Abstract
Background
The CpG island methylator phenotype (CIMP) is a distinct phenotype associated with microsatellite instability (MSI) and BRAF mutation in colon cancer. Recent investigations have selected 5 promoters (CACNA1G, IGF2, NEUROG1, RUNX3 and SOCS1) as surrogate markers for CIMP-high. However, no study has comprehensively evaluated an expanded set of methylation markers (including these 5 markers) using a large number of tumors, or deciphered the complex clinical and molecular associations with CIMP-high determined by the validated marker panel.
Metholodology/Principal Findings
DNA methylation at 16 CpG islands [the above 5 plus CDKN2A (p16), CHFR, CRABP1, HIC1, IGFBP3, MGMT, MINT1, MINT31, MLH1, p14 (CDKN2A/ARF) and WRN] was quantified in 904 colorectal cancers by real-time PCR (MethyLight). In unsupervised hierarchical clustering analysis, the 5 markers (CACNA1G, IGF2, NEUROG1, RUNX3 and SOCS1), CDKN2A, CRABP1, MINT31, MLH1, p14 and WRN were generally clustered with each other and with MSI and BRAF mutation. KRAS mutation was not clustered with any methylation marker, suggesting its association with a random methylation pattern in CIMP-low tumors. Utilizing the validated CIMP marker panel (including the 5 markers), multivariate logistic regression demonstrated that CIMP-high was independently associated with older age, proximal location, poor differentiation, MSI-high, BRAF mutation, and inversely with LINE-1 hypomethylation and β-catenin (CTNNB1) activation. Mucinous feature, signet ring cells, and p53-negativity were associated with CIMP-high in only univariate analysis. In stratified analyses, the relations of CIMP-high with poor differentiation, KRAS mutation and LINE-1 hypomethylation significantly differed according to MSI status.
Conclusions
Our study provides valuable data for standardization of the use of CIMP-high-specific methylation markers. CIMP-high is independently associated with clinical and key molecular features in colorectal cancer. Our data also suggest that KRAS mutation is related with a random CpG island methylation pattern which may lead to CIMP-low tumors.
Introduction
Epigenetic aberrations are important mechanisms in human carcinogenesis [1], [2]. A number of tumor suppressor genes are silenced by promoter CpG island methylation [3], [4]. A subset of colorectal cancers exhibit widespread promoter methylation, which is referred to as the CpG island methylator phenotype (CIMP) [4]–[7]. CIMP-high colorectal tumors have been associated with older age, female sex, proximal location, mucinous and poor differentiation, microsatellite instability (MSI), BRAF mutation, high LINE-1 methylation level, wild-type TP53, stable chromosomes, and inactive WNT/β-catenin [8]–[23]. However, many of these features are interrelated, and thus, it is essential to analyze a large number of tumors by multivariate analysis to decipher the complex relations between CIMP-high and these clinical/tumoral variables.
There is considerable heterogeneity of tumors with regard to CpG island methylation, and not all CpG islands are methylated in a similar manner in colorectal cancer [15]. Thus, choice of CpG islands can substantially influence the features of CIMP. In fact, different CIMP panels used in various studies have caused considerable confusion [7]. Weisenberger et al. [15] have screened 195 CpG islands, and selected 5 loci (CACNA1G, IGF2, NEUROG1, RUNX3 and SOCS1), which can serve as surrogate markers for CIMP-high. We have further validated the use of 8 markers (the above 5 plus CDKN2A (p16), CRABP1 and MLH1) as a CIMP-high diagnostic panel [24]. However, no study has comprehensively compared these CIMP-high-specific CpG islands and other CpG islands using a large number of tumors.
In this study, we have assessed 16 CpG islands including the new 5 CIMP markers as well as MINT (methylated in tumor) markers and other CpG islands, utilizing hierarchical clustering analysis on a large number of colorectal cancers. We have also assessed the characteristics of CIMP-high tumors determined by a validated marker panel, and interactions of various clinical and tumoral factors by multivariate logistic regression analysis. This study provides the rationale for standardization of CIMP-high-specific methylation markers.
Methods
Study Group
We utilized the databases of two large prospective cohort studies; the Nurses' Health Study (NHS, N = 121,700 women followed since 1976) [25], [26], and the Health Professionals Follow-up Study (HPFS, N = 51,500 men followed since 1986) [26]. A subset of cohort participants developed colorectal cancer during prospective follow-up. Thus, these colorectal cancers represented a population-based, relatively unbiased sample (compared to a single or few-hospital-based sample). Previous studies on the cohorts have described baseline characteristics of cohort participants and incident colorectal cancer cases, and confirmed that our colorectal cancers were well representative as a population-based sample [25], [26]. Clinical information was obtained through chart review by physicians. We collected paraffin-embedded tissue blocks from hospitals where participants had undergone resections of primary colorectal cancers. Based on availability of adequate tissue specimens, a total of 904 colorectal cancer cases (406 from the men's cohort and 498 from the women's cohort) were included. Clinical characteristics of the cases are described in Table 1 (on the left, under the column heading “All cases”). Among our cohort studies, there was no significant difference in demographic features between cases with tissue available and those without available tissue [26]. Most tumors have previously been characterized for statuses of MSI, CIMP, KRAS, BRAF, p53, β-catenin, LINE-1 methylation and 14 of the 16 methylation markers [19], [21], [24], [27]. However, none of our previous studies have comprehensively analyzed the 16 methylation markers in relation to each other, independent associations of CIMP with various clinical, pathological or tumoral molecular characteristics, or interactions of various factors on the associations with CIMP-high by comprehensive biostatistical methods. This study represents a unique novel study in term of 1) a large sample size; 2) the validated set of CIMP-specific methylation markers; 3) the number of other molecular events analyzed, including 8 CpG islands other than the CIMP-specific markers, MSI, KRAS, BRAF, p53, LINE-1 methylation and β-catenin; and 4) comprehensive statistical analyses including unsupervised hierarchical clustering, smoothing splines to assess nonlinearity, multivariate logistic regression, and stratified logistic regression. Thus, this study obtained novel data from the existing materials and database, analogous to novel studies using the well-described cell lines or mouse models. Informed consent was obtained from all study subjects. Tissue collection and analyses were approved by the Harvard School of Public Health and Brigham and Women's Hospital Institutional Review Boards.
