Abstract
Photorespiratory 2-phosphoglycolate (2PG) metabolism is essential for photosynthesis in higher plants but thought to be superfluous in cyanobacteria because of their ability to concentrate CO2 internally and thereby inhibit photorespiration. Here, we show that 3 routes for 2PG metabolism are present in the model cyanobacterium Synechocystis sp. strain PCC 6803. In addition to the photorespiratory C2 cycle characterized in plants, this cyanobacterium also possesses the bacterial glycerate pathway and is able to completely decarboxylate glyoxylate via oxalate. A triple mutant with defects in all 3 routes of 2PG metabolism exhibited a high-CO2-requiring (HCR) phenotype. All these catabolic routes start with glyoxylate, which can be synthesized by 2 different forms of glycolate dehydrogenase (GlcD). Mutants defective in one or both GlcD proteins accumulated glycolate under high CO2 level and the double mutant ΔglcD1/ΔglcD2 was unable to grow under low CO2. The HCR phenotype of both the double and the triple mutant could not be attributed to a significantly reduced affinity to CO2, such as in other cyanobacterial HCR mutants defective in the CO2-concentrating mechanism (CCM). These unexpected findings of an HCR phenotype in the presence of an active CCM indicate that 2PG metabolism is essential for the viability of all organisms that perform oxygenic photosynthesis, including cyanobacteria and C3 plants, at ambient CO2 conditions. These data and phylogenetic analyses suggest cyanobacteria as the evolutionary origin not only of oxygenic photosynthesis but also of an ancient photorespiratory 2PG metabolism.
It is well established that the photorespiratory C2 pathway, whereby 2-phosphoglycolate (2PG) is metabolized (1), is essential for photosynthesis in the majority of plants (2). In contrast, the functioning of the C2 pathway and its importance are still under discussion for cyanobacteria. These organisms were the first to have evolved oxygenic photosynthesis, and endosymbiotic engulfment of an ancient cyanobacterium led to the evolution of plant chloroplasts (3). In cyanobacteria, as in C3 plants, the primary carbon fixation is catalyzed by ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). Ribulose 1,5-bisphosphate reacts with either CO2, leading to the formation of 2 molecules of 3-phosphoglycerate (3PGA), or O2, generating 3PGA and 2PG. The latter compound is toxic to plant metabolism because it inhibits distinct steps in the carbon-fixing Calvin–Benson cycle (4, 5). Therefore, plants employ the so-called photorespiratory glycolate pathway (or C2 cycle), which degrades 2PG and converts 2 molecules of 2PG into 1 molecule each of 3PGA, CO2, and NH4+ (1, 6, 7). In a typical C3 plant, the ammonium is refixed at the expense of ATP, and 25% of the carbon entering the path is released as CO2. Generally, the photorespiratory cycle is indispensable for C3 plants, because mutations in single steps of the C2 cycle resulted in high-CO2-requiring (HCR) phenotypes (2, 8–10).
In contrast to plants, early studies on cyanobacterial 2PG metabolism indicated its absence or the occurrence of only the initial steps engaged in glycolate formation (11). This was difficult to understand because the affinity of the cyanobacterial Rubisco for CO2 is considerably lower than that of C3 plants (12). Today, it is widely accepted that the low CO2 affinity of Rubisco is compensated by an efficient inorganic carbon (Ci)-concentrating mechanism (CCM) that raises the concentration of CO2 in close proximity to Rubisco (13–15). Mutants impaired in functional components of the CCM, such as the carboxysomes (16–19) or transport and internal accumulation of Ci (20–22), show very low apparent photosynthetic affinity for external Ci and, thus, exhibit a HCR phenotype. These findings clearly revealed the essential function of the CCM for cyanobacterial survival under the present atmosphere and prompted the widely accepted notion that oxygenase activity of Rubisco is almost totally repressed in cyanobacteria. Therefore, metabolism of 2PG, the immediate product of this oxygenase function, seemed to be unnecessary in these organisms.
Recently, we provided evidence for combined action of a plant-like C2 cycle and a bacterial-like glycerate pathway (23) to metabolize 2PG in the cyanobacterial model strain Synechocystis sp. PCC 6803 (hereafter Synechocystis). Mutants defective in specific steps involved in these routes displayed growth retardation and accumulated intermediates of the photorespiratory metabolism already under high CO2 conditions [air enriched with 5% CO2 (HC)]. However, contrary to C3 plants, even the double mutants in the 2 known 2PG degrading routes operating in Synechocystis, the C2 cycle and glycerate pathway, were able to grow under ambient CO2 conditions [ambient air with 0.035% CO2 (LC)]. This ability was attributed to the activity of the CCM, which depresses the formation and hence metabolism of 2PG. Alternatively, this ability could also suggest the existence of additional routes for 2PG breakdown. Gene expression profiling, where the mRNA levels in LC- and HC-grown cells were compared, revealed the existence of hundreds of Ci-regulated genes (24, 25). Interestingly, some of the genes up-regulated under low CO2 encode for enzymes that form a third route of 2PG metabolism via a series of decarboxylations: glyoxylate is converted into oxalate, then to formate, and finally to CO2.
