Abstract
Mesenchymal cells recruited to damaged tissues must circulate through the bloodstream. The absolute numbers of circulating mesenchymal stem cells (cMSCs) in two different models of acute and chronic skeletal muscle injury were determined. cMSCs were present in significantly higher numbers in both models than in healthy controls. These results support the hypothesis that MSCs are mobilised into the bloodstream after skeletal muscle tissue damage. These two models (acute and chronic) would be of value in the search for molecular mediators of mobilisation of MSCs into the circulation.
Keywords: McArdle's disease, flow cytomery, mesenchymal stem cells, mobilisation, muscle damage
Mesenchymal stem cells (MSCs) are involved in the repair of damaged tissues and thus they have a crucial role in regenerative medicine. Several groups are developing treatments with MSCs for cardiac, bone, muscle, cartilage, and inflammatory diseases.1 MSCs reside in several adult tissues such as bone marrow, adipose tissue, cartilage, and skin.2 Under several physiological and pathological conditions, they migrate towards organs in which they do not normally reside.3 For instance, marrow MSCs have been shown to migrate to tumours,4 infarcted myocardium, and repair epithelial or neurological abnormalities.5,6,7,8 This requires that MSCs circulate in the blood to their final destination. We hypothesised that such mobilisation would be enhanced under conditions in which tissues are damaged. If so, circulating MSCs (cMSCs) should be detectable at higher levels under such conditions.
In this study, we determined the number of cMSCs in two models of skeletal muscle injury—acute and chronic—and compared them with those of healthy controls. We studied the circulation of MSCs in a model of acute muscle damage after strenuous muscular exercise involving eccentric contractions. We also studied patients with McArdle's disease under basal conditions. McArdle's disease is a rare genetic metabolic disorder (about one case per 100 000 population) which causes total deficiency of the skeletal muscle isoform of glycogen phosphorylase. The disease is characterised by considerable exercise intolerance, premature fatigue, myalgia, and cramps during exertion.9,10 Severe, chronic skeletal muscle injury—that is, rhabdomyolysis—even under basal conditions is one of the main problems associated with this disease.10 We quantified the magnitude of skeletal muscle damage by measuring serum creatine kinase activity, a well established marker of muscle injury.11
Methods
Written informed consent was obtained from each subject. The study was approved by the institutional ethics committee and adhered to the principles of the Declaration of Helsinki. Eleven patients (three women, eight men; mean (SEM) age 34 (2) years (range 25–45)) diagnosed with McArdle's disease by both muscle biopsy and molecular genetic analysis12 were chosen for the study of MSC mobilisation in the model of chronic skeletal muscle injury. Nineteen healthy volunteers of comparable age (35 (2) years (range 26–46)) with no basal skeletal muscle damage were used as controls. Eleven of these control subjects were also studied before and two hours after running a long distance (21 km) race. This type of event involves eccentric muscle contractions in large skeletal muscle mass resulting in an acute, reversible, stage of rhabdomyolysis.13 Thus blood samples obtained after the race represent an excellent model of acute skeletal muscle injury.
Peripheral blood was collected from all subjects in vacutainers containing EDTA and clot activator and gel for serum separation, and divided for biochemical and cellular studies. Serum total creatine kinase activity was measured in all samples. The serum was kept at −20°C until analysis with an automated analyser (Hitachi 911). Reference values for healthy adults used in our laboratory are 38–190 U/l (men) and 26–140 U/l (women). To exclude cardiac injury, we measured the cardiac isoform of troponin I (TnI‐c) in all samples with an Access Beckman immunoassay system. TnI‐c is an isoform of troponin I not found in skeletal muscle (even during development) that has been shown to have extremely high specificity for the detection of cardiac injury.14,15 The serum was kept at −20°C until assay with a highly specific immunoenzymometric assay (Access AccuTnI assay; Beckman, Fullterton, California, USA).
