Abstract
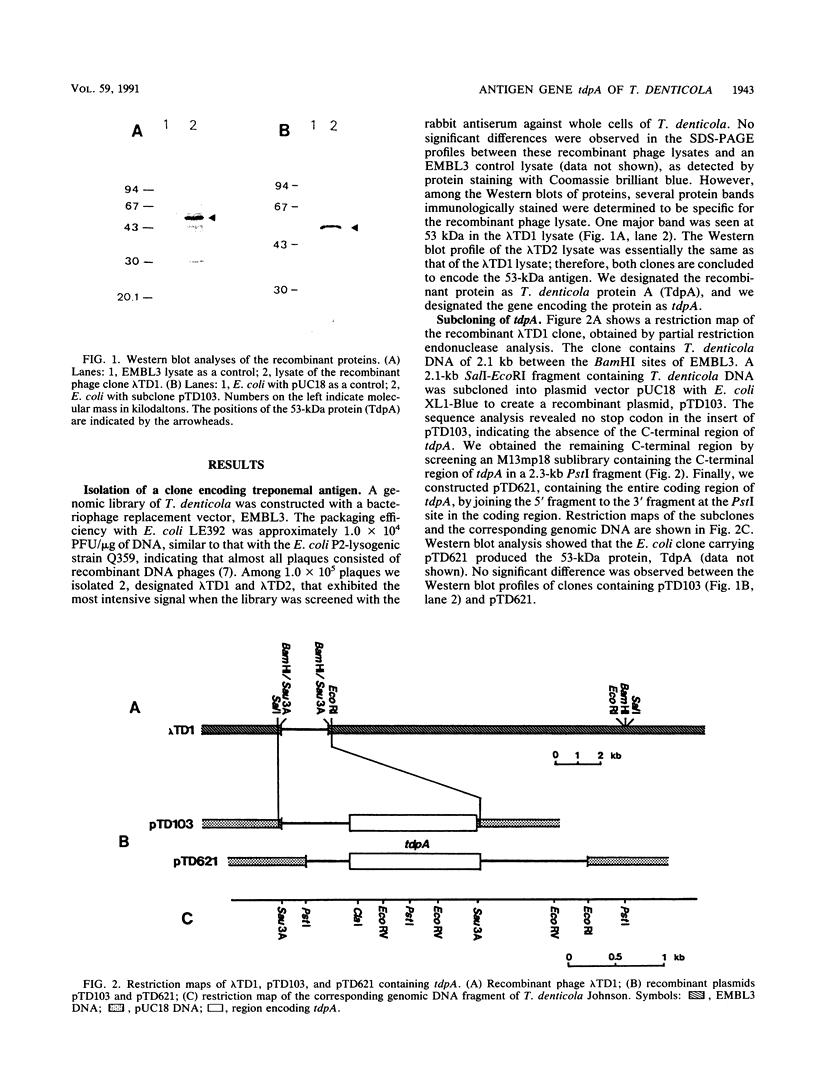
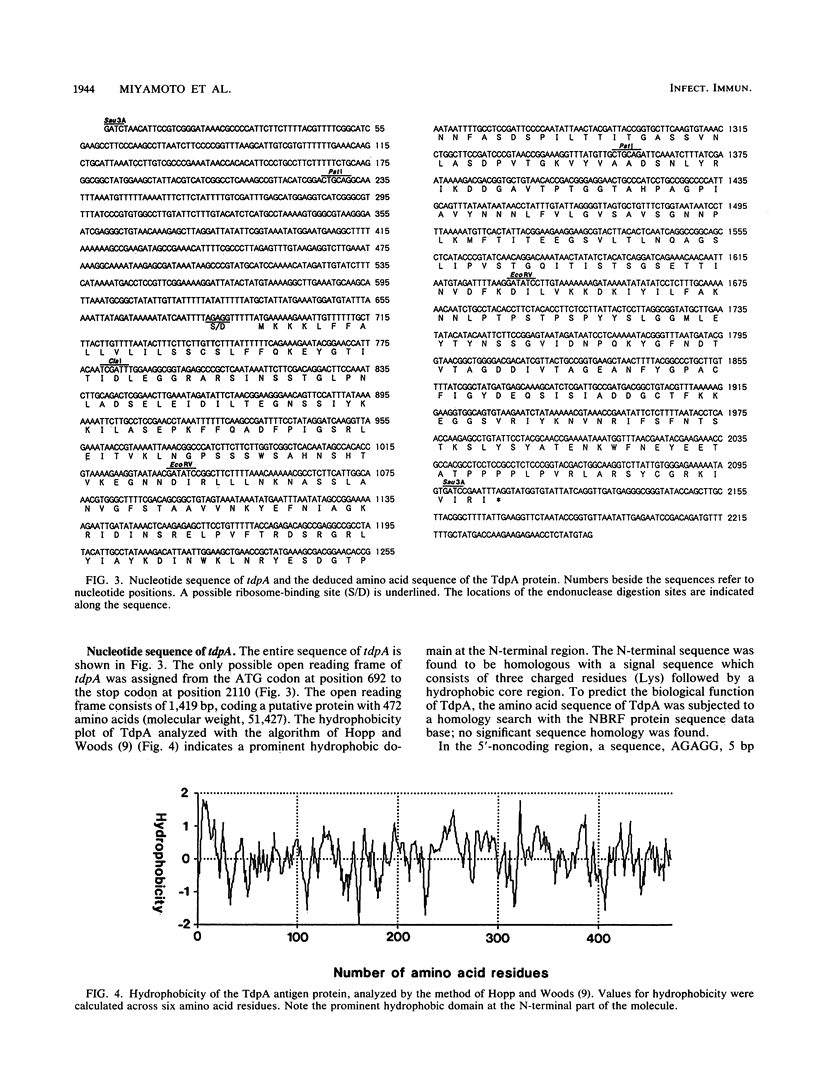
We isolated and characterized an immunogenic protein of an oral spirochete, Treponema denticola Johnson. A genomic DNA library constructed with bacteriophage lambda EMBL3 as a vector was immunologically screened with a rabbit antiserum against the whole cells. Using Western immunoblot analysis, we found a particular clone encoding an antigen with a molecular weight of 53,000; we designated the antigen as T. denticola protein A (TdpA). Complete sequence determination revealed an open reading frame of 1,419 bp and a signal peptide sequence that was homologous to that of bacterial lipoprotein. Southern hybridization analysis revealed that the tdpA gene is highly conserved in six tested strains of T. denticola species. Furthermore, we found that sera from some periodontitis patients contained antibody against the TdpA protein, although the reactivities of the antibodies varied among individuals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai A. [Studies on some morphological, biological and biochemical characteristics of the new cultivated oral spirochetes (author's transl)]. Shigaku. 1976 Aug;64(2):232–252. [PubMed] [Google Scholar]
- Boehringer H., Taichman N. S., Shenker B. J. Suppression of fibroblast proliferation by oral spirochetes. Infect Immun. 1984 Jul;45(1):155–159. doi: 10.1128/iai.45.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne A., Sanger R., Ivic A., Strugnell R. A., MacDougall J. H., Russell R. R., Penn C. W. Antigenic and structural analysis of Treponema denticola. J Gen Microbiol. 1989 Dec;135(12):3209–3218. doi: 10.1099/00221287-135-12-3209. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hansen E. B., Pedersen P. E., Schouls L. M., Severin E., van Embden J. D. Genetic characterization and partial sequence determination of a Treponema pallidum operon expressing two immunogenic membrane proteins in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1227–1237. doi: 10.1128/jb.162.3.1227-1237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E., Nauman R. K. Common antigens of Treponema denticola: chemical, physical, and serological characterization. Infect Immun. 1982 Aug;37(2):474–480. doi: 10.1128/iai.37.2.474-480.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M. [Some characters on 2 strains (E-30,E-31) of oral spirochetes]. Shigaku. 1971 Aug;59(3):193–215. [PubMed] [Google Scholar]