In the present study, we show unequivocally that an active 2PG metabolism not only exists but is essential for cyanobacterial growth in the present O2-containing atmosphere, despite the existence of the CCM. Also, the essential nature of the 2PG metabolism and its occurrence in all present-day cyanobacteria implies that this metabolism already existed in ancient cyanobacteria and might have been the starting point for the evolution of the plant photorespiratory 2PG metabolism after the engulfment of the primary cyanobacterial endosymbiont.
Results
A Triple Mutant in 3 Branches of 2PG Metabolism Exhibits an HCR Phenotype.
To examine the functioning and cooperation of all of the proposed photorespiratory pathways (Fig. 1), we raised a mutant in which these routes of 2PG metabolism were specifically inactivated downstream from glyoxylate. This mutant generation included the inactivation of odc encoding the oxalate decarboxylase involved in the decarboxlyation branch, of gcvT encoding the T-protein of glycine decarboxylase in the C2 cycle, and tsr encoding the tartronic semialdehyde reductase in the glycerate pathway. The triple mutant ΔgcvT/Δtsr/Δodc, isolated under HC, is completely segregated. PCR analysis proved that all of the WT copies of these 3 genes were inactivated, bearing the inserted cartridges encoding different antibiotics in all of the chromosome copies (Fig. 2A). Surprisingly, the triple mutant exhibited a HCR phenotype: it was unable to grow at air level of CO2 (Fig. 2B). In contrast, the single or double mutants defective in the decarboxylation branch and/or in the plant-like C2 cycle could acclimate and grow under LC.
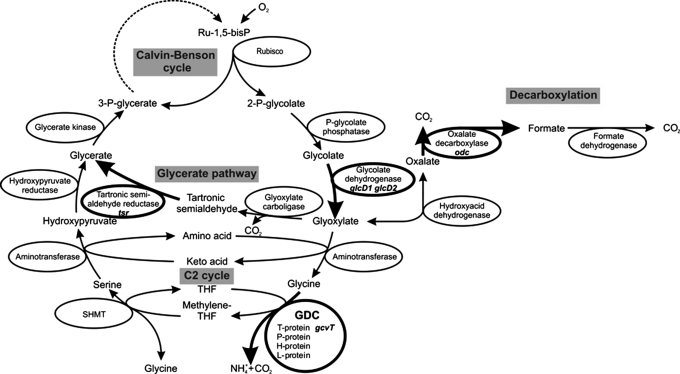
Fig. 1.
A scheme displaying the 2PG metabolism in Synechocystis sp. strain PCC 6803, which employs 3 different routes: C2 cycle, glycerate pathway, and the decarboxylating branch. Enzymatic steps mutated in strains used for this study are indicated in bold.
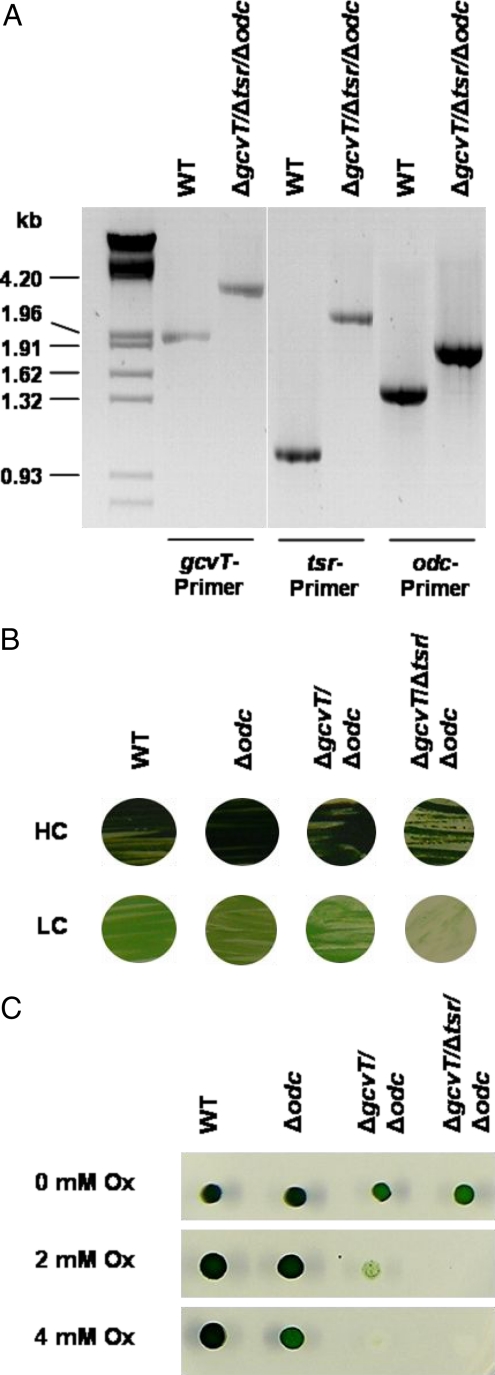
Fig. 2.