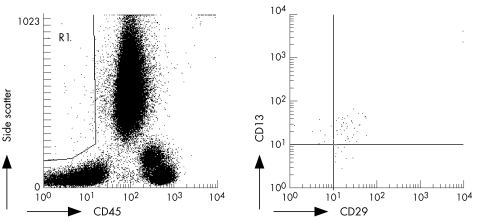
Our strategy for detecting cMSCs relied on flow cytometry analysis. There is no single marker that identifies MSCs, and so it is routinely assumed that they have a fibroblastoid phenotype in cultures and are non‐haematopoietic and negative for markers such as CD3, CD4, CD8, CD19, CD34, and CD62L, and positive for markers such as CD13, CD29, CD90, and CD105.16 Peripheral blood (100 μl) was stained for 25 minutes at 4°C with the following monoclonal antibodies: anti‐(human CD45‐PECy5) (clone J33; Immunotech, Marseille, France) in combination with anti‐(human CD13‐PE) (clone SJ1D1; Immunotech) and anti‐(human CD29‐FITC) (clone K20; Immunotech). As controls of non‐specific staining, cells were labelled with conjugated non‐specific isotype antibodies. Thereafter, red blood cells were lysed by adding 1 ml lysis solution (Quick Lysis; Vitro, Madrid, Spain) and incubated at room temperature for at least 10 minutes. Then 100 μl of blood containing Flow‐count fluorospheres (Beckman‐Coulter, Brea, California, USA) was added before flow cytometer acquisition to enable the determination of the absolute counts of cMSCs present in the peripheral blood samples. Cells were then analysed by flow cytometry (EPICS XL‐MCL cytometer; Coulter Electronics, Hialeah, Florida, USA). For each analysis, at least 100 000 cells with appropriate forward scatter/side scatter ratio were collected. Fluorospheres were collected in the FL3 channel. cMSCs were defined as medium‐high forward side, medium‐high side scatter, CD45 negative, CD29 positive, and CD13 positive events (fig 1).
Figure 1 Identification of circulating mesenchymal cells (cMSCs) by flow cytometry. Peripheral blood samples were stained with monoclonal antibodies as described in the text. A gate containing medium‐high forward and side scatter non‐haematopoietic cells was drawn (R1, left). cMSCs were defined as positive for the markers CD29 and CD13, within region R1 (right).
Statistical analysis was performed using the two sample Wilcoxon rank sum test (Mann‐Whitney two sample statistic) for comparisons. Results are expressed as mean (SEM). Results were considered significant if p⩽0.05. The relation between the number of cMSCs and creatine kinase activity was determined by calculating the Pearson's correlation coefficient.
Results
Overall, blood samples showed TnI‐c concentrations far below those indicative of myocardial injury (2–3 ng/ml). Except in four subjects after the race (with TnI‐c concentrations of 0.1–0.25 ng/ml), the concentration of TnI‐c was consistently well below cut‐off limits (0.1 ng/ml) in the rest of the samples. In particular, TnI‐c was <0.04 ng/ml in all patients with McArdle's disease, ruling out any possibility of myocardial damage.
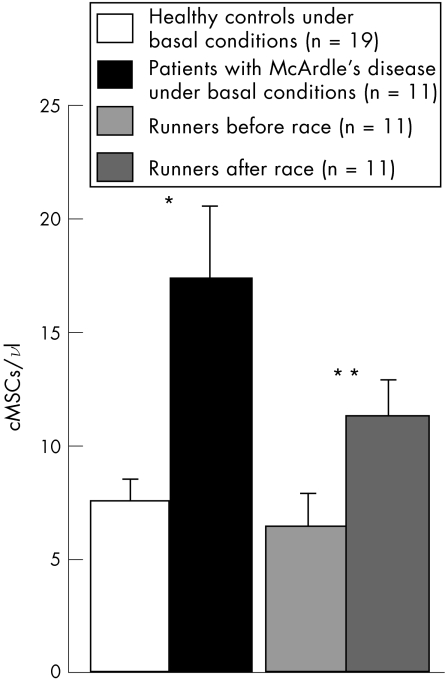
Patients with McArdle's disease had significantly more cMSCs than healthy controls under basal conditions (fig 2). On the other hand, the numbers of cMSCs increased above basal levels in the peripheral blood samples of healthy athletes obtained in the early (two hours) post‐race period (fig 2).
