in the nucleus during long-term depression (LTD) but not long-term potentiation of rodent hippocampal synapses, and during LTD but not long-term facilitation (LTF) of Aplysia sensory-motor synapses. Time-lapse microscopy of CREB2 tagged with a photoconvertible fluorescent protein further reveals retrograde transport of CREB2 from distal neurites to the nucleus of Aplysia SN during phenylalanine-methionine-arginine-phenylalanine-amide (FMRFamide)-induced LTD. Together, our findings indicate that CREB2 is a novel cargo of importin α that translocates from distal synaptic sites to the nucleus after stimuli that induce LTD of neuronal synapses.
Keywords: Aplysia, hippocampus, transcription, long-term depression, dendra
Synaptic plasticity is critical to cognition and memory formation. Long-lasting forms of synaptic plasticity require both transcription and translation (1–3), and specific patterns and strengths of synaptic stimulation trigger changes in neuronal gene expression (4). However, relatively little is known about how signals are transported from synapse to nucleus to regulate transcription. Many types of synaptic stimulation induce depolarization and electrochemical signaling to rapidly alter transcription in the nucleus (5). A slower, more persistent mechanism of signaling to the nucleus involves the transport of soluble molecules from stimulated synapses to the nucleus (6, 7). Recent studies have indicated that this type of transport may involve the active nuclear import pathway (8–10).
In the classical nuclear import pathway, proteins bearing nuclear localization signals (NLSs) bind an adaptor protein of the importin α family, which in turn binds the importin β1 nuclear transporter. Importin β1 mediates facilitated translocation of this heterotrimeric complex across the nuclear pore. In neurons, importins may also function to carry signals from distal neuronal processes to the soma and into the nucleus (8–11). This finding raises a critical question: what are the synaptically localized cargoes of importins that translocate to the nucleus to alter transcription during synaptic plasticity?
One attractive class of potential importin cargoes includes synaptically localized transcription factors, whose transport from synapse to nucleus would allow synaptic signals to be directly converted into changes in gene expression. In the present study, we investigated stimulus-induced changes in the subcellular localization of the transcriptional repressor CREB2/ATF4 in neurons. We focused on CREB2 because of its critical role in setting the threshold for long-term synaptic plasticity and memory in both Aplysia and mice (12–16). Thus, in Aplysia, CREB2 activity is specifically required for LTD of sensory-motor synapses induced by the neuropeptide FMRFamide (17–19). In rodent hippocampus, the translationally regulated expression of CREB2/ATF4 bidirectionally controls long-term plasticity and memory (16). Studies in rodent forebrain have also shown that CREB2 interacts with the metabotropic GABAB receptor by means of its b-ZIP domain, and that both proteins colocalize in neuronal dendrites (20–22).
Here, we performed studies in cultured rodent hippocampal and Aplysia sensory-motor neuronal cultures, 2 model systems for studying learning-related plasticity, to show that CREB2 is a novel cargo of importin α that translocates from sites of stimulation to the nucleus during LTD.
Results
CREB2 Is Present at Hippocampal Synapses and Binds Specific Importin α Isoforms.
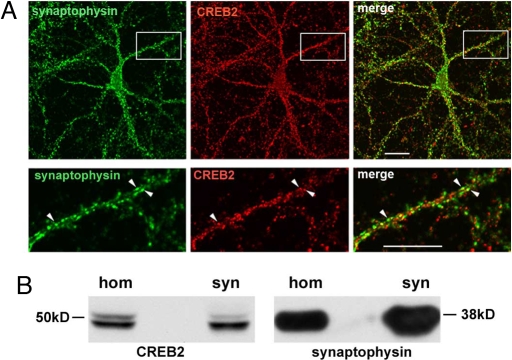
We first examined the subcellular localization of CREB2 in cultured hippocampal neurons to see whether it was appropriately localized for retrograde transport. Immunocytochemistry with anti-CREB2 rabbit polyclonal and mouse monoclonal antibodies [supporting information (SI) Fig. S1A] revealed that CREB2 was present in somatic and dendritic compartments. CREB2 immunoreactivity was punctate in the dendrite, and double label immunocytochemistry indicated that a fraction colocalized with the synaptic markers synaptophysin and postsynaptic density protein 95 (PSD95) [39% of CREB2 puncta colocalized with synaptophysin and 75% of synaptophysin puncta colocalized with CREB2; 24% of CREB2 puncta colocalized with PSD95 and 41% of PSD95 puncta colocalized with CREB2, Fig. 1A and Fig. S2A; see Materials and Methods]. To verify the synaptic localization of CREB2, we immunoblotted synaptoneurosome fractions from adult rat forebrain with anti-CREB-2 antibodies, and detected a band of 48 kD, corresponding to CREB2 (Fig. 1B). Together, these results indicate that CREB2 is present not only in the soma of mature neurons but also in distal dendrites and synapses.
Fig. 1.
CREB2 is present in distal neuronal processes and at synapses. (A) Immunocytochemistry of cultured hippocampal neurons (23 DIV) with anti-CREB2 (red) and p38 synaptophysin (green) antibodies revealed CREB2 immunoreactivity in dendrites where it colocalized with some p38 synaptophysin puncta (arrowheads). Shown are photomicrographs of confocal optical sections through the dendritic arbor; boxed area is shown at higher zoom (Lower). (B) Whole brain homogenate (hom) and synaptosome (syn) fractions immunoblotted with CREB2 and synaptophysin antibodies. [Scale bar, 20 μm (Upper); 10 μm (Lower).]