Genotypic and phenotypic characterization of the triple mutant ΔgcvT/Δtsr/Δodc. (A) Complete segregation of the mutant ΔgcvT/Δtsr/Δodc was verified by PCR with gene-specific primers (see SI Table 1). (B) Growth of WT, single mutant Δodc blocked in the decarboxylation branch, double mutant Δodc/ΔgcvT blocked in the decarboxylation branch and C2 cycle, and triple mutant ΔgcvT/Δtsr/Δodc blocked in all 3 branches of 2PG metabolism (see Fig. 1), respectively, under HC or LC. Strains were plated on BG11, pH 7, solidified by 0.9% Kobe agar, and incubated under continuous illumination of 30 μmol of photons per s per m2 at 30 °C for 7 d. (C) Resistance of WT, single mutant Δodc, double mutant Δodc/ΔgcvT, and triple mutant ΔgcvT/Δtsr/Δodc toward oxalate (Ox). Strains were plated on BG11 agar plates, pH 8, supplemented by different amounts of oxalate. Cells were incubated under continuous illumination of 30 μmol of photons per s per m2 at 30 °C and HC for 7 d. [C reproduced with permission from ref. 41].
Such a HCR phenotype is typical for plant photorespiratory mutants, for example the C3 plant Arabidopsis thaliana (2, 8–10), which do not possess a CCM. However, it was not expected in cyanobacteria, where the up-regulation of the CCM activity by LC is supposed to severely suppress photorespiration (13, 14). It is noteworthy that the HCR characteristic of the triple mutant was lost after a few cycles of cultivation at LC. However, with freshly generated triple-mutant clones, we were able to reproduce several times the initial HCR phenotype and its subsequent loss. These data clearly suggested that photorespiratory metabolism is essential for the ability of Synechocystis to grow under LC. A further apparent indication of the cooperation of these 3 metabolic routes was found in experiments where we tested the resistance toward external oxalate. An increasing sensitivity to oxalate (Δodc < ΔgcvT/Δodc < ΔgcvT/Δtsr/Δodc; Fig. 2C) was observed, suggesting that oxalate can also be converted back to glyoxylate and subsequently degraded by the C2 cycle and/or glycerate pathway.
Identification of an Alternative Glycolate Dehydrogenase.
In an earlier study (23), we showed that a mutant ΔglcD defective in a subunit of GlcD could grow under LC, albeit some 50% slower than the WT. In view of the HCR phenotype of the triple mutant, the growth characteristics of mutant ΔglcD raised the possibility of a potential bypass for glycolate oxidation in cyanobacteria. A search in the Synechocystis genome identified a gene, slr0806, potentially coding an alternative GlcD. The amino acid sequence of Slr0806 shares 46% similarity with GlcD1 (Sll0404) from Synechocystis and from Escherichia coli. Also, Slr0806 harbors a conserved consensus sequence GXGXXG [supporting information (SI) Fig. S1] thought to serve as a flavin-binding domain (26). In addition to GlcD1, the GlcD complex is comprised of a second dehydrogenase subunit GlcE (26), which is ≈42% similar to Slr0806. Also, phylogenetic analysis showed that Slr0806-like proteins from several microorganisms clearly cluster with the GlcD/E-group (Fig. S1) and is present in all of the available cyanobacterial genome sequences. Therefore, we assumed that Slr0806 may form a second GlcD, designated GlcD2, which acts together with GlcD1 in glycolate oxidation. This enzyme activity could explain the viability of ΔglcD1 mutant at LC in contrast to the HCR phenotype of the triple mutant.
To verify this assumption, we inactivated glcD2 in Synechocystis generated a double mutant in which both potential GlcDs were defective. The double mutant ΔglcD1/ΔglcD2 was isolated under HC, and PCR analyses proved that all the WT-copies of glcD1 and glcD2 were inactivated (data not shown). To examine possible involvement of GlcD2 in glycolate conversion, we quantified the internal glycolate levels in the WT and the mutants by using HPLC; 3 h after transfer from HC to LC, the glycolate content in the WT cells was very low (≈0.02 μmol of glycolate per mL of cell volume). It became ≈200-fold higher in mutant ΔglcD2 and even higher in mutant ΔglcD1 (Fig. 3A). Importantly, compared with the single mutants, the double mutant ΔglcD1/ΔglcD2 accumulated far more glycolate (Fig. 3A). It should be noted that glycolate was detected in cells of mutants ΔglcD1 and ΔglcD1/ΔglcD2 even when grown under HC, whereas only traces were observed in HC-cells of mutant ΔglcD2 and none in extracts from WT-cells grown under HC.
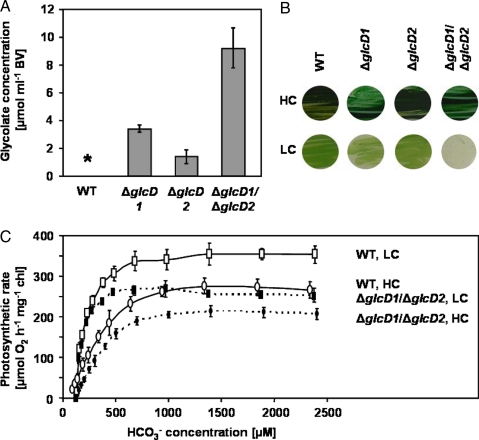
Fig. 3.