- Kokeguchi S., Kato K., Kurihara H., Murayama Y. Cell surface protein antigen from Wolinella recta ATCC 33238T. J Clin Microbiol. 1989 Jun;27(6):1210–1217. doi: 10.1128/jcm.27.6.1210-1217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leschine S. B., Canale-Parola E. Rifampin as a selective agent for isolation of oral spirochetes. J Clin Microbiol. 1980 Dec;12(6):792–795. doi: 10.1128/jcm.12.6.792-795.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Gojobori T., Aota S., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1986;14 (Suppl):r151–r197. doi: 10.1093/nar/14.suppl.r151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Murayama Y., Nagai A., Okamura K., Kurihara H., Nomura Y., Kokeguchi S., Kato K. Serum immunoglobulin G antibody to periodontal bacteria. Adv Dent Res. 1988 Nov;2(2):339–345. doi: 10.1177/08959374880020022401. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt M. A., Radolf J. D., Norgard M. V. The 34-kilodalton membrane immunogen of Treponema pallidum is a lipoprotein. Infect Immun. 1990 Feb;58(2):384–392. doi: 10.1128/iai.58.2.384-392.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt M. A., Riley B. S., Radolf J. D., Norgard M. V. Molecular characterization of the pathogen-specific, 34-kilodalton membrane immunogen of Treponema pallidum. Infect Immun. 1989 Nov;57(11):3314–3323. doi: 10.1128/iai.57.11.3314-3323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. [Isolation and pure cultivation of oral spirochetes, and electron microscopic studies on the cell structure as well as the biological characteris of pure cultivation strains]. Shigaku. 1968 Dec;56(3):299–332. [PubMed] [Google Scholar]
- Umemoto T., Namikawa I. Electron microscopy of the spherical body of oral spirochetes in vitro. Further studies. Microbiol Immunol. 1980;24(4):321–334. doi: 10.1111/j.1348-0421.1980.tb02835.x. [DOI] [PubMed] [Google Scholar]
- Umemoto T., Namikawa I., Suido H., Asai S. A major antigen on the outer envelope of a human oral spirochete, Treponema denticola. Infect Immun. 1989 Aug;57(8):2470–2474. doi: 10.1128/iai.57.8.2470-2474.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T., Zambon J. J., Genco R. J., Namikawa I. Major antigens of human oral spirochetes associated with periodontal disease. Adv Dent Res. 1988 Nov;2(2):292–296. doi: 10.1177/08959374880020021401. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- van Embden J. D., van der Donk H. J., van Eijk R. V., van der Heide H. G., de Jong J. A., van Olderen M. F., Osterhaus A. B., Schouls L. M. Molecular cloning and expression of Treponema pallidum DNA in Escherichia coli K-12. Infect Immun. 1983 Oct;42(1):187–196. doi: 10.1128/iai.42.1.187-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]