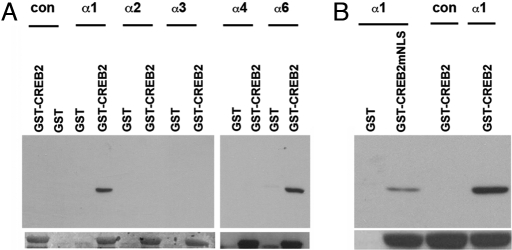
Although the mechanism whereby CREB2 is transported into the nucleus is unknown, Cibelli et al. (23) defined a bipartite NLS in CREB2, suggesting that it might be an importin α cargo. The mouse genome encodes 5 importin α isoforms, which are classified into 3 subclasses on the basis of sequence homology: S (α1 and 6), P (α2), and Q (α3 and 4) (24). We performed GST pull-down assays on lysates of 293T cells that had been individually transfected with each importin α isoform containing N-terminal FLAG tags. GST-CREB2 pulled down exclusively importin α1 and 6, which belong to the importin α S subclass (Fig. 2A). Previous study has shown that 5 amino acids (KKLKK, amino acids 280–284) within the bipartite NLS of CREB2 are crucial for its nuclear localization in COS cells (23). Mutation of the lysine residues (KKLKK to NSLNS) significantly reduced the binding of importin α1 in GST pull-down assays (Fig. 2B). The fact that binding was not abrogated may be due to the existence of a second NLS at amino acids 294 to 300 (RYRQKKR), or to the potential that GST-CREB2 dimerizes with endogenous CREB2, which in turn binds importin α. However, together these data indicate that CREB2 binds specific importin α isoforms by means of its NLS.
Fig. 2.
CREB2 binds importin α1 and 6. (A) FLAG-tagged importin α isoforms expressed in 293T cells were incubated with GST-CREB2 beads, and immunoblotted with anti-FLAG antibody. GST-CREB2 pulled down importin α1 and 6, but not α2, 3, or 4. (B) Mutations in the NLS of CREB2 (CREB2mNLS) reduced the amount of interacting importin α1. Immunoblotting with GST antibodies (A and B Lower) shows that equal amounts of GST-CREB2 were used in each pull-down assay (note that GST alone runs at a lower MW and is not shown).
Nuclear Import and Retrograde Transport of CREB2 Is Mediated by Importin α in Hippocampal Neurons.
To determine whether nuclear localization of CREB2 involved importin-mediated transport, we used a membrane-permeable peptide that contains the NLS of NF-kappa B p50 subunit (25) which has been shown to block nuclear import of several transcription factors (26). Incubation with the NLS peptide significantly reduced the nuclear accumulation of CREB2, compared with untreated control cultures or to cultures incubated with peptides in which the NLS was mutated so that it no longer binds to importin α (Fig. 3A). This finding is not only consistent with CREB2 being transported into the nucleus by importin α, but it also indicates that CREB2 shuttles between cytoplasm and nucleus in cultured hippocampal neurons. Notably, the NLS peptides did not simply block the nuclear import of newly synthesized CREB2, because a decrease in nuclear CREB2 was also observed when neurons were preincubated with the protein synthesis inhibitor emetine (Fig. S3A).
Fig. 3.
Retrograde transport and nuclear localization of CREB2 requires NLS- importin interactions. (A) Hippocampal neurons (10–12 DIV, serum-containing medium) were incubated with NLS peptide (50 μg/mL) or mutant peptide (mNLS) for 1h, and stained with anti-CREB2 (green) and anti-MAP2 (red) antibodies. Nuclear CREB2 (green) was reduced with the NLS but not the mNLS peptide (P < 0.0001, Student's t test; n = 56 and 49, respectively, t = 4.1, df = 103). (B) Dendra-CREB2 was transfected into hippocampal neurons (9–10 DIV) and imaged after photoconversion of a small region in the dendrite (arrow). An increase in photoconverted Dendra-CREB2 (red) was observed in the nucleus within 3 min after photoconversion (arrowhead), with a reduction in the dendrite. Neurons transfected with Dendra-CREB2 were preincubated with NLS peptide or mutant peptide (mNLS) for 30 min before imaging. The percentage of photoconverted red signal that persisted at the site of photoconversion in the dendrite, and the percentage increase in the nucleus were quantified for all 3 conditions (line graph, n = 7, 11, and 9 for no peptide, NLS, and mNLS peptides respectively). The decrease in the dendrite and increase in the nucleus in the no peptide and mNLS peptide groups was statistically significant (P < 0.01) at all time points by ANOVA and Dunnett's posthoc multiple comparisons test (F = 7.19 and df = 24 for increase in nucleus; F = 119 and df = 30 for decrease in dendrite). [Scale bar, 10 μm (A); 20 μm (B).]