Phenotypic characterization of single mutants and a double mutant of Synechocystis defective in GlcDs. (A) Quantification of intracellular glycolate in cells of single- (ΔglcD1, ΔglcD2) and double- (ΔglcD1/ΔglcD2) mutants in the glycolate converting step. Samples were taken 3 h after shift from HC to LC and glycolate was quantified by HPLC. *, WT cells contained only traces of glycolate under these conditions. (B) Growth of WT, single mutants ΔglcD1 or ΔglcD2, and double mutant ΔglcD1/ΔglcD2 under HC or LC. Strains were plated on BG11, pH 7, solidified by 0.9% Kobe agar, and incubated under continuous illumination of 30 μmol of photons per s per m2 at 30 °C for 7 d. (C) Photosynthesis rates of cells of the WT and the double mutant ΔglcD1/ΔglcD2 at different concentrations of HCO3− as a source for Ci. The cells were grown in liquid BG11 medium at HC or transferred to aeration by ambient air (LC) for 6 h.
The Double Mutant Impaired in both GlcD1 and GlcD2 Exhibits a HCR Phenotype.
Because the level of glycolate accumulated was higher in mutant ΔglcD1 than in ΔglcD2 (Fig. 3A), we conclude that the former is the major GlcD in Synechocystis. This assumption is supported by the fact that the single ΔglcD1 mutant, but not ΔglcD2, grew slower than the WT under both HC and LC. Interestingly, the double mutant ΔglcD1/ΔglcD2 could grow under HC, albeit somewhat slower than the WT, but could not grow under LC (Fig. 3B). In contrast to the triple mutant, the HCR phenotype of the double mutant was stable for many generations. After a few days under LC, the cell suspensions of mutant ΔglcD1/ΔglcD2 were completely bleached and could not recover after transfer back to HC. The HCR phenotype of this double mutant corresponds to the same phenotype described above for the triple mutant ΔgcvT/Δtsr/Δodc impaired in the 2PG metabolism downstream of glyoxylate. The fact that the newly-constructed double and triple mutants showed a HCR phenotype clearly indicates and supports our view that a photorespiratory 2PG metabolism is essential for cyanobacteria at LC despite the action of the CCM.
Characterization of Photosynthesis in the GlcD1/D2 Mutants.
In earlier studies, HCR phenotypes were observed in CCM mutants of cyanobacteria defective in the ability to accumulate Ci internally (22, 27) or in the structural organization of the carboxysomes (16, 28, 29). In both cases, the phenotype emerged from the very low apparent photosynthetic affinity for external Ci. Analysis of the photosynthetic parameters showed that this was not the case in the ΔglcD mutants (Fig. 3C). When grown under HC, the apparent photosynthetic affinity for extracellular Ci and the Vmax were somewhat lower in the ΔglcD1/ΔglcD2 mutant as compared with WT. The K1/2 for Ci of the double mutant was ≈0.35 mM HCO3−, whereas this value increased ≈100 times to 40 mM HCO3− in the ΔccmM mutant (19) or to >20 mM HCO3− in the Δ5 mutant (30), where the carboxysomes were absent and all the genes encoding known Ci uptake systems were inactivated, respectively. Therefore, the HCR phenotype of the double mutant cannot be explained by a reduced affinity of the cell for Ci. In this respect, the double mutant ΔglcD1/ΔglcD2 resembled the ΔpurK mutant of Synechococcus sp. strain PCC 7942, where the HCR phenotype emerged from an inability to produce purines under LC (31). Last, after exposure of mutant ΔglcD1/ΔglcD2 to LC for 6 h, the photosynthetic K1/2 (Ci) decreased considerably as in the WT (Fig. 3C), indicating that the induction of a more efficient CCM was not sufficient to enable growth under LC. Interestingly, the photosynthetic Vmax of the mutant was lower than that of the WT, particularly under LC, possibly reflecting inhibition of Calvin–Benson cycle enzymes by the accumulating 2PG (4, 5).
Discussion
Results presented here point to a common concept for C3 plants and cyanobacteria, namely that 2PG metabolism is an essential partner for oxygenic photosynthesis in O2-containing environments. We believe this to be a novel view arising from the generation and characterization of 2 different sets of cyanobacterial mutants impaired in 2PG metabolism. To gain a comprehensive molecular description of the 2PG metabolism in cyanobacteria, we have identified the complete decarboxylation of glyoxylate as the third route for 2PG degradation (Fig. 1) and constructed the triple mutant ΔgcvT/Δtsr/Δodc that showed the HCR phenotype. This phenotype, which contradicted the ability of mutant ΔglcD to grow under LC, urged us to identify an alternative GlcD, GlcD2, involved in the conversion of glycolate to glyoxylate. The generation of the double mutant ΔglcD1/ΔglcD2 resulted again in the HCR phenotype and confirmed that 2PG conversion is indeed essential for growth.