Table 1. Clinical, pathologic and molecular characteristics of colon cancer cases according to CIMP status.
| Clinical, pathological or molecular feature | All cases | CIMP-low/0 | CIMP-high | P value |
| Total N | 904 | 771 | 133 | |
| Sex | 0.003 | |||
| Male | 406 (45%) | 362 (47%) | 44 (33%) | |
| Female | 498 (55%) | 409 (53%) | 89 (67%) | |
| Mean age±SD | 66.3±8.4 | 65.7±8.5 | 69.4±6.9 | <0.0001# |
| Tumor location∧ | <0.0001 | |||
| Proximal | 386 (45%) | 270 (37%) | 116 (89%) | |
| Distal (excluding rectum) | 282 (33%) | 270 (37%) | 12 (9.2%) | |
| Rectum | 199 (23%) | 196 (27%) | 3 (2.3%) | |
| Tumor stage | <0.0001 | |||
| I | 203 (25%) | 188 (28%) | 15 (12%) | |
| II | 261 (33%) | 193 (29%) | 68 (54%) | |
| III | 229 (29%) | 203 (30%) | 26 (21%) | |
| IV | 106 (13%) | 90 (13%) | 16 (13%) | |
| Tumor differentiation | <0.0001 | |||
| Well/moderate | 803 (91%) | 710 (94%) | 93 (70%) | |
| Poor | 83 (9.4%) | 43 (5.7%) | 40 (30%) | |
| Mucinous component | <0.0001 | |||
| 0% | 601 (66%) | 545 (71%) | 56 (42%) | |
| 1–49% | 188 (21%) | 152 (20%) | 36 (27%) | |
| ≥50% | 115 (13%) | 74 (9.6%) | 41 (31%) | |
| Signet ring cells | <0.0001 | |||
| 0% | 844 (93%) | 733 (95%) | 111 (83%) | |
| ≥1% | 60 (6.6%) | 38 (4.9%) | 22 (17%) | |
| MSI | <0.0001 | |||
| Non-MSI-high | 748 (85%) | 707 (95%) | 41 (31%) | |
| MSI-high | 127 (15%) | 37 (5.0%) | 90 (69%) | |
| Mean LINE-1 methylation (%)±SD | 61.4±9.6 | 60.7±9.6 | 65.1±8.3 | <0.0001# |
| BRAF | <0.0001 | |||
| Wild-type | 748 (87%) | 697 (95%) | 51 (40%) | |
| Mutated | 113 (13%) | 36 (4.9%) | 77 (60%) | |
| KRAS | <0.0001 | |||
| Wild-type | 557 (63%) | 445 (59%) | 112 (85.5%) | |
| Mutated | 326 (37%) | 307 (41%) | 19 (14.5%) | |
| p53* | <0.0001 | |||
| Negative | 506 (57%) | 403 (53%) | 103 (77%) | |
| Positive | 386 (43%) | 356 (47%) | 30 (23%) | |
| β-catenin* | <0.0001 | |||
| Inactive (score 0–2) | 499 (64%) | 403 (60%) | 96 (91%) | |
| Active (score 3–5) | 278 (36%) | 269 (40%) | 9 (8.6%) |
(%) indicates the proportion of cases with a specific clinical feature within each MSI/CIMP subtype.
Proximal colon includes cecum to transverse colon, and distal colon includes splenic flexure to sigmoid colon.
p53 and β-catenin status was determined by immunohistochemistry. Active β-catenin was defined as β-catenin score ≥3, where the β-catenin score was the sum of nuclear (0, 1+ or 2+), cytoplasmic (0, 1+ or 2+) and membrane (0 or 1+ if expression is lost) scores as originally described by Jass et al.[35].
t-test assuming unequal variances.
CIMP, CpG island methylator phenotype; LINE-1, long interspersed nucleotide element-1; MSI, microsatellite instability; SD, standard deviation.
Pathologic Examination, DNA Extraction and Sequencing of KRAS and BRAF
For all cases, pathologic features including tumor differentiation, mucinous features and signet ring cells were examined by a pathologist (S.O.). Poor differentiation was defined as the presence of <50% glandular area. Genomic DNA was extracted from paraffin tissue, and PCR and Pyrosequencing targeted for KRAS codons 12 and 13, and BRAF codon 600 were performed as previously described [28], [29].
Microsatellite Instability (MSI) Analysis
MSI status was determined by the MSI panel including D2S123, D5S346, D17S250, BAT25, BAT26, BAT40, D18S55, D18S56, D18S67 and D18S487 (i.e., 10-marker panel) as previously described [24]. A “high degree of MSI” (MSI-high) was defined as the presence of instability in ≥30% of the markers.
Real-time PCR (MethyLight) for Quantitative DNA Methylation Analysis
Sodium bisulfite treatment on DNA and subsequent real-time PCR (MethyLight [30]) was validated and performed as previously described [31]. We quantified DNA methylation in 5 CIMP-specific promoters (CACNA1G, IGF2, NEUROG1, RUNX3 and SOCS1) and 11 other CpG islands [CDKN2A (p16), CHFR, CRABP1, HIC1, IGFBP3, MGMT, MINT1, MINT31, MLH1, p14 (CDKN2A/ARF), and WRN]. COL2A1 (the collagen 2A1 gene) was used to normalize for the amount of template bisulfite-converted DNA [31]. Primers and probes were previously described [15], [27], except for IGFBP3, p14 and WRN: IGFBP3-F, 5′-GTT TCG GGC GTG AGT ACG A-3′ (Genbank No. M35878, nucleotide Nos. 1692-1710); IGFBP3-R, GAA TCG ACG CAA ACA CGA CTA C(nucleotide Nos. 1789-1810) and IGFBP3-probe, 6FAM-TCG GTT GTT TAG GGC GAA GTA CGG G-BHQ-1(nucleotide Nos. 1760-1784) (bisulfite-converted nucleotides are highlighted by bold face and italics); P14 (CDKN2A/ARF)-F, 5′- TTG GAG GCG GCG AGA ATA T-3′ (Genbank No. L41934, nucleotide Nos. 238-256); P14-R, 5′- CCC CGT AAA CCG CGA AAT A-3′ (nucleotide Nos. 332-350); P14-probe, 6FAM-5′- CGG TTC GTC GCG AGT GAG GGT T-3′ –BHQ-1 (nucleotide Nos. 299-320); WRN-F, 5′-GTA TCG TTC GCG GCG TTT AT-3′ (Genbank No. AY442327, nucleotide Nos. 1827-1846); WRN-R, 5′-ACG AAA CCG ATA TCC GAA ATC A -3′ (nucleotide Nos. 1887-1908) and WRN-probe, 6FAM-TTT TTT TTG CGG TCG TTG CGG G-BHQ-(nucleotide Nos. 1855-1876). The PCR condition was initial denaturation at 95°C for 10 min followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. A standard curve was made for each PCR plate by duplicated PCR amplifications for COL2A1 on bisulfite-converted human genomic DNA at 4 different concentrations (in a 5-fold dilution series). The percentage of methylated reference (PMR, i.e., degree of methylation) at a specific locus was calculated by dividing the GENE:COL2A1 ratio of template amounts in a sample by the GENE:COL2A1 ratio of template amounts in SssI-treated human genomic DNA (presumably fully methylated) and multiplying this value by 100. Methylation positivity was set as PMR≥4 as previously validated [31].