To determine whether importin α transported CREB2 from distal sites to the soma, we used live cell imaging of cultured hippocampal neurons. To follow the retrograde movement of dendritically localized CREB2, we tagged CREB2 at its N terminus with the photoconvertible fluorescent protein Dendra, which converts from green to red with brief UV irradiation (27). After photoconverting a small (≈20 μm) dendritic region (40 to 60 μm from the soma), we followed the photoconverted CREB2 over time (Fig. 3B). Consistent with retrograde transport of CREB2 from dendrite to nucleus, the red signal in the nucleus increased over time, accompanied by a rapid decrease from the site of photoconversion in the dendrite (Fig. 3B). Dendra-CREB2 that was photoconverted in the dendrite was predominantly nuclear by 20 min (nuclear to cytoplasmic ratio of 26 ± 7, n = 7). To determine whether this nuclear concentration required an intact NLS, we mutated the NLS in CREB2 (Dendra-CREB2-mNLS), and found that it distributed more evenly between the cytoplasm and nucleus 20 min after photoconversion in the dendrite (nuclear to cytoplasmic ratio of 1.5 ± 0.2, n = 6,), as did dendra itself (not fused to CREB2) (nuclear to cytoplasmic ratio at 20 min of 1.2 ± 0.3, n = 6; Fig. S4).
To confirm that importin α mediates the retrograde transport of CREB2 in dendrites, neurons transfected with Dendra-CREB2 were incubated with the cell-permeable NLS peptide. Incubation with the NLS peptide significantly impeded the retrograde movement of Dendra-CREB2, whereas incubation with the mutant NLS peptide did not (Fig. 3B). In contrast, the passive diffusion of Dendra (not fused to CREB2) was not affected by the NLS peptide (data not shown). Together, these observations indicate that CREB2 is transported from distal dendrites to the nucleus in an importin α dependent manner.
Nuclear Import of CREB2 During LTD but Not LTP of Cultured Hippocampal Synapses.
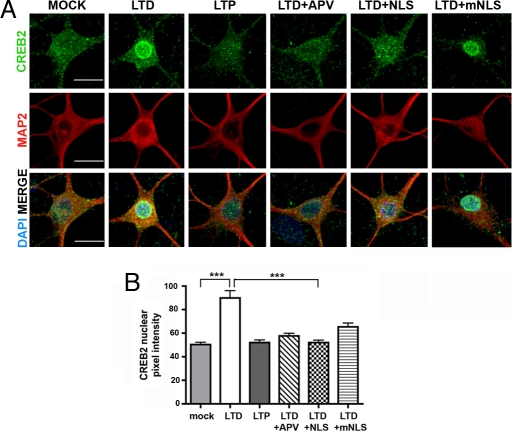
To determine whether CREB2 nuclear translocation is regulated by stimuli that elicit synaptic plasticity, we used protocols previously shown to produce LTD or LTP of miniature excitatory postsynaptic currents (mEPSCs) in cultured hippocampal neurons (28). These experiments were conducted in older, serum-free cultures (21–28 DIV; Fig. 4), where the nuclear concentration of CREB2 in unstimulated neurons was lower than in younger (10–12 DIV) cultures grown in serum-containing medium (Fig. 3). We induced LTD of mEPSCs with a brief application of NMDA together with glycine, and LTP of mEPSCs with a brief application of glycine, fixed the cultures, and processed them for immunocytochemistry with anti-CREB2 antibodies. As shown in Fig. 4, nuclear CREB2 immunoreactivity was significantly increased by stimuli inducing LTD but not LTP of hippocampal neurons. The increase in the nucleus was accompanied by a loss of CREB2 immunoreactivity from dendrites (Fig. S3B). Incubation with the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV), which blocks LTD (28), blocked the NMDA/glycine-induced increase in nuclear CREB2. To determine whether this increase involved importin-mediated nuclear import, we incubated the cultures with membrane permeable NLS peptides, or as a control with membrane permeable mutant peptides, before and during stimulation. Consistent with a requirement for importins, the NLS peptides blocked the LTD-induced increase in nuclear CREB2 immunoreactivity, whereas the mutant peptides had no effect.
Fig. 4.
Importin-mediated nuclear accumulation of CREB2 during NMDA-induced LTD of hippocampal neurons. (A) Hippocampal neurons (23 DIV) were incubated with NMDA (20 μM) and glycine (20 μM) for 5 min to induce LTD or with glycine (200 μM) for 5 min to induce LTP. In some cultures, APV (100 μM) was added to block the NMDA receptor. To examine the role of importins in CREB2 nuclear translocation, membrane permeant NLS peptides (50 μg/mL) or mutant NLS peptides (mNLS; 50 μg/mL) were added for 30 min before, during and after LTD stimulation. Mock treated cultures received vehicle (H2O). We stained cultures 25 min after the end of stimulation with anti-CREB2 (green) and anti-MAP2 (red) antibodies, and nuclei with DAPI. (Scale bar, 10 μm.) (B) Quantification of the nuclear pixel intensity of CREB2 reveals that LTD but not LTP induces CREB2 nuclear translocation and nuclear import can be blocked by NLS peptides (***, P < 0.001; n = 15 each, F = 18.89, df = 99 1-way ANOVA and Newman–Keul's multiple comparison test).
CREB2 Interacts with Importin α in Aplysia Neurons and Is Concentrated at Synapses in SN/MN Cocultures.
To further investigate the function of importin-mediated CREB2 nuclear import during synaptic plasticity, we turned to the Aplysia sensory-motor neuronal culture preparation, where specific stimulation protocols elicit transcription-dependent LTD and LTF (1). Of particular interest to us, previous studies have established a role for CREB2 during FMRFamide-induced LTD of sensory-motor synapses (18, 19).