Although the newly identified GlcD2 is similar to the earlier recognized GlcD1, it could not fully replace GlcD1 in the ΔglcD1 mutant, because the latter mutant accumulated glycolate and vice versa. In addition, the significant increase in glycolate in the double mutant defective in both GlcDs also supports the notion that both GlcD proteins are active in glycolate oxidation. Correspondingly, the HCR phenotype was only obtained in the double mutant where the conversion of glycolate to glyoxylate is completely blocked. The high accumulation of glycolate may have been toxic for the cells, as was shown for higher plants (10). Accumulation of glycolate in these mutants indicated that the oxygenase activity of Rubisco was not fully inhibited even under the HC applied here. Also, because the photosynthetic parameters were close to those observed in the WT (Fig. 3C) and differed from those observed in “classical HCR mutants” defective in CCM, we conclude that the functioning and even activation of the CCM was insufficient to allow growth of GlcD mutants at air level of CO2. Recently, it was shown that 2PG, the product of oxygenase reaction by Rubisco, serves as a signal to trigger acclimation of cyanobacteria to LC (32). In agreement with this observation, we found indications for LC acclimation already under HC in cells of the GlcD1 mutant, which accumulates glycolate and possibly also increased amounts of 2PG under HC (25).
Apparently, the glyoxylate produced by GlcD1 and GlcD2 is metabolized by the cooperation of 3 different routes operating in Synechocystis and presumably other cyanobacteria. The presence of the third route, the complete decarboxylation of 2PG, was verified here by the generation and characterization of the triple mutant ΔgcvT/Δtsr/Δodc, which is unable to grow under LC. The observed unstable HCR phenotype of the triple mutant is not exceptional. Pseudoreversions of mutants originally showing HCR phenotype were reported in several cases including a mutant lacking carboxysomes (33) and a double mutant in the 2 2PG phosphatases of Synechocystis (23). It is important to note that these mutants neither excreted 2PG nor glycolate (23), an observation supported by the marked glycolate accumulation inside the GlcD mutants.
In view of the HCR phenotype of the double and triple mutants we conclude the following: (i) the 2PG metabolism in Synechocystis comprises 3 cooperating routes, the C2 cycle, the glycerate pathway, and complete decarboxylation; (ii) the 2PG metabolism is active and essential in cells grown under atmospheric level of CO2, indicating that the CCM does not block photorespiration as efficiently as was postulated; and (iii) the main function of 2PG metabolism seems to be related to the reduction of the amount of toxic intermediates to below critical threshold levels, although we cannot rule out the importance of 3PGA regeneration.
The third conclusion is supported by the close correlation observed between growth retardation and accumulation of glycolate (shown here) or glycine (34). Similar indications exist for C3 plants, where the extent of phenotypical changes correlated with the amount of internally accumulated intermediates of 2PG metabolism (10). Our suggestion that metabolism of 2PG is not only beneficial but essential for cyanobacteria is supported by the fact that all the enzymes necessary for the plant-like C2 cycle, as well as for the glycerate pathways, are present in all the presently-known complete genome sequences. This includes the smallest genomes of marine Prochlorococcus (Table 1) and Synechococcus strains, which are thought to possess only genes essential for their survival in a constant environment as photoautotrophic organisms (35).
Table 1.
Proteins involved in 2-phosphoglycolate metabolism in the cyanobacteria Synechocystis sp. strain PCC 6803 (ORF in 6803) and Nostoc sp. strain PCC 7120 (ORF in 7120), and the presence of close homologs (e-values higher than e−25) in Prochlorococcus sp. strain SS120 (SS120) and Arabidopsis thaliana (A. thal.)
| Protein | ORF in 6803 | ORF in 7120 | Present in SS120 | Present in A. thal. | C2 cycle in A. thal. | Cyano origin? |
|---|---|---|---|---|---|---|
| 2-phosphoglycolate phosphatase | slr0458 | − | + | − | At5 g36790* | |
| sll1349 | alr4944 | + | − | |||
| Glycolate DH | (At5g06580‡) | |||||
| GlcD1 | sll0404 | alr5269 | − | + | ||
| GlcD2 | slr0806 | all4443 | + | − | ||
| Glycolate oxidase | − | all0170 | − | + | At4g18360† | At4 g18360 |
| Serine/glyoxylate aminotransferase | sll1559 | alr1004 | + | + | At2g13360† | |
| Glycine decarboxlyase | ||||||
| P-protein | slr0293 | all4607 | + | + | At4g33010‡ | At4g33010 |
| T-protein | sll0171 | all4609 | + | + | At1g11860‡ | (At3g16950*) |
| H-protein | slr0879 | all4608 | + | + | At2g35370‡ | |
| L-protein | slr1096 | alr4745 | + | + | At3g17240‡ | |
| Serine hydroxymethyl-transferase | sll1931 | alr4806 | + | + | At4g37930‡ | (At4g32520*) |
| Glutamate/glyoxylate aminotransferase | slr0006 | alr2765 | + | − | At1g23310† | |
| Hydroxypyruvate reductase | sll1908 | alr1890 | + | + | At1g68010† | (At1g17745*) |
| Glycerate kinase | ||||||
| Bacterial type | slr1840 | − | − | − | ||
| Plant type | − | alr2873 | + | + | At1g80380* | At1g80380 |
| Glyoxylate carboligase | sll1981 | all3555 | + | − | ||
| Tartronic semialdehyde reductase | slr0229 | alr3358 | + | + | ||
| Hydroxyacid reductase | sll1556 | alr0058 | − | + | ||
| sll0891 | alr4322 | − | + | |||
| Oxalate decarboxylase | sll1358 | − | − | − | ||
| Formate dehydrogenase | sll1359 | − | − | − |
The column ″C2 cycle in A. thal.″ lists proteins that are experimentally proven to participate in the plant-like photorespiratory C2 cycle. The column ″Cyano origin?″ indicates genes for enzymes acting in the plant C2 cycle that possibly originate from the cyanobacterial endosymbiont because these plant proteins cluster closest to homolog proteins from cyanobacteria. Amino acid similarity searches and cluster analyses were done by using the BLAST algorithm (40) at the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/blast/Blast.cgi) or CyanoBase (http://bacteria.kazusa.or.jp/cyanobase/). ORFs written in bold are probably of cyanobacterial origin and involved in the plant C2 cycle, whereas ORFs in brackets probably originated also from cyanobacteria but are not directly involved in the plant C2 cycle because these proteins are located in other cellular compartments. +, present; −, absent.