Pyrosequencing to Measure LINE-1 Methylation
In order to accurately quantify relatively high LINE-1 methylation levels, we utilized Pyrosequencing as previously described [21]. LINE-1 methylation level measured by Pyrosequencing has been shown to correlate well with overall 5-methylcytosine level (i.e., genome-wide DNA methylation level) in tumor cells [32], [33].
Immunohistochemistry for p53 and β-catenin
Tissue microarrays (TMAs) were constructed and immunohistochemistry for p53 and β-catenin was performed as previously described [19], [34]. Appropriate positive and negative controls were included in each run of immunohistochemistry. Cytoplasmic and nuclear β-catenin expression was recorded separately as either no expression (0), weak expression (1+), or moderate/strong expression (2+). The β-catenin activation score was calculated as the sum of nuclear score (0–2), cytoplasmic score (0–2) and membrane score (0 if membrane staining was positive, +1 if membrane expression was lost), as originally described by Jass et al. [35]. All immunohistochemically-stained slides were examined by one of the investigator (β-catenin by K.N.; p53 by S.O.) unaware of other data. Random samples of 402 and 118 tumors were re-examined for β-catenin and p53, respectively, by a second observer (β-catenin by S.O., p53 by K.N.) unaware of other data, and the concordances between the two observers were 0.83 for β-catenin (κ = 0.65, p<0.0001), and 0.87 for p53 (κ = 0.75, p<0.0001), indicating substantial agreement.
Statistical Analysis
For cluster analysis of biomarkers including the 16 methylation markers, MSI, KRAS and BRAF, we utilized average linkage hierarchical clustering with a Euclidean distance metric as implemented in MeV (http://www.tm4.org) [36]. The chi square test was used to examine an association between CIMP and other categorical variables of interest. The t-test assuming unequal variances was performed to compare mean age and mean LINE-1 methylation level. The κ coefficient was calculated to assess agreement between each of the 16 markers (positive vs. negative) and the 16-marker CIMP panel (CIMP-high positive vs. negative).
To examine the relations of a given variable and CIMP-high, we utilized unconditional logistic regression models to calculate odds ratios (ORs) for CIMP-high, according to the status of the given variable, unadjusted and adjusted for age, sex, tumor location, stage, differentiation, LINE-1 methylation level, and status of MSI, KRAS, BRAF, p53 and β-catenin. To adjust for potential confounding, age and LINE-1 methylation level were used as continuous variables, and all of the other variables were used as categorical variables.
For age and LINE-1, we assessed non-linearity by the likelihood ratio test that compared a regression model including a quadratic (or cubic) term with a model excluding it. The likelihood ratio test showed that including the quadratic term did not significantly alter model fit (p = 0.86 for age, p = 0.078 for LINE-1), and that including the cubic term did not significantly alter model fit (p = 0.87 for age, p = 0.084 for LINE-1). We also examined the possibility of a non-linear relation between age (or LINE-1 methylation) and CIMP-high, non-parametrically with restricted cubic splines [37].
We dichotomized tumor location (proximal vs. distal), tumor differentiation (poor vs. well/moderate), signet ring cells (present vs. absent), MSI (high vs. non-MSI-high), p53 (positive vs. negative), KRAS (mutated vs. wild-type), BRAF (mutated vs. wild-type) and β-catenin (active vs. inactive). There were 3 categories for mucinous feature (0%, 1–49%, and ≥50%) in the initial main analysis ( Table 2 ). We dichotomized mucinous feature (present vs. absent) in secondary stratified analyses and analyses of interactions, because multivariate ORs for CIMP-high were similar across the 1–49% mucinous and ≥50% mucinous categories (in reference to the non-mucinous category). There were 4 categories for stage (I, II, III and IV) in the initial main analysis ( Table 2 ). We dichotomized tumor stage (I vs. II–IV) in secondary stratified analyses and analyses of interactions, because multivariate ORs for CIMP-high were similar across stage II–IV (in reference to stage I).
Table 2. Associations with CIMP-high in colorectal cancer by univariate and multivariate analyses.
| Clinical, pathological, or molecular characteristics | Univariate OR (95% CI) | P value | OR adjusted for major confounder(s) | Major confounder(s) (in the order of strength of effect) | Multivariate OR (95% CI) | P value |
| Age (10-year increment) | 1.78 (1.40–2.27) | <0.0001 | 2.53 (1.69–3.78) | BRAF, MSI | 3.16 (1.93–5.18) | <0.0001 |
| Female (vs. male) | 1.79 (1.22–2.64) | 0.003 | - | - | 1.96 (0.95–4.08) | 0.071 |
| Proximal (vs. distal)∧ | 13.3 (7.64–23.3) | <0.0001 | 5.58 (2.80–11.1) | MSI, BRAF | 3.39 (1.57–7.34) | 0.002 |
| Tumor stage (vs. I) | tumor location, MSI, BRAF | |||||
| Stage II | 4.42 (2.44–8.00) | <0.0001 | 2.53 (1.06–6.08) | 1.83 (0.69–4.83) | 0.23 | |
| Stage III | 1.61 (0.83–3.12) | 0.16 | 1.91 (0.72–5.12) | 1.83 (0.63–5.28) | 0.26 | |
| Stage IV | 2.23 (1.06–4.71) | 0.04 | 2.28 (0.78–6.65) | 1.72 (0.53–5.62) | 0.37 | |
| Poor differentiation | 7.28 (4.50–11.8) | <0.0001 | 2.96 (1.42–6.19) | MSI, BRAF | 2.98 (1.15–7.69) | 0.024 |
| Mucinous feature (vs. 0%) | MSI, BRAF | |||||
| 1–49% | 2.31 (1.46–3.64) | 0.0003 | 1.72 (0.89–3.29) | 1.53 (0.71–3.32) | 0.28 | |
| ≥50% | 5.39 (3.37–8.63) | <0.0001 | 2.09 (1.03–4.24) | 2.06 (0.81–5.22) | 0.13 | |
| Signet ring cells, any | 3.82 (2.18–6.71) | <0.0001 | 0.68 (0.32–1.41) | mucin, differentiation | 0.98 (0.30–3.17) | 0.97 |
| MSI-high | 41.9 (25.5–68.9) | <0.0001 | 27.6 (16.2–47.1) | tumor location | 21.2 (9.79–45.8) | <0.0001 |
| BRAF mutation | 29.2 (18.0–47.6) | <0.0001 | 18.7 (11.0–31.7) | tumor location | 15.9 (6.60–38.4) | <0.0001 |
| KRAS mutation | 0.25 (0.15–0.41) | <0.0001 | 0.71 (0.36–1.40) | BRAF, MSI | 0.97 (0.44–2.10) | 0.93 |
| β-catenin activation* | 0.14 (0.07–0.28) | <0.0001 | 0.21 (0.10–0.44) | tumor location | 0.21 (0.070–0.62) | 0.005 |
| LINE-1 hypomethylation (30% decline) | 0.20 (0.10–0.39) | <0.0001 | 0.30 (0.13–0.69) | MSI | 0.27 (0.094–0.75) | 0.012 |
| p53 positivity | 0.34 (0.22–0.52) | <0.0001 | 0.66 (0.39–1.13) | MSI | 0.73 (0.35–1.51) | 0.39 |
Multivariate logistic regression model includes all of the above variables. Note that female, tumor stage, signet ring cells, mucinous feature, KRAS and p53 are not significantly related with CIMP-high after adjusting for other variables.
