Abstract
The differentiation of physiological left ventricular hypertrophy (LVH) from hypertrophic cardiomyopathy (HCM) can prove challenging for even the most experienced cardiologists. The case is presented of a 17 year old elite swimmer who had electrocardiographic and echocardiographic features that were highly suggestive of HCM. However, indices of diastolic function were normal and cardiopulmonary exercise testing revealed high peak oxygen consumption in keeping with physiological LVH. To resolve the diagnostic dilemma, the patient underwent detraining for eight weeks, after which, there was complete resolution of the changes seen on electrocardiogram and echocardiogram, indicating physiological LVH rather than HCM.
Keywords: hypertrophic cardiomyopathy, detraining, elite adolescent athlete, left ventricular hypertrophy
Sudden cardiac death from hypertrophic cardiomyopathy (HCM) is well recognised.1,2 The death of some high profile athletes from HCM in the past few years has galvanised some sporting authorities to implement cardiovascular screening in all recruits before selection. However, cardiovascular adaptation to intense physical training itself can cause significant left ventricular hypertrophy (LVH) that may mimic HCM.3,4 A common and challenging scenario involves the differentiation of physiological LVH (“athlete's heart”) from HCM.
Case report
A 17 year old elite male swimmer was referred with an abnormal 12 lead electrocardiogram (ECG) during cardiovascular screening. The athlete trained for about 14 hours and swam 16 miles a week. He was asymptomatic. There was no history of illicit drug abuse or a family history of premature sudden cardiac death. Blood pressure measured 110/70 mm Hg, and cardiovascular examination was normal.
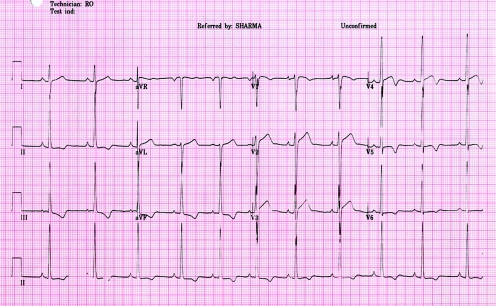
The 12 lead ECG showed voltage criteria for left ventricular hypertrophy and deep T wave inversions (>0.2 mV) in the inferior and lateral leads (fig 1). A two dimensional echocardiogram revealed significant concentric LVH with a maximal left ventricular wall thickness of 14 mm. The left ventricular cavity size was normal measuring 48 mm in end diastole. Indices of systolic and diastolic functions were normal (E wave 0.9 m/s; A wave 0.36 m/s; E/A 2.63; E deceleration time 226 milliseconds). The valves and right ventricle appeared normal.
Figure 1 A 12 lead electrocardiogram from a 17 year old swimmer showing sinus rhythm with deep T wave inversion (>0.2 mV) in inferior and lateral leads and isolated Sokolow voltage criterion for left ventricular hypertrophy.
The differential diagnosis was between HCM and athlete's heart. The distinction between the two entities is crucial as a false diagnosis of HCM in an athlete calls for disqualification from competitive sport, conversely a false diagnosis of athlete's heart could jeopardise a young life.
A cardiac magnetic resonance imaging scan excluded apical HCM. A 12 lead ECG and echocardiography performed on both parents and two siblings were normal. Although this excluded familial HCM, it did not rule out the possibility of a sporadic disease causing mutation.
During a cardiopulmonary exercise stress test, the athlete achieved a peak oxygen consumption of 68 ml/kg/min (141% of predicted). The blood pressure response to exercise was normal with the systolic blood pressure rising from 110 mm Hg at rest to 180 mm Hg at peak exercise. A 48 hour Holter monitor failed to reveal any pathological ventricular arrhythmias.
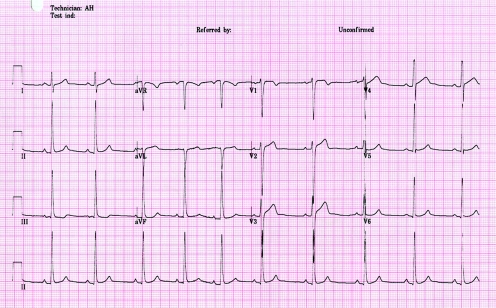
The athlete was persuaded to detrain for eight weeks, and three cardiovascular evaluations were performed in the interim, during which two consecutive ECGs revealed a gradual but complete resolution of the T wave inversions (fig 2). A subsequent echocardiogram showed regression of LVH from 14 mm to 11 mm. The normalisation of the ECG and echocardiographic abnormalities after detraining confirmed that the changes observed in our athlete reflected extreme physiological adaptation to exercise, rather than HCM. The patient has resumed training and continues to compete at regional level.
Figure 2 A 12 lead electrocardiogram from the same athlete after detraining showing complete resolution of T wave inversions in inferior and lateral leads.