*The mature protein is predicted to be localized in the Arabidopsis chloroplast.
†The mature protein is predicted to be localized in the Arabidopsis peroxisome.
‡The mature protein is predicted to be localized in the Arabidopsis mitochondrion.
Last, in view of our findings, we propose that the 2PG metabolism is an essential partner of oxygenic photosynthesis early on from its evolution in cyanobacteria. This hypothesis does not necessarily contradict the suggestion that the oxygenic photosynthesis evolved 2–3 billion years ago in an oxygen-free atmosphere. It is quite likely that inside the cyanobacterial cell oxygen concentration could rise significantly (15), leading to an elevated oxygenase activity of Rubisco already in ancient cyanobacteria. This may have been the case particularly within mats or stromatolith-like structures, where the ancient cyanobacterial cells were shielded by extracellular polysaccharides and inorganic matter. Also, it was suggested that the CCM evolved only ≈400 million years ago (14, 15), certainly long after the primary engulfment of an ancient cyanobacterium by the endosymbiosis ≈1.2 billion years ago (3, 36). If this is the case, it was necessary to develop the means to overcome the formation and accumulation of toxic amounts of intermediates such as 2PG, glycolate, or glycine soon after the evolution of oxygenic photosynthesis.
Taking this hypothetical scenario into consideration, it is possible that an active photorespiratory 2PG metabolism existed already in ancient cyanobacteria and was transferred into present-day higher plants by the engulfment of the primary cyanobacterial endosymbiont. To examine this assumption, we performed phylogenetic analyses of genes for the cyanobacterial 2PG metabolism searching for homologous proteins in the Arabidopsis genome (Table 1). Phylogenetic trees, where the cyanobacterial and corresponding plant proteins cluster closest together, are usually taken as evidence that those genes might have been transferred by means of endosymbiosis into the plant genome (3, 36). Such genomic searches suggested that glycolate oxidase and the plant-type glycerate kinase (Fig. S2), which are missing in Synechocystis but present in other cyanobacteria such as Nostoc sp. strain PCC 7120 and the P-protein subunit of glycine decarboxylase complex, originated from cyanobacteria. Also, close homologs were found for GlcD1, the L-protein subunit of glycine decarboxylase complex, serine hydroxymethyltransferase, and hydroxypyruvate reductase. However, these plant homologs are localized in other cells compartments (e.g., the cyanobacterial-like serine hydroxymethyltransferase is directed to the chloroplast; see Table 1) and are probably not directly involved in the present day plant-type C2 cycle. These analyses also proposed the presence of proteins probably homologous to enzymes of the bacterial-type glycerate pathway in Arabidopsis, but their function has yet to be characterized (Table 1).
Materials and Methods
Strains and Culture Conditions.
The cyanobacterial strains used in this work are listed in Table S1. The glucose-tolerant strain of Synechocystis sp. PCC 6803 was obtained from N. Murata (National Institute for Basic Biology, Okazaki, Japan) and served as the WT. Cultivation of mutants was performed at 50 μg·mL−1 kanamycin (Km), 20 μg·mL−1 spectinomycin (Sp) or at 25 μg·mL−1 chloramphenicol (Cm) as required. Axenic cultures of Synechocystis (≈108 cells per mL) were grown photoautotrophically in batch cultures (3-cm glass vessels with 5-mm glass tubes for aeration) at 29 °C under continuous illumination of 130 μmol of photons per s per m2 (warm light; Osram L58 W32/3) bubbling (flow rate ≈5 mL·min−1) with air enriched with CO2 (HC) in the BG11 medium at pH 7.0. Ci limitation was set by transferring exponentially growing cultures (OD750 0.9, volume 130 mL) from bubbling with CO2-enriched air to bubbling with ambient air (≈0.035%, LC). Growth was monitored by measurements of the optical density at 750 nm (OD750). Agar plates (BG 11, pH 7, solidified by 0.9% Kobe agar) were incubated under continuous illumination of 30 μmol of photons per s per m2 at 30 °C for 7 d in air or HC. Contamination by heterotrophic bacteria was checked by spreading of 0.2 mL of culture on LB plates. The E. coli strain TG1 (37), cultured in LB medium at 37 °C, was used for routine DNA manipulations.
Generation of Mutants.