β-catenin activation is defined as β-catenin score ≥3. The β-catenin score is the sum of nuclear (0, 1+ or 2+), cytoplasmic (0, 1+ or 2+) and membrane (0 or 1+) scores as originally described by Jass et al.[35].
Proximal colon includes cecum to transverse colon, and distal colon includes splenic flexure to rectum.
CI, confidence interval; CIMP, CpG island methylator phenotype; LINE-1, long interspersed nucleotide element-1; MSI, microsatellite instability; OR, odds ratio.
When there was missing information on tumor stage (12%), LINE-1 (3.9% missing), MSI (3.2% missing), p53 (1.3% missing), KRAS (2.3% missing) or BRAF (4.7% missing), we assigned a separate (“missing”) indicator variable and included those cases in the multivariate analysis models. We confirmed that excluding cases with a missing variable did not significantly alter results (data not shown). There was no missing information in other variables.
An association of each variable with CIMP-high was also assessed in strata of important clinical or molecular features, including age (<65 year old vs. ≥65 year old), sex, tumor location (proximal vs. distal), MSI status, and BRAF status. For stratified analysis, each multivariate logistic regression model included a variable of interest that was stratified by a given stratifying variable (e.g., age) and adjusted for all of the remaining variables (SAS codes available upon request).
An interaction was assessed by including the cross product term of a given variable (e.g., MSI) and another variable of interest in a regression model, and the likelihood ratio test compared a model including the cross product term with that excluding it. In addition to interactions of any given variable with MSI, location, age, sex and BRAF, we examined all possible remaining two-way interactions, and found no significant interactions (data not shown).
All statistical analyses except for clustering analysis used SAS version 9.1 (SAS Institute, Cary, NC). All p values were two-sided, and statistical significance was defined as p≤0.05. Nonetheless, multiple hypotheses testing was considered when interpreting the data, especially in examining multiple two-way interactions.
Results
Evaluation of 16 methylation markers
We obtained 904 colorectal cancer specimens and quantified DNA methylation in the 16 loci [CACNA1G, IGF2, NEUROG1, RUNX3, SOCS1, CDKN2A (p16), CRABP1, MLH1, CHFR, HIC1, IGFBP3, MGMT, MINT1, MINT31, p14 (CDKN2A/ARF), and WRN] by real-time PCR (MethyLight [30]) assays. The first 5 loci (up to SOCS1) were selected as good predictors of CIMP (CpG island methylator phenotype) by screening of 195 CpG islands [15]. The use of the first 8 loci (up to MLH1) as a CIMP-high diagnostic panel has been previously validated [24].
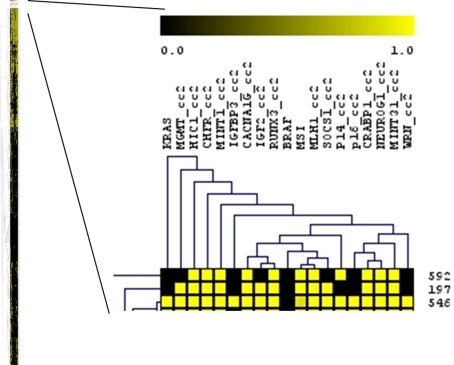
To evaluate 16 methylation markers in an unbiased fashion, we conducted an unsupervised hierarchical clustering analysis of the 16 methylation markers and status of MSI (microsatellite instability), and KRAS and BRAF oncogenes, using 860 tumors with all of these results available ( Figure 1 ). The 8 CIMP-high markers (CACNA1G, CDKN2A (p16), CRABP1, IGF2, MLH1, NEUROG1, RUNX3 and SOCS1) were generally clustered together, indicating good concordance of methylation patterns in these markers and supporting these 8 markers as good CIMP-high markers. In addition, p14, MINT31 and WRN were also clustered with the 8 markers. The other 5 methylation markers (MGMT, HIC1, CHFR, MINT1 and IGFBP3) were not clustered closely with each other or the 8 markers. The BRAF and MSI variables, which have been known to be associated with CIMP-high [15], [18], [24], were also clustered together with these 8 markers, indicating tight associations with CIMP-high. Notably, KRAS mutation was not clustered with any of the methylation markers, suggesting its association with a random methylation pattern (particularly in CIMP-low tumors which have been associated with KRAS mutation [29]; see also Supplemental Figure S1 ). We used clustering analysis only for the examination of marker clustering, but not for tumor classification. That was because clustering of markers was very stable with the large number of tumors (i.e., excluding a few tumors did not substantially influence results) while tumor classification by clustering analysis based on the 16 markers was not stable.
Figure 1. Hierarchical clustering analysis of 16 methylation makers, MSI, KRAS and BRAF in colorectal cancers.
Horizontal and vertical axes represent markers and cases, respectively. The expanded view of clustering tree for the markers is shown on the right. The 8 markers in our CIMP-high diagnostic panel (CACNA1G, IGF2, RUNX3, MLH1, SOCS1, CDKN2A (p16), CRABP1 and NEUROG1) are clustered closely, supporting that these markers are good CIMP-high markers. Also note the close relationship between MSI, BRAF and the 8 CIMP-high markers. KRAS mutation is not clustered with any of the methylation markers analyzed, suggesting that KRAS mutation (which is associated with CIMP-low [24], [29]; see Supplemental Figure) is probably associated with a random methylation pattern.