Discussion
The distinction between physiological LVH from HCM is crucial, especially when one considers that HCM accounts for approximately one third of all exercise related sudden cardiac death in young athletes. Our athlete had some ECG and echocardiographic features suggestive of HCM based on two large studies performed in adolescent athletes. An ECG study of 1000 highly trained adolescent athletes suggested that deep T wave inversions (>0.2 mV), in any lead and even minor T wave inversions in the lateral leads are generally absent in adolescent athletes.5 An echocardiographic study of 720 athletes showed that LVH was present in 4% of adolescent athletes. However, in terms of absolute values, a wall thickness >12 mm was rare and present in just two (<0.5%) athletes. Furthermore, adolescent athletes with a LVH also had an enlarged left ventricular cavity size, with measurements of 52–60 mm,6 whereas our athlete had a non‐dilated left ventricular cavity.
However, unlike patients with HCM, our athlete had normal indices of diastolic function and a high peak oxygen consumption, indicating that the LVH may be physiological. Patients with HCM have low peak oxygen consumption irrespective of symptoms.7 The mechanisms include impaired myocardial relaxation and failure to augment stroke volume effectively during exercise. On the other hand, elite athletes have efficient left ventricular filling and can augment stroke volume even at rapid heart rates. In general, a peak oxygen consumption of >50 ml/kg/min or >120% of that predicted for age and size favours physiological LVH.8
What is already known on this topic
The presence of echocardiographic ventricular hypertrophy in an adolescent athlete in association with a non‐dilated left ventricular cavity size and deep T wave inversions on the 12 lead ECG is highly suggestive of hypertrophic cardiomyopathy
Differentiation between physiological left ventricular hypertrophy from athletic training and hypertrophic cardiomyopathy is crucial as hypertrophic cardiomyopathy is the most common cause of exercise related sudden cardiac death in adolescent athletes
What this study adds
This study exemplifies the role of detraining in differentiating athlete's heart from hypertrophic cardiomyopathy
All athletes with left ventricular hypertrophy and deep T wave inversions should be reassessed after a period of detraining before erroneoulsy diagnosed as having hypertrophic cardiomyopathy
In summary, our athlete had some objective evidence to suggest the diagnosis of HCM and others to indicate athlete's heart. Given the persistence of diagnostic uncertainty and the potential implications of a false diagnosis, he was persuaded to detrain for a period of eight weeks, which resolved the dilemma.
Detraining is a definitive method of differentiating physiological LVH from HCM. It is well recognised that physiological LVH in athletes regresses after a short period of detraining.9,10 Unfortunately, most athletes who are striving to achieve honours in competitive sport are not willing to detrain, as it compromises fitness and may prove costly for team selection. This case shows the importance of implementing detraining to resolve diagnostic uncertainty in adolescent athletes with ECG and echocardiographic features suggestive of HCM. In the absence of routine availability of genetic testing for HCM, detraining enables a correct diagnosis and prevents subjective diagnostic errors with potentially serious consequences.
Abbreviations
ECG - electrocardiogram
HCM - hypertrophic cardiomyopathy
LVH - left ventricular hypertrophy
Footnotes
Competing interests: none declared
References
- 1.Sharma S, Whyte G, McKenna W J. Sudden death from cardiovascular disease in young athletes: fact or fiction. Br J Sports Med 199731269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maron B, Epstein S, Roberts W. Causes of sudden death in young competitive athletes. J Am Coll Cardiol 19867204–214. [DOI] [PubMed] [Google Scholar]
- 3.Pellicia A, Maron B J, Spataro A.et al The upper limit of physiological hypertrophy in highly trained elite athletes. N Engl J Med 1991324295–301. [DOI] [PubMed] [Google Scholar]
- 4.Maron B J, Pellicia A, Spirito P. Cardiac disease in young trained athletes. Insights into methods for distinguishing athletes heart from structural heart disease, with particular emphasis on hypertrophic cardiomyopathy. Circulation 1995911596–1601. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Whyte G, Elliott P.et al Electrocardiographic changes in 1000 highly trained junior elite athletes. Br J Sports Med 199933319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S, Maron B J, Whyte G.et al Physiologic limits of left ventricular hypertrophy in elite junior athletes: relevance to differential diagnosis of athlete's heart and hypertrophic cardiomyopathy. J Am Coll Cardiol 2002401431–1436. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Elliott P, Whyte G.et al Utility of cardiopulmonary exercise in the assessment of clinical determinants of functional capacity in hypertrophic cardiomyopathy. Am J Cardiol 200086162–168. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Elliott P M, Whyte G.et al Utility of metabolic exercise testing in distinguishing hypertrophic cardiomyopathy from physiologic left ventricular hypertrophy in athletes. J Am Coll Cardiol 200036864–870. [DOI] [PubMed] [Google Scholar]
- 9.Maron B, Pelliccia A, Sparato A.et al Reduction in left ventricular wall thickness after deconditioning in highly trained Olympic athletes. Br Heart J 199369125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehsani A A, Hagberg J M, Hickson R C. Rapid changes in left ventricular dimensions and mass in response to physical conditioning and deconditioning. Am J Cardiol 19784252–56. [DOI] [PubMed] [Google Scholar]