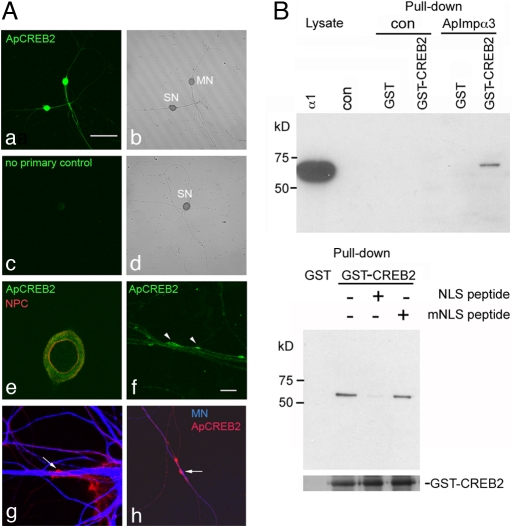
We previously identified an isoform of importin α, ApImp-α3, in Aplysia (9). In pull-down assays in which GST-ApCREB2 was incubated with lysates of 293T cells expressing FLAG-tagged ApImp-α3, we detected an interaction between the 2 proteins (Fig. 5B Upper). The interaction between ApImp-α3 and ApCREB2 was specific and required the NLS of ApCREB2, because incubation of the pull-down reaction with excess NLS peptide abolished the interaction (Fig. 5B Lower).
Fig. 5.
Aplysia CREB2 localizes to distal neurites of Aplysia SNs and interacts with Aplysia importin α3. (A) ApCREB2 was present in soma and distal neurites of Aplysia SN-MN cultures as detected by immunocytochemistry with anti-ApCREB2 antibodies (a; ref. 12). No signal was detected in controls (cultured SN) lacking primary antibody (c). Higher magnification revealed little ApCREB2 staining in the nucleus, outlined with anti-nuclear pore complex (NPC) antibodies (e), but positive CREB2 immunoreactivity in distal neurites and varicosities (arrowheads, f). Labeling of the MN with Alexa Fluor 647 (blue) shows that ApCREB2 immunoreactivity (red) was present in SN varicosities (arrows in g and h). [Scale bar, 200 μm (a–d); 20 μm (e–h).] (B) Lysates of 293T cells expressing FLAG-ApImp-α3 were incubated with GST-ApCREB2 beads. ApImp-α3 was pulled down by GST-ApCREB2 but not GST beads (Upper). Incubating the lysate with excess NLS peptide prevented the interaction between ApImp-α3 and ApCREB2, whereas mNLS peptide had no effect. Immunoblotting with GST antibodies (Lower) shows that equal amounts of GST-CREB2 were used (note that GST alone runs at a lower MW and is not shown).
To establish whether ApCREB2, like its mammalian homolog, localized to distal neurites and synapses of Aplysia SN-motor neuron (MN), we performed immunocytochemistry of cultured sensory-motor synapses by using 2 polyclonal antibodies (12, 29) (Fig. S1B). As shown in Fig. 5A and Fig. S2B, immunocytochemistry with both antibodies revealed that ApCREB2 was present in the somatic cytoplasmic with little immunoreactivity in the nucleus (Fig. 5Ae). Notably, it was also present in distal neurites, where it appeared to concentrate in varicosities (Fig. 5Af and Fig. S2B). We labeled the motor neurons with a volume-filling fluorescent dye (dextran Alexa Fluor 647, blue, Fig. 5 Ag and Ah) to distinguish between immunoreactivity in the SN and MN. These experiments revealed that ApCREB2 was concentrated at SN varicosities (Fig. 5 Ag and Ah).
FMRFamide Induced Retrograde Trafficking and Nuclear Translocation of ApCREB2 in SNs.
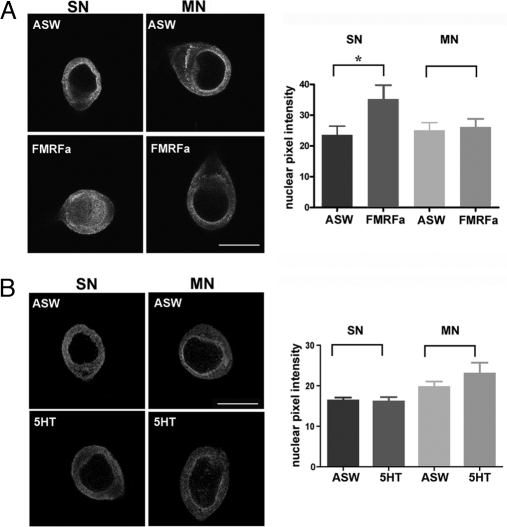
Last, we asked whether the retrograde transport and nuclear import of ApCREB2 were regulated by stimuli that induce transcription-dependent synaptic plasticity. Because ApCREB2 was predominantly cytoplasmic in unstimulated SNs, we reasoned that FMRFamide, which induces CREB2-dependent LTD of sensory-motor synapses (18, 19), should induce nuclear translocation of ApCREB2. Indeed, 5 pulses of FMRFamide significantly increased the concentration of ApCREB2 in the SN nucleus (Fig. 6A). This nuclear translocation was specific to SNs, and did not occur in MNs. Also, the nuclear translocation was specific to LTD, as it was not observed after 5 pulses of serotonin (5HT), which induce LTF of sensory-motor synapses (Fig. 6B).