To describe performance of each of the 16 markers in an unbiased way, we calculated κ coefficient (for agreement statistics), sensitivity and specificity of each maker for CIMP-high diagnosis determined by the 16 markers (Supplemental Table S1 ). The cutoff for CIMP-high was set as ≥11/16 or ≥10/16 methylated markers based on the distribution of KRAS and BRAF mutations (Supplemental Figure), and on the previous findings that CIMP-high is associated with BRAF mutation and CIMP-low is associated with KRAS mutation [24], [29]. Sensitivity and specificity of each marker reflected overall concordance of a methylation pattern with the remaining 15 markers. It was evident that performance of the 8 CIMP-panel markers (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3 and SOCS1) was generally good. The κ coefficient was greater than 0.5 for all of these 8 markers. RUNX3 was the single best marker for CIMP-high diagnosis. Among the other 8 markers (CHFR, HIC1, IGFBP3, MGMT, MINT1, MINT31, p14 and WRN), only MINT31 and p14 consistently showed the κ coefficient greater than 0.5, and good sensitivity/specificity. This was in agreement with clustering analysis, which showed that MINT31 and p14 clustered with the 8 CIMP-panel markers.
We also compared the all-16-marker panel with the 8-marker CIMP panel. Using the 8-maker panel, or the 16-maker panel, CIMP-high was defined as ≥6/8 or ≥11/16 methylated markers, respectively. Among the 904 cases, 879 cases (97.2%) showed concordant diagnosis of CIMP status between the 16-marker panel and the 8-marker panel (κ = 0.89, p<0.0001). When the 16-marker CIMP panel was used, the associations of CIMP-high with clinical and molecular features were very similar to the CIMP-high associations by the 8-marker CIMP panel (data not shown). We also confirmed a high degree of agreement (98.6% concordant; κ = 0.94) between the 8-marker panel and the 5-marker panel described by Weisenberger et al. [15]. Thus, in subsequent analyses, we used the 8-marker CIMP panel which we had extensively validated [24].
CIMP-high in colorectal cancer
We assessed clinical, pathologic and molecular features of CIMP-high colorectal cancer ( Table 1 ). By univariate analysis, CIMP-high was associated with female sex, older age, proximal location, poor differentiation, mucin, signet ring cells, MSI-high and BRAF mutation, and inversely with stage I, KRAS mutation, LINE-1 hypomethylation, positive p53, and active β-catenin (all p<0.004).
Age was linearly associated with CIMP-high in logistic regression analysis (p for trend <0.0001). We did not show significant non-linearity by the likelihood ratio test that compared a model including a quadratic (or cubic) term with a model excluding it (p>0.85). Likewise, LINE-1 hypomethylation was inversely linearly associated with CIMP-high (p for trend <0.0001), and there was no significant non-linearity by the likelihood ratio test, using a quadratic (or cubic) term (p≥0.078). Non-parametric restricted cubic splines also supported a linear relation between age and CIMP-high ( Figure 2 ) and an inverse linear relation between LINE-1 hypomethylation and CIMP-high ( Figure 3 ).
Figure 2. Smoothing spline for the age/CIMP-high association.
Loge(OR for CIMP-high) (y axis) according to age (x axis) is shown (with young age as a referent). Broken lines indicate 95% confidence interval. Note the linear relation between age and CIMP-high. CIMP, CpG island methylator phenotype; OR, odds ratio.
Figure 3. Smoothing spline for the LINE-1/CIMP-high association.
Loge(OR for CIMP-high) (y axis) according to LINE-1 methylation (x axis) is shown (with high-level LINE-1 methylation as a referent). Broken lines indicate 95% confidence interval. Note the inverse linear relation between LINE-1 hypomethylation and CIMP-high. CIMP, CpG island methylator phenotype; LINE-1, long interspersed nucleotide element-1; OR, odds ratio.
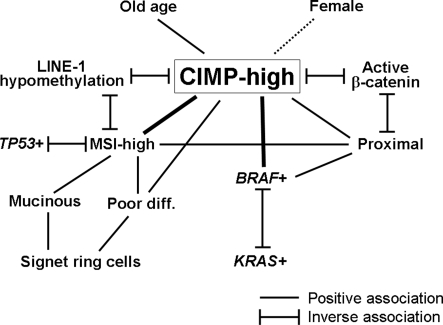
In multivariate logistic regression analysis, CIMP-high was significantly associated with older age, proximal location, poor differentiation, MSI-high and BRAF mutation, and inversely with active β-catenin and LINE-1 hypomethylation ( Table 2 ). However, all of the other features (female, stage, mucin, signet ring cells, KRAS and p53) were no longer significantly associated with CIMP-high in multivariate analysis. We further examined for potential confounders in the association of each variable with CIMP-high. Except for sex, all of the other variables showed substantial changes in odds ratio (OR) for the association with CIMP-high after adjusting for MSI, BRAF and/or tumor location (or other variables) ( Table 2 ). These results indicated the existence of complex relations between clinical and molecular features (including CIMP) in colorectal cancer, which are summarized in Figure 4 .
Figure 4. Summary of associations of CIMP-high with clinical and molecular features.
The broken line indicates the relatively weak association.
Associations with CIMP-high in strata of MSI
Molecular classification by MSI status is increasingly important in colorectal cancer [38]–[40]. Thus, we examined the relations of clinical and tumoral variables with CIMP-high in MSI-high tumors and non-MSI-high tumors ( Table 3 ). Older age, proximal location and BRAF mutation were significantly associated with CIMP-high in both MSI-high and non-MSI-high tumors. In contrast, the relations of CIMP-high with poor differentiation, KRAS mutation and LINE-1 hypomethylation appeared to be different according to MSI status (p for interaction <0.005). CIMP-high was associated with poor differentiation and inversely with KRAS mutation in MSI-high tumors, but not in non-MSI-high tumors. LINE-1 hypomethylation was inversely associated with CIMP-high in non-MSI-high tumors, but not in MSI-high tumors.
Table 3. Associations with CIMP-high in MSI-high and non-MSI-high colorectal cancers.