Figure 2 Absolute counts of circulating mesenchymal cells (cMSCs) under normal conditions (healthy controls and runners before a race) or under conditions of acute (runners after a race) or chronic (patients with McArdle's disease) skeletal muscle damage. The absolute counts of the cMSCs present in the peripheral blood samples were determined by following the strategy described in fig 1, with the use of Count fluorospheres. *p = 0.028; **p = 0.007.
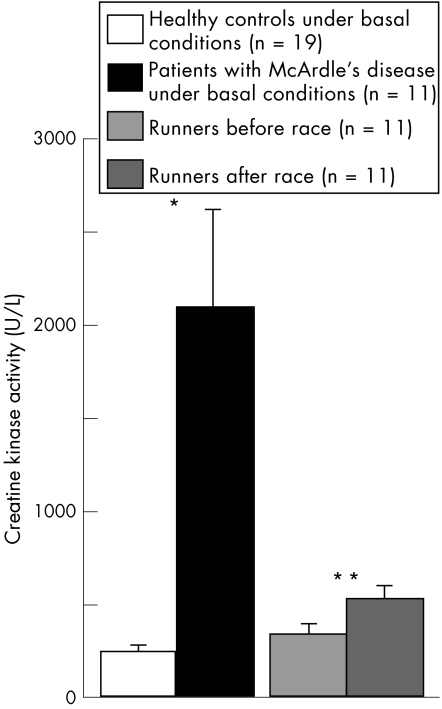
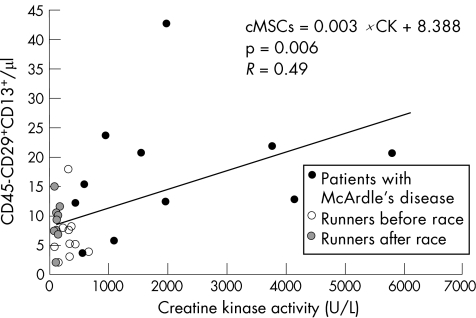
Creatine kinase activity had increased in runners after the race, and was significantly higher in patients with McArdle's disease than in healthy controls (fig 3). We found a significant, positive correlation between the number of cMSCs and creatine kinase activity in all samples (R = 0.49; p = 0.006; fig 4).
Figure 3 Creatine kinase activity in the different groups. *p<0.00005; **p = 0.0069.
Figure 4 Correlation between the absolute count of circulating mesenchymal cells (cMSCs) and creatine kinase activity.
Discussion
What is already known on this topic
Mesenchymal stem cells are involved in the repair of damaged tissues (particularly ischaemic myocardium) and thus they have a crucial role in regenerative medicine
What this study adds
Mesenchymal stem cells are mobilised into the bloodstream in response to acute and chronic skeletal muscle injury
Although more research is needed, these cells may play a role in the repair of damaged skeletal muscle tissue
Our results support the hypothesis that both acute and chronic skeletal muscle injury may be factors that contribute to mobilisation of MSCs into the circulation. Either of these two models would be of value in the search for molecular mediators of MSC mobilisation. These cells appear in the blood in response to relatively low—for example, runners after a race—or high—for example, patients with McArdle's disease—amounts of skeletal muscle damage, as shown in fig 4, where the amount of muscle damage is reflected by creatine kinase activity. Although more research is needed, as no evidence of heart damage was found, our preliminary findings suggest that cMSCs may migrate, at least to a certain extent, to damaged skeletal muscles in an attempt to participate in the process of muscle repair. This possibility is of potential interest for future regenerative treatments using MSCs in neuromuscular disorders—for example, McArdle's disease—associated with chronic muscle wasting. To date it is generally accepted that a population of myogenic progenitors termed satellite cells (which are located inside skeletal muscle, surrounding normal skeletal muscle cells) are solely in charge of undertaking postnatal development and repair of skeletal muscle.17
In summary, this is the first report to show evidence of mobilisation of MSCs into blood after skeletal muscle damage. Such damage was not associated with damage in other tissues (particularly the myocardium) in which MSCs are thought to play a regenerative role. Although more studies are needed, our report suggests a role for MSCs in skeletal muscle regeneration. This may open up new possibilities in regenerative medicine against human diseases associated with chronic muscle breakdown. Beyond this, the response of MSCs observed here suggests that acute and chronic skeletal muscle injury may be a valuable model for studying the factors that regulate the MSC response to injury.