Fig. 6.
ApCREB2 accumulates in the nucleus of SNs during FMFRamide-induced LTD of sensory-motor synapses. SN-MN cultures (5DIV) received 5 spaced applications of FMFRamide (5xFMFRa) to induce LTD (A) or 5 spaced applications of 5HT (5x5HT) to induce long-term facilitation (B), or 5 applications of ASW (5xASW) as control. Cultures were immediately fixed and processed for immunocytochemistry with anti-ApCREB2 antibodies. Representative confocal optical sections through the nucleus are shown on the left and group data on the right. (A) Spaced application of FMFRamide increased the concentration of ApCREB2 in the SN nucleus but not in the MN nucleus, whereas ASW had no effect (*, P < 0.05, ASW SN, n = 23 vs. FMFRamide SN, n = 19, unpaired t test, t = 2.2, df = 40). (Scale bar, 40 μm.) (B) Spaced application of 5HT did not significantly alter the nuclear concentration of CREB2 in either SN or MN (n = 11 for ASW, and 7 for 5HT for both SN and MN).
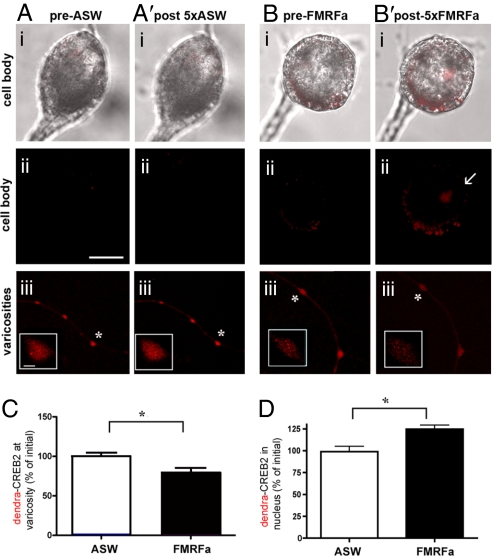
To determine whether the increase in nuclear ApCREB2 after 5 pulses of FMRFamide treatment might result from the transport of ApCREB2 from varicosities, we expressed Dendra-ApCREB2 in the SN of the SN-MN coculture. Dendra-ApCREB2 was photoconverted in distal neurites, and the coculture was then treated with 5 pulses of artificial seawater (ASW) or FMRFamide. Significantly less photoconverted Dendra-ApCREB2 remained at the varicosities (Fig. 7B′iii) and more photoconverted Dendra-ApCREB2 was present in the nucleus (Fig. 7B′i and ii) after the fifth pulse of FMRFamide, as compared with control cultures mock treated with ASW (Fig. 7A). These results suggest that 5 pulses of FMFRamide trigger the translocation of CREB2 from varicosities to the nucleus during LTD.
Fig. 7.
Time-lapse microscopy reveals translocation of ApCREB2 from varicosity to nucleus in Aplysia SNs during LTD. SNs were microinjected with Dendra-ApCREB2 and, 24 h later, Dendra-ApCREB2 was photoconverted in neurites and the cultures were stimulated with 5 pulses of FMRFamide or ASW. Representative images showing phase and photoconverted red dendra-ApCREB2 in cell bodies before (Ai) and after (A′i) 5xASW or before (Bi) and after (B′i) 5xFMRFamide; photoconverted red dendra-ApCREB2 alone in the cell body before (Aii) and after (A′ii) 5xASW or before (Bii) and after (B′ii) 5xFMRFa; and photoconverted red dendra-ApCREB2 in distal varicosities before (Aiii) and after (A′iii) 5xASW or before (Biii) or after (B′iii) 5xFMRFa. Photoconversion was done immediately before the first application of ASW or FMRFa; photoconverted (red) Dendra-CREB2 is reduced in varicosities and increased in the nucleus after the fifth pulse of FMRFamide, but not ASW. Note that Dendra-CREB2 accumulates in large puncta in the nucleus. Asterisks indicate varicosities that are digitally enlarged in Aiii and Biii (Inset). [Scale bar, 20 μm (Aii); 2.5 μm (Aiii).] Quantification of the difference in intensity of the red photoconverted signal in varicosities remaining after the fifth stimulation revealed a significant decrease in varicosities (*, P < 0.05, n = 38 and 47 for ASW and FMRFamide treatments, respectively; unpaired t test, t = 2.6, df = 17) and a significant increase in the nucleus (*, P < 0.05, n = 3 for ASW and 5 for FMRFamide, t = 3.6, df = 5, unpaired t test) with FMRFa compared with ASW.
Discussion
Extracellular stimuli produce long-lasting changes in cellular function by altering transcription in the nucleus. Frequently, this process involves the convergence of signal transduction cascades within the nucleus, where they modulate the activity of transcriptional regulators. Stimulus-induced nuclear import of transcription factors provides a potentially more direct means of signaling from sites of stimulation to the nucleus (30, 31). A number of transcription factors and transcriptional regulators, including NFκB, Elk-1, NFAT, and CAMAP have indeed been shown to translocate from distal processes to the nucleus in neurons (32–36).