| Clinical, pathological, or molecular characteristics | P for interaction with MSI | Among non-MSI-high tumors | Among MSI-high tumors | ||
| Multivariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value | ||
| Age (10-year increment) | 0.033 | 2.12 (1.23–3.65) | 0.007 | 6.14 (2.49–15.1) | <0.0001 |
| Female (vs. male) | 0.46 | 1.41 (0.57–3.47) | 0.45 | 2.03 (0.69–6.06) | 0.20 |
| Proximal (vs. distal)∧ | 0.33 | 3.01 (1.22–7.40) | 0.016 | 7.17 (1.74–29.5) | 0.006 |
| Tumor stage II–IV (vs. I) | 0.064 | 0.97 (0.35–2.68) | 0.95 | 4.92 (1.18–20.5) | 0.029 |
| Poor differentiation | 0.002 | 0.84 (0.21–3.30) | 0.80 | 25.6 (3.43–191) | 0.002 |
| Mucinous feature, any | 0.29 | 2.02 (0.86–4.74) | 0.11 | 0.95 (0.30–2.99) | 0.93 |
| Signet ring cells, any | 0.22 | 1.65 (0.44–6.17) | 0.46 | 0.42 (0.065–2.71) | 0.36 |
| BRAF mutation | 0.21 | 11.7 (4.34–31.6) | <0.0001 | 49.8 (4.71–527) | 0.001 |
| KRAS mutation | 0.004 | 2.24 (0.81–6.17) | 0.12 | 0.17 (0.039–0.78) | 0.022 |
| p53 positivity | 0.65 | 0.72 (0.31–1.70) | 0.46 | 0.50 (0.12–2.00) | 0.33 |
| LINE-1 hypomethylation (30% decline) | 0.002 | 0.095 (0.026–0.34) | 0.0003 | 3.20 (0.47–22.0) | 0.24 |
| β-catenin activation* | 0.18 | 0.32 (0.097–1.04) | 0.058 | 0.063 (0.008–0.51) | 0.009 |
Each multivariate logistic regression model assesses a variable of interest (stratified by MSI status in a given model), adjusting for all of the above remaining variables. An interaction was assessed by the likelihood ratio test that compares a model including a cross product term (of the MSI variable and another variable of interest) with a model excluding it.
β-catenin activation is defined as β-catenin score ≥3, where the β-catenin score is the sum of nuclear (0, 1+ or 2+), cytoplasmic (0, 1+ or 2+) and membrane (0 or 1+) scores as originally described by Jass et al.[35].
Proximal colon includes cecum to transverse colon, and distal colon includes splenic flexure to rectum.
CI, confidence interval; CIMP, CpG island methylator phenotype; LINE-1, long interspersed nucleotide element-1; MSI, microsatellite instability; OR, odds ratio.
Associations with CIMP-high in strata of tumor location
There is accumulating evidence for a molecular difference between proximal and distal colorectal cancers [38], [41]. Therefore, we examined the relations of clinical and tumoral variables with CIMP-high in proximal tumors and distal tumors ( Table 4 ). The relations of CIMP-high with the variables did not appear to differ according to tumor location (all p for interaction >0.23).
Table 4. Associations with CIMP-high in proximal and distal colorectal cancers.
| Clinical, pathological, or molecular characteristics | P for interaction with location | Among proximal tumors∧ | Among distal tumors∧ | ||
| Multivariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value | ||
| Age (10-year increment) | 0.32 | 2.62 (1.58–4.34) | 0.0002 | 4.62 (1.61–13.2) | 0.004 |
| Female (vs. male) | 0.71 | 1.93 (0.87–4.30) | 0.11 | 1.21 (0.32–4.58) | 0.78 |
| Tumor stage II–IV (vs. I) | 0.31 | 2.48 (0.79–7.79) | 0.12 | 1.00 (0.24–4.15) | 0.99 |
| Poor differentiation | 0.46 | 2.54 (0.91–7.10) | 0.075 | 5.81 (0.89–37.8) | 0.066 |
| Mucinous feature, any | 0.24 | 1.25 (0.58–2.72) | 0.57 | 3.14 (0.79–12.5) | 0.10 |
| Signet ring cells, any | 0.56 | 0.86 (0.25–2.94) | 0.81 | 2.01 (0.18–22.6) | 0.57 |
| MSI-high | 0.33 | 31.0 (13.2–73.2) | <0.0001 | 14.3 (3.28–62.5) | 0.0004 |
| BRAF mutation | 0.56 | 13.7 (5.21–36.1) | <0.0001 | 22.8 (4.78–109) | <0.0001 |
| KRAS mutation | 0.39 | 1.20 (0.48–2.99) | 0.70 | 0.57 (0.13–2.44) | 0.44 |
| p53 positivity | 0.37 | 0.89 (0.39–2.06) | 0.79 | 0.37 (0.083–1.63) | 0.19 |
| LINE-1 hypomethylation (30% decline) | 0.26 | 0.19 (0.055–0.63) | 0.007 | 0.65 (0.098–4.24) | 0.65 |
| β-catenin activation* | -# | 0.35 (0.11–1.13) | 0.078 | 0# | -# |
Each multivariate logistic regression model assesses a variable of interest (stratified by tumor location in a given model), adjusting for all of the above remaining variables. An interaction was assessed by the likelihood ratio test that compares a model including a cross product term (of the location variable and another variable of interest) with a model excluding it.
β-catenin activation is defined as β-catenin score ≥3, where the β-catenin score is the sum of nuclear (0, 1+ or 2+), cytoplasmic (0, 1+ or 2+) and membrane (0 or 1+) scores as originally described by Jass et al.[35].
Proximal colon includes cecum to transverse colon, and distal colon includes splenic flexure to rectum.
Because there was no CIMP-high β-catenin-active tumors in distal colon, stable p for interaction and 95% CI for OR could not be estimated.
CI, confidence interval; CIMP, CpG island methylator phenotype; LINE-1, long interspersed nucleotide element-1; MSI, microsatellite instability; OR, odds ratio.
Associations with CIMP-high in other stratified analyses
We examined the relations of clinical and tumoral variables with CIMP-high in strata of sex, age (<65 year old vs. ≥65 year old) and BRAF status. Considering multiple hypotheses testing (12-hypotheses testing each), the effect of the variables did not appear to significantly differ according to age (all p for interaction >0.03) and sex (all p for interaction >0.02). Notably, the effect of LINE-1 hypomethylation did appear to differ according to BRAF status (p for interaction = 0.001) ( Table 5 ). A significant inverse association of LINE-1 hypomethylation with CIMP-high was present in BRAF-mutated tumors [adjusted OR = 0.022; 95% confidence interval (CI), 0.003–0.17], but not in BRAF-wild-type tumors (adjusted OR = 0.87; 95% CI, 0.25–3.06).
