Abstract
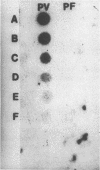
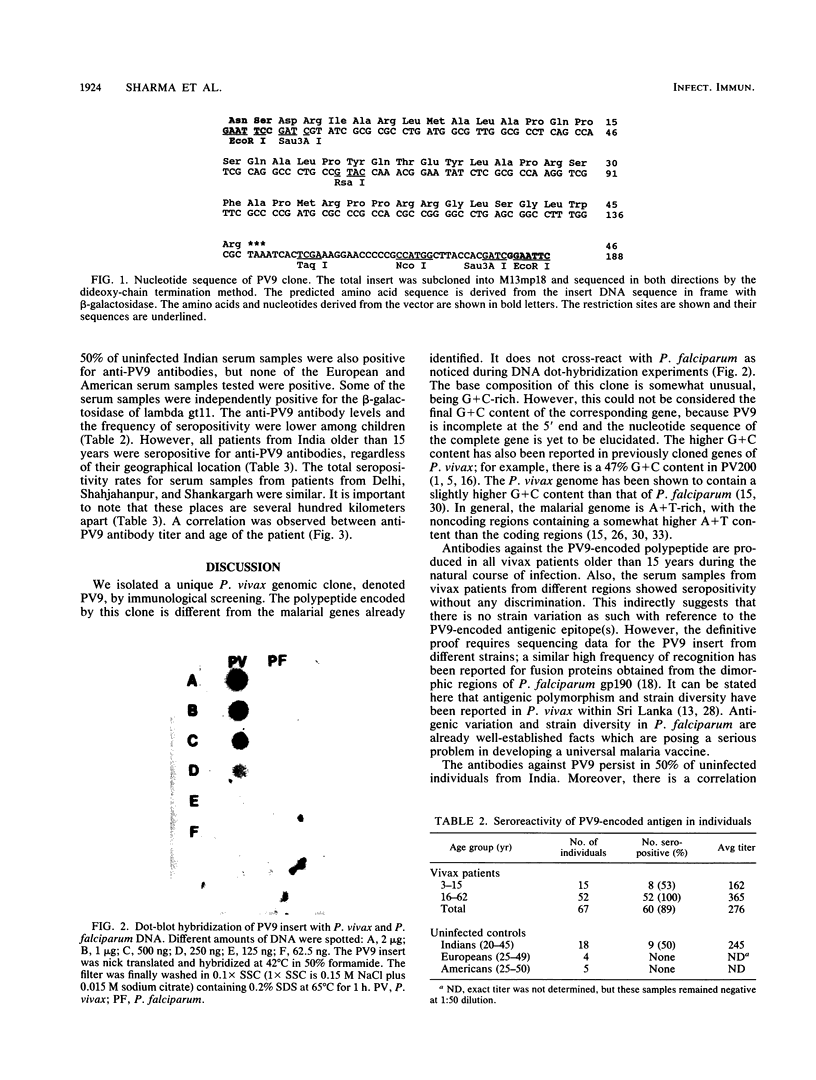
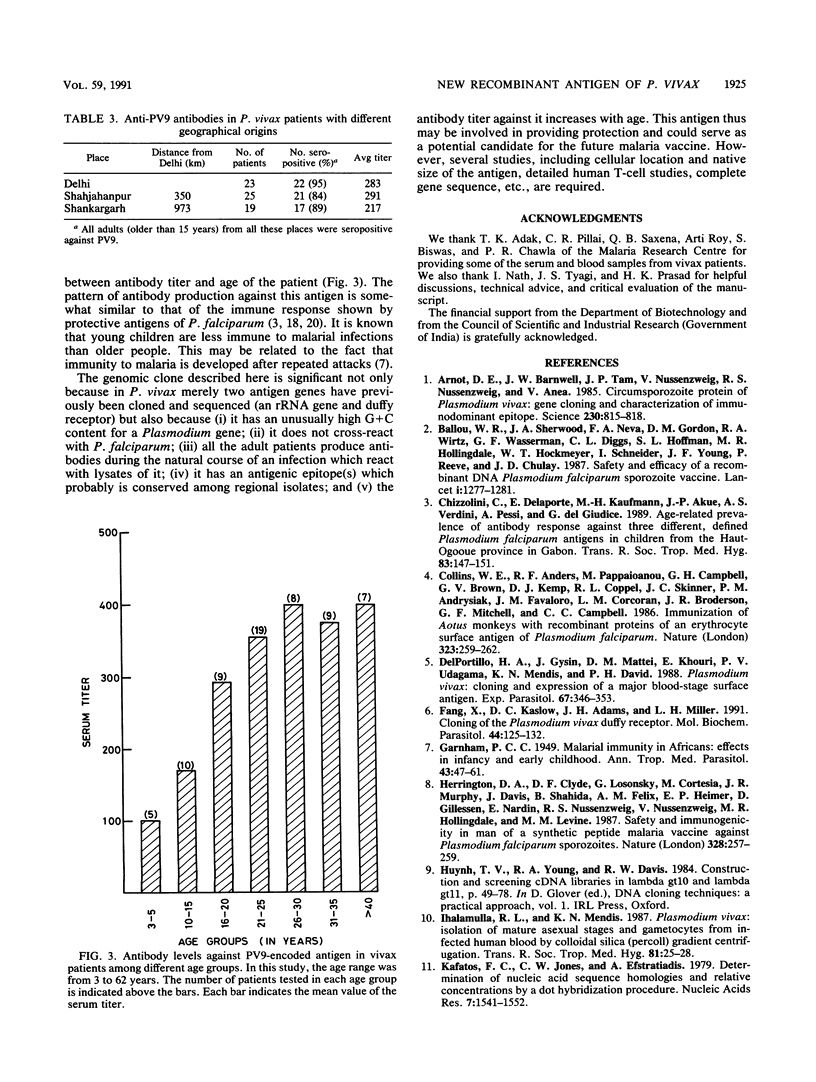
A genomic library for Plasmodium vivax was constructed in lambda gt11 and immunologically screened with pooled serum samples from vivax patients. Six seroreactive clones were isolated, and one clone, denoted PV9, was studied further. This clone has an unusual base composition (65% G + C), does not share any homology with P. falciparum, and codes for an entirely new antigenic determinant. Antibodies (immunoglobulin G type) against the PV9-encoded polypeptide were produced in all vivax patients older than 15 years. This seroreactivity was lower among patients younger than 15 years (53%). The antigenic epitope(s) of the PV9-encoded polypeptide was recognized at a similar rate by serum samples from P. vivax patients who were living 350 to 973 km apart. Fifty percent of uninfected Indian adults were also seropositive, whereas all European and American (United States) sera tested were negative, suggesting that anti-PV9 antibodies persist after infection. The seroreactivity pattern of this antigen is similar to that of the immunity developed in malaria after repeated infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D. E., Barnwell J. W., Tam J. P., Nussenzweig V., Nussenzweig R. S., Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985 Nov 15;230(4727):815–818. doi: 10.1126/science.2414847. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Chizzolini C., Delaporte E., Kaufmann M. H., Akue J. P., Verdini A. S., Pessi A., del Giudice G. Age-related prevalence of antibody response against three different, defined Plasmodium falciparum antigens in children from the Haut-Ogooué province in Gabon. Trans R Soc Trop Med Hyg. 1989 Mar-Apr;83(2):147–151. doi: 10.1016/0035-9203(89)90619-6. [DOI] [PubMed] [Google Scholar]
- Collins W. E., Anders R. F., Pappaioanou M., Campbell G. H., Brown G. V., Kemp D. J., Coppel R. L., Skinner J. C., Andrysiak P. M., Favaloro J. M. Immunization of Aotus monkeys with recombinant proteins of an erythrocyte surface antigen of Plasmodium falciparum. Nature. 1986 Sep 18;323(6085):259–262. doi: 10.1038/323259a0. [DOI] [PubMed] [Google Scholar]