The finding that importin α localizes to synapses and translocates to the nucleus after synaptic stimulation (9) suggested that synapse-to-nucleus trafficking of NLS-containing cargoes might underlie long-term synaptic plasticity. Consistent with this possibility, a recent study identified Jacob, a caldendrin binding partner, as a synaptically localized cargo of importin α1 that translocates to the nucleus after extrasynaptic NMDA receptor activation (10). In the present study, we show that the transcriptional repressor CREB2 is a synaptically localized cargo of importins. Specifically, we show that CREB2 localizes to distal dendrites of hippocampal neurons and to distal neurites of Aplysia neurons and that this pool of CREB2 translocates to the nucleus in an importin-mediated manner. We further provide evidence that LTD-inducing stimuli increase the concentration of CREB2 in the nucleus both in rodent hippocampal and in Aplysia SNs-MNs.
The regulated nucleocytoplasmic trafficking of CREB2 is of particular interest because CREB2 is postulated to set the threshold for plasticity and memory in both mouse and Aplysia. Costa-Mattioli et al. (15, 16) have shown that regulated translation of CREB2 controls CREB-dependent transcription and modulates hippocampal plasticity and memory. Our studies indicate that the nucleocytoplasmic trafficking of CREB2 provides an additional means of regulating the activity of CREB2. Thus, we found that NLS-containing peptides reduced the nuclear accumulation of CREB2 in cultured hippocampal neurons even in the presence of protein synthesis inhibition (Fig. S3A), suggesting that the nuclear import of preexisting CREB2 is regulated independently of new translation. Whereas the increased nuclear concentration of CREB2 after LTD may result in part from regulated nuclear export, our findings that (i) this increase is blocked by excess NLS peptides, which saturate importin α and inhibit classical nuclear import (Figs. 3 and 4), and (ii) that dendra-ApCREB2 translocates from neurite to nucleus in Aplysia SNs specifically during FMRFamide-induced LTD (Figs. 6 and 7) provide strong evidence that LTD-inducing stimuli specifically regulate the importin-mediated nuclear transport of CREB2.
Recent studies (35, 37) have reported that the mRNAs encoding 2 transcription factors, CREB1 and Elk-1, localize to axons and dendrites of neurons, respectively, and that both transcription factors are locally translated and transported to the nucleus. We explored the possibility that CREB2 mRNA was also synaptically localized and translated but did not detect CREB2 mRNA in hippocampal dendrites or Aplysia neurites by in situ hybridization (Fig. S5). Thus, our data are more consistent with the regulated nuclear transport of somatically synthesized but dendritically localized CREB2.
Experiments conducted in cultured hippocampal neurons (10–12 DIV) indicate that CREB2 shuttles dynamically in and out of the nucleus, because in the absence of any stimuli, NLS peptides decrease nuclear CREB2 immunoreactivity, and overexpressed Dendra-CREB2 photoconverted in the dendrite translocates into the nucleus (Fig. 3). However, that the nuclear import of CREB2 is specifically regulated by LTD-inducing stimuli is indicated by 3 findings. First, the concentration of nuclear CREB2 increases significantly after stimuli that induce LTD (but not LTP) in hippocampal neurons, and this increase is inhibited by excess NLS peptides (which block importin-mediated nuclear import). Second, ApCREB2 nuclear immunoreactivity increases in Aplysia SNs after 5 pulses of FMRFamide, which induce LTD, but not after 5 pulses of ASW (control), or 5 pulses of 5HT, which induce LTF. Last, dendra-apCREB2 photoconverted in Aplysia sensory neurites translocates from the neurite to the nucleus after 5 pulses of FMRFamide, but not after 5 pulses of ASW. Notably, unlike CREB2 in hippocampal neurons, ApCREB2 does not appear to undergo constitutive shuttling in and out of the nucleus. This difference is likely due to the fact that Aplysia SNs-MNs exhibit extremely low levels of activity in culture in the absence of stimulation, whereas spontaneous neurotransmission and recurrent action potential firing are high in high-density hippocampal cultures, and this “basal” activity may alter the nucleocytoplasmic distribution of CREB2. Our finding that the nuclear concentration of CREB2 in hippocampal neurons is greater in younger cultures grown in serum-containing media also suggests that CREB2 nuclear localization is regulated developmentally and/or in response to serum.
During LTP of hippocampal synapses and during 5HT-induced LTF of Aplysia sensory-motor synapses, upstream kinases such as MAPK and PKA translocate to the nucleus to phosphorylate and activate CREB1 (38). In contrast, we find that the transcription factor CREB2 itself undergoes regulated nuclear translocation during LTD. In both Aplysia SNs and rodent CA1 hippocampal neurons, p38 MAPK is the upstream kinase for CREB2 during LTD (19, 39), and one plausible hypothesis is that p38 MAPK phosphorylates CREB2 in the cytoplasm, which might promote binding of CREB2 to importin α and lead to the retrograde movement of CREB2 to the nucleus. Alternatively, p38 MAPK might also translocate to the nucleus after stimulation, perhaps together with CREB2. Whether and how CREB2 nuclear import is regulated by phosphorylation will require further investigation. Given the critical role of CREB2 during long-term synaptic plasticity and memory, future studies on the regulation of CREB2 nucleocytoplasmic trafficking by distinct stimuli is likely to provide significant insights into the cell biological mechanisms of long-term synaptic plasticity.
Materials and Methods
For details, see SI Materials and Methods.
Antibodies.