Table 5. Associations with CIMP-high in BRAF-wild-type and BRAF-mutated colorectal cancers.
| Clinical, pathological, or molecular characteristics | P for interaction with BRAF | Among BRAF-wild-type tumors | Among BRAF-mutated tumors | ||
| Multivariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value | ||
| Age (10-year increment) | 0.13 | 3.60 (2.02–6.43) | <0.0001 | 1.80 (0.81–4.02) | 0.15 |
| Female (vs. male) | 0.23 | 2.27 (1.01–5.09) | 0.047 | 0.70 (0.14–3.51) | 0.67 |
| Proximal (vs. distal)∧ | 0.56 | 3.87 (1.57–9.50) | 0.003 | 2.33 (0.52–10.4) | 0.27 |
| Tumor stage II–IV (vs. I) | 0.043 | 2.79 (0.92–8.45) | 0.69 | 0.37 (0.070–1.94) | 0.24 |
| Poor differentiation | 0.27 | 3.84 (1.27–11.6) | 0.017 | 1.43 (0.32–6.49) | 0.64 |
| Mucinous feature, any | 0.98 | 1.56 (0.69–3.56) | 0.29 | 1.54 (0.45–5.30) | 0.50 |
| Signet ring cells, any | 0.25 | 0.67 (0.15–3.09) | 0.61 | 2.55 (0.40–16.3) | 0.32 |
| MSI-high | 0.21 | 21.6 (9.56–48.6) | <0.0001 | 82.6 (8.48–804) | 0.0001 |
| KRAS mutation | 0.37 | 1.02 (0.44–2.35) | 0.96 | 0.18 (0.005–6.69) | 0.35 |
| p53 positivity | 0.52 | 0.55 (0.21–1.40) | 0.21 | 0.90 (0.26–3.06) | 0.86 |
| LINE-1 hypomethylation (30% decline) | 0.001 | 0.87 (0.25–3.06) | 0.83 | 0.022 (0.003–0.17) | 0.0003 |
| β-catenin activation* | 0.44 | 0.20 (0.052–0.76) | 0.018 | 0.49 (0.076–3.21) | 0.46 |
Each multivariate logistic regression model assesses a variable of interest (stratified by BRAF status in a given model), adjusting for all of the above remaining variables. An interaction was assessed by the likelihood ratio test that compares a model including a cross product term (of the BRAF variable and another variable of interest) with a model excluding it.
β-catenin activation is defined as β-catenin score ≥3, where the β-catenin score is the sum of nuclear (0, 1+ or 2+), cytoplasmic (0, 1+ or 2+) and membrane (0 or 1+) scores as originally described by Jass et al.[35].
Proximal colon includes cecum to transverse colon, and distal colon includes splenic flexure to rectum.
CI, confidence interval; CIMP, CpG island methylator phenotype; LINE-1, long interspersed nucleotide element-1; MSI, microsatellite instability; OR, odds ratio.
We also examined all of the remaining potential two-way interactions by the available clinical and tumoral variables, and found no significant interactions with regard to the associations with CIMP-high (data not shown).
Discussion
In this study utilizing a large sample size, we evaluated 16 methylation makers in an unbiased fashion. The 16 methylation markers included the 5 markers (CACNA1G, IGF2, NEUROG1, RUNX3 and SOCS1) that were selected by screening of 195 CpG islands [15] and further validated to be included in the CIMP-high diagnostic panel (the above 5 plus CDKN2A, CRABP1 and MLH1) [24]. By unsupervised hierarchical clustering analysis, the 5 methylation markers were clustered with each other as well as with MSI (microsatellite instability) and BRAF mutation. Analysis of κ coefficient, sensitivity and specificity also demonstrated good performance of the 5 methylation markers with generally concordant methylation pattern. Utilizing the validated CIMP panel, we have deciphered the complex relations of CIMP-high with various clinical, pathologic and molecular features in colorectal cancer. Our data provide a rationale for the of the validated CIMP-specific methylation marker panel.
This study is the first extensive investigation to compare the 5 new CIMP-high markers [15] with MINT1, MINT31 and other CpG islands, using a large sample size. Performance of the 5 new markers (CACNA1G, IGF2, NEUROG1, RUNX3, and SOCS1), CRABP1 and MLH1 was consistently superior to that of WRN, MINT1, CHFR, IGFBP3, HIC1 and MGMT. MINT31, CDKN2A, and p14 showed intermediate performance characteristics, and in hierarchical clustering analysis, were generally clustered with the new 5 CIMP markers, MSI and BRAF mutation. We have provided valuable data for standardization of methylation markers for the detection of CIMP-high in colorectal cancer.
Studying epigenetic and genetic aberrations is important in cancer research [42]–[46]. We used quantitative PCR assays (MethyLight [30]) to determine the degree of DNA methylation, which is robust enough to reproducibly differentiate low-level methylation from high-level methylation [31]. Our resource of a large colorectal cancer sample obtained from two large prospective cohorts (representing a relatively unbiased sample compared to a single-hospital-based sample) has provided a sufficient power to evaluate the 16 methylation markers, and to simultaneously assess independent relations of CIMP-high with multiple clinical and tumoral molecular variables.
Interestingly, unsupervised clustering analysis using a large number of tumors revealed that KRAS mutation was not clustered with any of the 16 methylation markers. However, as shown in our previous studies [24], [29], KRAS mutation was more common in CIMP-low tumors compared to CIMP-high and CIMP-0 tumors. Although these findings appeared to be discrepant, we believe that KRAS mutation is perhaps associated with a random pattern of CpG island methylation, indicated by the non-clustering phenomenon in clustering analysis. In contrast, our clustering analysis has clearly shown that BRAF mutation is clustered with CIMP-high specific markers, indicating that BRAF mutation is perhaps associated with a non-random pattern of CpG island methylation.
Previous studies identified various factors associated with CIMP-high, including old age, female, proximal location, poor differentiation, mucin, signet ring cells, MSI-high, BRAF mutation, wild-type KRAS, inactive β-catenin, wild-type APC, high LINE-1 methylation level, and wild-type TP53 [9]–[19], [21], [47], [48]. However, many of these factors are interrelated. Thus, in order to properly decipher the relations with CIMP-high, it is necessary to use a large number of tumors, determine a number of molecular features, and perform comprehensive biostatistical analysis. We were able to utilize a large colorectal cancer sample that has been examined for multiple molecular events, and appropriate biostatistical methods. Figure 3 summarizes our current knowledge on the associations of clinical, pathologic and molecular features including CIMP in colorectal cancer. It is very important to keep in mind these relations, when analyzing the association between any of these factors and an outcome (e.g., molecular changes in colorectal cancer, patient mortality, etc.). These factors may confound the relationship of interest. Indeed, we have demonstrated confounding effects of MSI, BRAF and tumor location in a number of the associations in Table 2 . In particular, signet ring cells, KRAS and p53 were no longer associated with CIMP-high after adjusting for the confounders.
We have shown that the relations of CIMP-high with tumor differentiation, KRAS mutation and LINE-1 hypomethylation appear to differ according to MSI status. MSI is a major molecular classifier in colorectal cancer [38]–[40]. MSI-high tumors have been shown to exhibit widespread mutations in nucleotide repeat sequences such as those in TGFBR2 and BAX [49], [50]. Thus, it is likely that overall genomic changes in MSI-high tumors are dissimilar to those of non-MSI-high tumors. That may explain why there are some pathologic and molecular features that are differentially associated with CIMP-high according to MSI status.