To generate mutation in the ORF slr0806 (designated glcD2), the Sp resistance cartridge derived from pUC4S was integrated into the coding sequence at the unique BamHI restriction site. The products were checked by restriction analysis. Plasmid DNA of these constructs was isolated from E. coli by using the illustra plasmidPrep Mini Spin Kit (GE Healthcare); ≈1 μg of DNA was used for transformation of Synechocystis and Sp-resistant clones were selected (38). To show alterations in the genotype, PCR with gene-specific oligonucleotides (see Table S1) was carried out by using the Taq-PCR Master Mix (Qiagen).
Quantification of Internal Glycolate Concentrations.
Glycolate was extracted from frozen cyanobacterial cell pellets of 50 mL of culture with 80% ethanol at 65 °C for 3 h. After centrifugation, the supernatants were dried by lyophilization and redissolved in 350 μL of water. The content of glycolate was determined by HPLC in ion-exclusion mode as described in ref. 23.
Characterization of Photosynthesis.
The rate of CO2-dependent O2 evolution as a function of Ci concentration was determined by using a Clark type O2 electrode (PS2108, Passport dissolved O2 sensor) essentially as described in ref. 39. The cells were harvested by centrifugation and resuspended in a CO2-free medium containing 10 mM NaCl and 20 mM Hepes, pH 7.5. They were then placed in the O2 electrode chamber at 30 °C, 300 μmol of photons per s per m2 and allowed to use the Ci in their medium until they reached the CO2 compensation point. Aliquots of NaHCO3 of known concentrations were injected to raise the Ci concentration by known increments while measuring the resulting rise in the rate of O2 concentration in the chamber.
Supplementary Material
Acknowledgments.
M.H. was supported by Deutsche Forschungsgemeinschaft Grant HA2002/7. A.K. was supported by a grant from the Israeli Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807043105/DCSupplemental.
References
- 1.Tolbert NE. The C-2 oxidative photosynthetic carbon cycle. Ann Rev Plant Physiol Plant Mol Biol. 1997;48:1–25. doi: 10.1146/annurev.arplant.48.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Somerville CR. An early Arabidopsis demonstration. Resolving a few issues concerning photorespiration. Plant Physiol. 2001;125:20–24. doi: 10.1104/pp.125.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deusch O, et al. Genes of cyanobacterial origin in plant nuclear genomes point to a heterocyst-forming plastid ancestor. Mol Biol Evol. 2008;25:748–761. doi: 10.1093/molbev/msn022. [DOI] [PubMed] [Google Scholar]
- 4.Husic DW, Husic HD, Tolbert NE. The oxidative photosynthetic carbon cycle or C2 cycle. CRC Crit Rev Plant Sci. 1987;5:45–100. [Google Scholar]
- 5.Norman EG, Colman B. Purification and characterization of phosphoglycolate phosphatase from the cyanobacterium Coccochloris peniocystis. Plant Physiol. 1991;95:693–698. doi: 10.1104/pp.95.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowes G, Ogren WL, Hageman RH. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971;45:716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- 7.Ogren WL. Photorespiration—Pathways, regulation, and modification. Ann Rev Plant Physiol. 1984;35:415–442. [Google Scholar]
- 8.Boldt R, et al. D-GLYCERATE 3-KINASE, the last unknown enzyme in the photorespiratory cycle in Arabidopsis, belongs to a novel kinase family. Plant Cell. 2005;17:2413–2420. doi: 10.1105/tpc.105.033993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel N, et al. Deletion of glycine decarboxylase in Arabidopsis is lethal under nonphotorespiratory conditions. Plant Physiol. 2007;144:1328–1335. doi: 10.1104/pp.107.099317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarte S, Bauwe H. Identification of the photorespiratory 2-phosphoglycolate phosphatase, PGLP1, in Arabidopsis. Plant Physiol. 2007;144:1580–1586. doi: 10.1104/pp.107.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colman B. Photosynthetic carbon assimilation and the suppression of photorespiration in the cyanobacteria. Aquatic Botany. 1989;34:211–231. [Google Scholar]
- 12.Badger MR. Kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase from Anabaena variabilis. Arch Biochem Biophys. 1980;201:247–255. doi: 10.1016/0003-9861(80)90509-3. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan A, Reinhold L. CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:539–570. doi: 10.1146/annurev.arplant.50.1.539. [DOI] [PubMed] [Google Scholar]
- 14.Badger MR, Price GD, Long BM, Woodger FJ. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J Exp Bot. 2006;57:249–265. doi: 10.1093/jxb/eri286. [DOI] [PubMed] [Google Scholar]
- 15.Raven JA, Cockell CS, De La Rocha CL. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos Trans R Soc London Ser B. 2008;363:2641–2650. doi: 10.1098/rstb.2008.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus Y, Schwarz R, Friedberg D, Kaplan A. High CO2 requiring mutant of Anacystis nidulans R2. Plant Physiol. 1986;82:610–612. doi: 10.1104/pp.82.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omata T, Ogawa T, Marcus Y, Friedberg D, Kaplan A. Adaptation to low CO2 level in a mutant of Anacystis nidulans R2 which require high CO2 for growth. Plant Physiol. 1987;83:892–894. doi: 10.1104/pp.83.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marco E, Martinez I, Ronen-Tarazi M, Orus MI, Kaplan A. Inactivation of ccmO in Synechococcus sp. strain PCC 7942 results in a mutant requiring high levels of CO2. Appl Environ Microbiol. 1994;60:1018–1020. doi: 10.1128/aem.60.3.1018-1020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry S, Fischer JH, Kruip J, Hauser M, Wildner GF. Monitoring cytosolic pH of carboxysome-deficient cells of Synechocystis sp. PCC 6803 using fluorescence analysis. Plant Biol. 2005;7:342–347. doi: 10.1055/s-2005-837710. [DOI] [PubMed] [Google Scholar]