Two antibodies against Aplysia CREB2, one raised against the recombinant protein (12), and the other against a peptide that includes the 2 putative MAP kinase phosphorylation sites (Ser152 and Ser237; the peptide sequence is SPPDSPEQGPSSPET; ref. 29) were kind gifts from Eric Kandel (Columbia University, New York, NY) and Jack Byrne (University of Texas, Austin, TX), respectively. Anti-mammalian CREB2 antibodies included SC200 rabbit anti-human CREB2 antibodies from Santa Cruz Biotechnologies and 2B3 mouse monoclonal anti-human CREB2 antibodies from Genetex. Additional primary antibodies used include: mouse MA1–045 anti-PSD95 from Affinity Bioreagents; mouse SY38 anti-synaptophysin from Chemicon; rabbit Z66 anti-synaptophysin from Zymed/Invitrogen; mouse H2 MAP2 antibodies from Sigma; mouse-mAB414 nuclear pore complex antibodies from Covance. Secondary antibodies include Alexa-fluor 488 or 546 conjugated goat anti-rabbit or anti-mouse IgGs from Molecular Probes/Invitrogen and HRP goat anti-rabbit or anti-mouse IgGs from Cell Signaling Technologies.
DNA Constructs.
The pDendra2-N vector was from Evrogen. The entire Dendra sequence was amplified by Pfu turbo polymerase (Stratagene), with the addition of 8 aa linker sequence SGDSGVYK, and multiple cloning sites to the C terminus of Dendra. The DNA fragment was then subcloned into pNEX3 vector. Full-length ApCREB2 cDNA was amplified from pGEX-ApCREB2 (12) and subcloned into the pNEX3-Dendra vector. Full-length mouse CREB2 cDNA was amplified from mouse brain RNA and subcloned into pGEX-3X (Amersham) and pDendra vector. GST and Dendra were fused to the N terminus of CREB2 for all of the fusion proteins.
Cell Culture, Transfection, GST Pull-Down, and Microinjection.
Dissociated rat hippocampal neurons were cultured as previously described (9). Transfection was done at 7–9 DIV by using calcium phosphate precipitation. Aplysia SN-MN cocultures were prepared as previously described (9). On 3 DIV, SNs were microinjected with 0.5 mg/mL plasmid; 293T cells were transfected by using lipofectamine 2000 as recommended by the manufacturer (Invitrogen).
Immunofluorescence Staining and in Situ Hybridization.
Immunofluorescence staining (9) and FISH (40, 41) were performed as previously described.
Pharmacological Stimulation of Aplysia Neurons and Live Cell Imaging.
FMRFamide from Sigma or Bachem was dissolved in ASW to 1 mM. Five pulses of FMRFamide (10 μM) were bath-applied to SN-MN coculture (5 DIV) as previously described (42), and neurons were fixed 5 min after the 5th pulse. For time-lapse imaging, neurons expressing dendra-ApCREB2 were switched to ASW before taking images (prephotoconversion). The entire field was illuminated with UV light (mercury bulb, filter 405 nm) for 6 sec, and images were taken in the red channel. Optical sections of the same field were taken with the same parameters 5 min after the first and fifth pulse of ASW/FMRFamide.
Pharmacological Treatment and Live Cell Imaging of Hippocampal Neurons.
Hippocampal neurons (10–12 DIV for Fig. 3; 21–23 DIV for Fig. 4) were incubated with NLS or mNLS peptides (50 μg/mL, BioMol) for 1 h at 37°C. To block protein synthesis, neurons were preincubated with emetine (50 μM; Calbiochem) for 30 min at 37 °C before adding the NLS or mNLS peptides. For live cell imaging, a small segment (≈20 μm) of distal neurites (40–60 μm from the soma) of hippocampal neurons (8–13DIV) transfected with Dendra, Dendra-CREB2 or Dendra-CREB2-mNLS was photoconverted with UV light (405 nm) by using a mercury bulb (100 W) for ≈5 sec. To test the effect of the NLS peptide, neurons were preincubated with 50–75 μg/mL NLS or mutated NLS peptide at 37 °C for 1 h before photoconversion. These experiments were performed at room temperature.
Image Acquisition and Analysis.
Confocal fluorescence images were obtained by using a Zeiss Pascal scanning laser microscope (Figs. 1, 3, and 5–7) or on Marianas spinning disk confocal microscope (Fig. 4).
Supplementary Material
Acknowledgments.
We thank Carrie Heusner and Rachel Jeffrey for synaptoneurosomes, Ohtan Wang for assistance with figures, Peter Lin for help with image analysis, all members of the K.C.M. laboratory for discussions, and Jack Byrne, Dusan Bartsch (Central Institute of Mental Health, Mannheim, Germany), and Eric Kandel for anti-ApCREB2 antibodies. K.-O.L. was supported by Croucher Foundation fellowship (Hong Kong) and K.C.M. by National Institutes of Health Grant R01 MH077022.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803906105/DCSupplemental.