Abbreviations
cMSC - circulating mesenchymal stem cell
MSC - mesenchymal stem cell
TnI‐c - cardiac isoform of troponin I
Footnotes
Competing interests: none declared
Financial support: supported by grants from Fondo de Investigaciones Sanitarias (FIS) numbers p1040487, p1041157, p1040362, and p1052217 and Fundacíon Oncohematogía Infantil.
References
- 1.Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy 2005736–45. [DOI] [PubMed] [Google Scholar]
- 2.Conget P A, Minguell J J. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol 199918167–73. [DOI] [PubMed] [Google Scholar]
- 3.Prockop D J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 199727671–74. [DOI] [PubMed] [Google Scholar]
- 4.Studeny M, Marini F C, Champlin R E.et al Bone marrow‐derived mesenchymal stem cells as vehicles for interferon‐beta delivery into tumors. Cancer Res 2002623603–3608. [PubMed] [Google Scholar]
- 5.Bruder S P, Fink D J, Caplan A I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem 199456283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin H K, Carter J E, Huntley G W, Schuchman E H. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase‐deficient mice delays the onset of neurological abnormalities and extends their life span. J Clin Invest 20021091183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spees J L, Olson S D, Ylostalo J.et al Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA 20031002397–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S.et al Bone marrow cells regenerate infarcted myocardium. Nature 2001410701–705. [DOI] [PubMed] [Google Scholar]
- 9.McArdle B. Myophaty due to a defect in muscle glycogen breakdown. Clin Sci 19511013–33. [PubMed] [Google Scholar]
- 10.Tsujino S, Shanske S, DiMauro S. Molecular genetic heterogeneity of myophosphorylase deficiency (McArdle disease). N Engl J Med 1993329241–245. [DOI] [PubMed] [Google Scholar]
- 11.Sorichter S, Puschendorf B, Mair J. Skeletal muscle injury induced by eccentric muscle action: muscle proteins as markers of muscle fiber injury. Exerc Immunol Rev 199955–21. [PubMed] [Google Scholar]
- 12.Martin M A, Rubio J C, Buchbinder J.et al Molecular heterogeneity of myophosphorylase deficiency (McArdle's disease): a genotype‐phenotype correlation study. Ann Neurol 200150574–581. [PubMed] [Google Scholar]
- 13.Peake J M, Suzuki K, Wilson G.et al Exercise‐induced muscle damage, plasma cytokines, and markers of neutrophil activation. Med Sci Sports Exerc 200537737–745. [DOI] [PubMed] [Google Scholar]
- 14.Vidotto C, Tschan H, Atamaniuk J.et al Responses of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) and cardiac troponin I (cTnI) to competitive endurance exercise in recreational athletes. Int J Sports Med 200526645–650. [DOI] [PubMed] [Google Scholar]
- 15.Adams J E, 3rd, Bodor G S, Davila‐Roman V G.et al ardiac troponin I. A marker with high specificity for cardiac injury. Circulation 199388101–106. [DOI] [PubMed] [Google Scholar]
- 16.Lee R H, Kim B, Choi I.et al Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 200414311–324. [DOI] [PubMed] [Google Scholar]
- 17.McKinnell I W, Parise G, Rudnicki M A. Muscle stem cells and regenerative myogenesis. Curr Top Dev Biol 200571113–130. [DOI] [PubMed] [Google Scholar]