- 20.Omata T, et al. Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc Natl Acad Sci USA. 1999;96:13571–13576. doi: 10.1073/pnas.96.23.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonfil DJ, et al. A putative HCO3− transporter in the cyanobacterium Synechococcus sp. strain PCC 7942. FEBS Lett. 1998;430:236–240. doi: 10.1016/s0014-5793(98)00662-0. [DOI] [PubMed] [Google Scholar]
- 22.Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L. Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc Natl Acad Sci USA. 2004;101:18228–18233. doi: 10.1073/pnas.0405211101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhut M, et al. The plant-like C2 glycolate cycle and the bacterial-like glycerate pathway cooperate in phosphoglycolate metabolism in cyanobacteria. Plant Physiol. 2006;142:333–342. doi: 10.1104/pp.106.082982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HL, Postier BL, Burnap RL. Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J Biol Chem. 2004;279:5739–5751. doi: 10.1074/jbc.M311336200. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhut M, et al. Long-term response toward inorganic carbon limitation in wild type and glycolate turnover mutants of the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol. 2007;144:1946–1959. doi: 10.1104/pp.107.103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellicer MT, Badía J, Aguilar J, Baldomà L. glc locus of Escherichia coli: Characterization of genes encoding the subunits of glycolate oxidase and the glc regulator protein. J Bacteriol. 1996;178:2051–2059. doi: 10.1128/jb.178.7.2051-2059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa T, Kaplan A. Inorganic carbon acquisition systems in cyanobacteria. Photosyn Res. 2003;77:105–115. doi: 10.1023/A:1025865500026. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa T, Marco E, Orus MI. A gene (ccmA) required for carboxysome formation in the cyanobacterium Synechocystis sp. strain PCC6803. J Bacteriol. 1994;176:2374–2378. doi: 10.1128/jb.176.8.2374-2378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarz R, Reinhold L, Kaplan A. Low activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase in carboxysome-defective Synechococcus mutants. Plant Physiol. 1995;108:183–190. doi: 10.1104/pp.108.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu M, et al. Properties of mutants of Synechocystis sp. strain PCC 6803 lacking carbon sequestering systems. Plant Cell Physiol. 2008 doi: 10.1093/pcp/pcn139. in press. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz R, Lieman-Hurwitz J, Hassidim M, Kaplan A. Phenotypic complementation of high CO2-requiring mutants of the cyanobacterium Synechococcus sp. strain PCC 7942 by inosine 5′-monophosphate. Plant Physiol. 1992;100:1987–1993. doi: 10.1104/pp.100.4.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura T, et al. Mechanism of low CO2-induced activation of the cmp bicarbonate transporter operon by a LysR family protein in the cyanobacterium Synechococcus elongatus strain PCC 7942. Mol Microbiol. 2008;68:98–109. doi: 10.1111/j.1365-2958.2008.06137.x. [DOI] [PubMed] [Google Scholar]
- 33.Emlyn-Jones D, Woodger FJ, Andrews TJ, Price GD, Whitney SM. A Synechococcus PCC7942 Delta ccmM (Cyanophyceae) mutant pseudoreverts to air growth without regaining carboxysomes. J Phycol. 2006;42:769–777. [Google Scholar]
- 34.Eisenhut M, Bauwe H, Hagemann M. Glycine accumulation is toxic for the cyanobacterium Synechocystis sp. strain PCC 6803, but can be compensated by supplementation with magnesium ions. FEMS Microbiol Lett. 2007;277:232–237. doi: 10.1111/j.1574-6968.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 35.Dufresne A, et al. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc Natl Acad Sci USA. 2003;100:10020–10025. doi: 10.1073/pnas.1733211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin W, et al. Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc Natl Acad Sci USA. 2002;99:12246–12251. doi: 10.1073/pnas.182432999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 38.Hagemann M, Schoor A, Jeanjean R, Zuther E, Joset F. The stpA gene form Synechocystis sp. strain PCC 6803 encodes the glucosylglycerol-phosphate phosphatase involved in cyanobacterial osmotic response to salt shock. J Bacteriol. 1997;179:1727–1733. doi: 10.1128/jb.179.5.1727-1733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan A, Marcus Y, Reinhold L. Inorganic carbon uptake by cyanobacteria. Methods Enzymol. 1988;167:534–539. [Google Scholar]
- 40.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenhut M, et al. Oxalate decarboxylase is involved in turnover of 2-phosphoglycolate in Synechocystis sp. strain PCC 6803. In: Allen JF, Gantt E, Golbeck JH, Osmond B, editors. Photosynthesis: Energy from the sun. Netherlands: Springer; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.