- Fang X. D., Kaslow D. C., Adams J. H., Miller L. H. Cloning of the Plasmodium vivax Duffy receptor. Mol Biochem Parasitol. 1991 Jan;44(1):125–132. doi: 10.1016/0166-6851(91)90228-x. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Ihalamulla R. L., Mendis K. N. Plasmodium vivax: isolation of mature asexual stages and gametocytes from infected human blood by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg. 1987;81(1):25–28. doi: 10.1016/0035-9203(87)90271-9. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A., Sharma Y. D., Karoui H., Naslund L. Histidine-rich domain of the knob protein of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7938–7941. doi: 10.1073/pnas.83.20.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsley G., Patarapotikul J., Handunnetti S., Khouri E., Mendis K. N., David P. H. Plasmodium vivax: karyotype polymorphism of field isolates. Exp Parasitol. 1988 Dec;67(2):301–306. doi: 10.1016/0014-4894(88)90077-x. [DOI] [PubMed] [Google Scholar]
- Marshall E. Malaria research--what next? Science. 1990 Jan 26;247(4941):399–402. doi: 10.1126/science.2300799. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Dame J. B., Miller L. H., Barnwell J. Evolutionary relatedness of Plasmodium species as determined by the structure of DNA. Science. 1984 Aug 24;225(4664):808–811. doi: 10.1126/science.6382604. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Lal A. A., de la Cruz V. F., Miller L. H., Maloy W. L., Charoenvit Y., Beaudoin R. L., Guerry P., Wistar R., Jr, Hoffman S. L. Sequence of the immunodominant epitope for the surface protein on sporozoites of Plasmodium vivax. Science. 1985 Dec 20;230(4732):1381–1383. doi: 10.1126/science.2416057. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Müller H. M., Früh K., von Brunn A., Esposito F., Lombardi S., Crisanti A., Bujard H. Development of the human immune response against the major surface protein (gp190) of Plasmodium falciparum. Infect Immun. 1989 Dec;57(12):3765–3769. doi: 10.1128/iai.57.12.3765-3769.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarroyo M. E., Amador R., Clavijo P., Moreno A., Guzman F., Romero P., Tascon R., Franco A., Murillo L. A., Ponton G. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988 Mar 10;332(6160):158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Berzins K., Wåhlin B., Troye-Blomberg M., Hagstedt M., Andersson I., Högh B., Petersen E., Björkman A. Dissection of the human antibody response to the malaria antigen Pf155/RESA into epitope specific components. Immunol Rev. 1989 Dec;112:115–132. doi: 10.1111/j.1600-065x.1989.