References
- 1.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 2.Kelleher RJ, III, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Stough S, Shobe JL, Carew TJ. Intermediate-term processes in memory formation. Curr Opin Neurobiol. 2006;16:672–678. doi: 10.1016/j.conb.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 4.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 5.Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: The logic behind the mechanisms. Curr Opin Neurobiol. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 6.Heusner C, Martin KC. Signaling from the synapse to the nucleus. In: Hell JW, Ehlers MD, editors. Structural and Functional Organization of the Synapse. Springer, NY: 2008. pp. 601–620. [Google Scholar]
- 7.Otis KO, Thompson KR, Martin KC. Importin-mediated nuclear transport in neurons. Curr Opin Neurobiol. 2006;16:329–335. doi: 10.1016/j.conb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Hanz S, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 9.Thompson KR, et al. Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway. Neuron. 2004;44:997–1009. doi: 10.1016/j.neuron.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Dieterich DC, et al. Caldendrin-Jacob: A protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol. 2008;6:e34. doi: 10.1371/journal.pbio.0060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlson E, et al. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch D, et al. Aplysia CREB2 represses long-term facilitation: Relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen A, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 14.Lee JA, Kim H, Lee YS, Kaang BK. Overexpression and RNA interference of Ap-cyclic AMP-response element binding protein-2, a repressor of long-term facilitation, in Aplysia kurodai sensory-to-motor synapses. Neurosci Lett. 2003;337:9–12. doi: 10.1016/s0304-3940(02)01285-5. [DOI] [PubMed] [Google Scholar]
- 15.Costa-Mattioli M, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa-Mattioli M, et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montarolo PG, Kandel ER, Schacher S. Long-term heterosynaptic inhibition in Aplysia. Nature. 1988;333:171–174. doi: 10.1038/333171a0. [DOI] [PubMed] [Google Scholar]
- 18.Guan Z, et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- 19.Guan Z, et al. p38 MAP kinase mediates both short-term and long-term synaptic depression in Aplysia. J Neurosci. 2003;23:7317–7325. doi: 10.1523/JNEUROSCI.23-19-07317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nehring RB, et al. The metabotropic GABAB receptor directly interacts with the activating transcription factor 4. J Biol Chem. 2000;275:35185–35191. doi: 10.1074/jbc.M002727200. [DOI] [PubMed] [Google Scholar]
- 21.White JH, et al. The GABAB receptor interacts directly with the related transcription factors CREB2 and ATFx. Proc Natl Acad Sci USA. 2000;97:13967–13972. doi: 10.1073/pnas.240452197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vernon E, et al. GABA(B) receptors couple directly to the transcription factor ATF4. Mol Cell Neurosci. 2001;17:637–645. doi: 10.1006/mcne.2000.0960. [DOI] [PubMed] [Google Scholar]
- 23.Cibelli G, Schoch S, Thiel G. Nuclear targeting of cAMP response element binding protein 2 (CREB2) Eur J Cell Biol. 1999;78:642–649. doi: 10.1016/S0171-9335(99)80049-1. [DOI] [PubMed] [Google Scholar]
- 24.Yoneda Y. Nucleocytoplasmic protein traffic and its significance to cell function. Genes Cells. 2000;5:777–787. doi: 10.1046/j.1365-2443.2000.00366.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 26.Torgerson TR, Colosia AD, Donahue JP, Lin YZ, Hawiger J. Regulation of NF-kappa B, AP-1, NFAT, and STAT1 nuclear import in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-kappa B p50. J Immunol. 1998;161:6084–9092. [PubMed] [Google Scholar]
- 27.Gurskaya NG, et al. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotech. 2006;24:461–465. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- 28.Lu W, et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed HA, Yao W, Fioravante D, Smolen PD, Byrne JH. cAMP-response elements in Aplysia creb1, creb2, and Ap-uch promoters: Implications for feedback loops modulating long term memory. J Biol Chem. 2005;280:27035–27043. doi: 10.1074/jbc.M502541200. [DOI] [PubMed] [Google Scholar]
- 30.Turpin P, Ossareh-Nazari B, Dargemont C. Nuclear transport and transcriptional regulation. FEBS Lett. 1999;452:82–86. doi: 10.1016/s0014-5793(99)00533-5. [DOI] [PubMed] [Google Scholar]
- 31.Komeili A, O'Shea EK. Nuclear transport and transcription. Curr Opin Cell Biol. 2000;12:355–360. doi: 10.1016/s0955-0674(00)00100-9. [DOI] [PubMed] [Google Scholar]
- 32.Graef IA, et al. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- 33.Wellmann H, Kaltschmidt B, Kaltschmidt C. Retrograde transport of transcription factor NF-kappa B in living neurons. J Biol Chem. 2001;276:11821–11829. doi: 10.1074/jbc.M009253200. [DOI] [PubMed] [Google Scholar]
- 34.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 35.Barrett LE, et al. Region-directed phototransfection reveals the functional significance of a dendritically synthesized transcription factor. Nat Methods. 2006;3:455–460. doi: 10.1038/nmeth885. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, et al. Nuclear translocation of CAM-associated protein activates transcription for long-term facilitation in Aplysia. Cell. 2007;129:801–812. doi: 10.1016/j.cell.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 37.Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol. 2008;10:149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin KC, et al. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 39.Bolshakov VY, Carboni L, Cobb MH, Siegelbaum SA, Belardetti F. Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3-CA1 synapses. Nat Neurosci. 2000;3:1107–1112. doi: 10.1038/80624. [DOI] [PubMed] [Google Scholar]
- 40.Lyles V, Zhao Y, Martin KC. Synapse formation and mRNA localization in cultured Aplysia neurons. Neuron. 2006;49:349–356. doi: 10.1016/j.neuron.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 41.Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J Neurosci. 2006;26:13390–13399. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montarolo PG, et al. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.