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., Taverne J., Bate C. A., de Souza J. B. The malaria vaccine: anti-parasite or anti-disease? Immunol Today. 1990 Jan;11(1):25–27. doi: 10.1016/0167-5699(90)90007-v. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V. P. Community-based malaria control in India. Parasitol Today. 1987 Jul;3(7):222–226. doi: 10.1016/0169-4758(87)90066-4. [DOI] [PubMed] [Google Scholar]
- Sharma V. P., Mehrotra K. N. Return of malaria. Nature. 1982 Jul 8;298(5870):210–210. doi: 10.1038/298210a0. [DOI] [PubMed] [Google Scholar]
- Sharma Y. D., Kilejian A. Structure of the knob protein (KP) gene of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Nov;26(1-2):11–16. doi: 10.1016/0166-6851(87)90124-1. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagama P. V., David P. H., Peiris J. S., Ariyaratne Y. G., Perera K. L., Mendis K. N. Demonstration of antigenic polymorphism in Plasmodium vivax malaria with a panel of 30 monoclonal antibodies. Infect Immun. 1987 Nov;55(11):2604–2611. doi: 10.1128/iai.55.11.2604-2611.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A. P., McCutchan T. F. Partial sequence of the asexually expressed SU rRNA gene of Plasmodium vivax. Nucleic Acids Res. 1989 Mar 11;17(5):2135–2135. doi: 10.1093/nar/17.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L. Molecular biology of malaria parasites. Exp Parasitol. 1988 Aug;66(2):143–170. doi: 10.1016/0014-4894(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Wyler D. J. Malaria--resurgence, resistance, and research (second of two parts). N Engl J Med. 1983 Apr 21;308(16):934–940. doi: 10.1056/NEJM198304213081605. [DOI] [PubMed] [Google Scholar]
- Wyler D. J. Malaria--resurgence, resistance, and research. (First of two parts). N Engl J Med. 1983 Apr 14;308(15):875–878. doi: 10.1056/NEJM198304143081505. [DOI] [PubMed] [Google Scholar]
- Yang Y. F., Tan-ariya P., Sharma Y. D., Kilejian A. The primary structure of a Plasmodium falciparum polypeptide related to heat shock proteins. Mol Biochem Parasitol. 1987 Nov;26(1-2):61–67. doi: 10.1016/0166-6851(87)90130-7. [DOI] [PubMed] [Google Scholar]
- del Portillo H. A., Gysin J., Mattei D. M., Khouri E., Udagama P. V., Mendis K. N., David P. H. Plasmodium vivax: cloning and expression of a major blood-stage surface antigen. Exp Parasitol. 1988 Dec;67(2):346–353. doi: 10.1016/0014-4894(88)90081-1. [DOI] [PubMed] [Google Scholar]