In summary, using the 16 methylation markers and a large population-based sample, we have evaluated performance of each of the 16 methylation markers in an unbiased fashion. Our current study provides valuable data for standardization of the use of CIMP-high-specific markers. Using the validated CIMP-specific methylation marker panel, we have comprehensively analyzed the clinical, pathologic and molecular features of CIMP-high colorectal cancer by comprehensive biostatistical methods. We have provided the rationale to use the validated CIMP-high-specific methylation marker panel in clinical and research settings. Further studies are necessary to elucidate fundamental molecular defects that lead to CIMP-high colorectal cancer.
Supporting Information
Distribution of colorectal cancers according to the number of methylated markers and KRAS/BRAF mutational status. Note that KRAS mutation is associated with CIMP-low (rather than CIMP-high and CIMP-negative), in agreement with studies using more limited CIMP-specific methylation markers [24], [29].
(0.12 MB TIF)
Markers are listed in the order of the kappa coefficient. * Sensitivity of each marker is defined as “[the number of CIMP-high cases positive for a given marker] / [the number of all CIMP-high cases]”. ∧ Specificity of each marker is defined as “[the number of non-CIMP-high cases negative for a given marker] / [the number of all non-CIMP-high cases]”.
(0.15 MB DOC)
Acknowledgments
We deeply thank the Nurses' Health Study and Health Professionals Follow-up Study cohort participants who have generously agreed to provide us with biological specimens and information through responses to questionnaires; hospitals and pathology departments throughout the U.S. for generously providing us with tumor tissue materials; Frank Speizer, Walter Willett, Graham Colditz, Susan Hankinson, and many other staff members who implemented and have maintained the cohort studies.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by U.S. National Institute of Health (NIH) grants P01 CA87969, P01 CA55075, P50 CA127003 and K07 CA122826 (to S.O.), and in part by grants from the Bennett Family Fund and from the Entertainment Industry Foundation (EIF) through the EIF National Colorectal Cancer Research Alliance (NCCRA). K.N. was supported by a fellowship grant from the Japanese Society for Promotion of Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCI or NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 3.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer – a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 4.Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004;4:988–993. doi: 10.1038/nrc1507. [DOI] [PubMed] [Google Scholar]
- 5.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grady WM. CIMP and colon cancer gets more complicated. Gut. 2007;56:1498–1500. doi: 10.1136/gut.2007.125732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teodoridis JM, Hardie C, Brown R. CpG island methylator phenotype (CIMP) in cancer: Causes and implications. Cancer Lett. 2008;268:177–186. doi: 10.1016/j.canlet.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Toyota M, Ohe-Toyota M, Ahuja N, Issa JP. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci U S A. 2000;97:710–715. doi: 10.1073/pnas.97.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rijnsoever M, Grieu F, Elsaleh H, Joseph D, Iacopetta B. Characterisation of colorectal cancers showing hypermethylation at multiple CpG islands. Gut. 2002;51:797–802. doi: 10.1136/gut.51.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehall VL, Wynter CV, Walsh MD, Simms LA, Purdie D, et al. Morphological and molecular heterogeneity within nonmicrosatellite instability-high colorectal cancer. Cancer Res. 2002;62:6011–6014. [PubMed] [Google Scholar]
- 11.Hawkins N, Norrie M, Cheong K, Mokany E, Ku SL, et al. CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology. 2002;122:1376–1387. doi: 10.1053/gast.2002.32997. [DOI] [PubMed] [Google Scholar]
- 12.Kambara T, Simms LA, Whitehall VLJ, Spring KJ, Wynter CVA, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagasaka T, Sasamoto H, Notohara K, Cullings HM, Takeda M, et al. Colorectal cancer with mutation in BRAF, KRAS, and wild-type with respect to both oncogenes showing different patterns of DNA methylation. J Clin Oncol. 2004;22:4584–4594. doi: 10.1200/JCO.2004.02.154. [DOI] [PubMed] [Google Scholar]
- 14.Iacopetta B, Grieu F, Li W, Ruszkiewicz A, Caruso M, et al. APC gene methylation is inversely correlated with features of the CpG island methylator phenotype in colorectal cancer. Int J Cancer. 2006;119:2272–2278. doi: 10.1002/ijc.22237. [DOI] [PubMed] [Google Scholar]
- 15.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 16.Goel A, Nagasaka T, Arnold CN, Inoue T, Hamilton C, et al. The CpG Island Methylator Phenotype and Chromosomal Instability Are Inversely Correlated in Sporadic Colorectal Cancer. Gastroenterology. 2007;132:127–138. doi: 10.1053/j.gastro.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Ogino S, Cantor M, Kawasaki T, Brahmandam M, Kirkner G, et al. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut. 2006;55:1000–1006. doi: 10.1136/gut.2005.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samowitz W, Albertsen H, Herrick J, Levin TR, Sweeney C, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki T, Nosho K, Ohnishi M, Suemoto Y, Kirkner GJ, et al. Correlation of beta-catenin localization with cyclooxygenase-2 expression and CpG island methylator phenotype (CIMP) in colorectal cancer. Neoplasia. 2007;9:569–577. doi: 10.1593/neo.07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen L, Toyota M, Kondo Y, Lin E, Zhang L, et al. Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci U S A. 2007;104:18654–18659. doi: 10.1073/pnas.0704652104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogino S, Kawasaki T, Nosho K, Ohnishi M, Suemoto Y, et al. LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG methylator phenotype in colorectal cancer. Int J Cancer. 2008;122:2767–2773. doi: 10.1002/ijc.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derks S, Postma C, Carvalho B, van den Bosch SM, Moerkerk PT, et al. Integrated analysis of chromosomal, microsatellite and epigenetic instability in colorectal cancer identifies specific associations between promoter methylation of pivotal tumour suppressor and DNA repair genes and specific chromosomal alterations. Carcinogenesis. 2008;29:434–439. doi: 10.1093/carcin/bgm270. [DOI] [PubMed] [Google Scholar]
- 23.Barault L, Charon-Barra C, Jooste V, de la Vega MF, Martin L, et al. Hypermethylator phenotype in sporadic colon cancer: study on a population-based series of 582 cases. Cancer Res. 2008;68:8541–8546. doi: 10.1158/0008-5472.CAN-08-1171. [DOI] [PubMed] [Google Scholar]
- 24.Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, et al. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9:305–314. doi: 10.2353/jmoldx.2007.060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 26.Chan AT, Ogino S, Fuchs CS. Aspirin and the Risk of Colorectal Cancer in Relation to the Expression of COX-2. New Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki T, Ohnishi M, Nosho K, Suemoto Y, Kirkner GJ, et al. CpG island methylator phenotype-low (CIMP-low) colorectal cancer shows not only few methylated CIMP-high-specific CpG islands, but also low-level methylation at individual loci. Mod Pathol. 2008;21:245–255. doi: 10.1038/modpathol.3800982. [DOI] [PubMed] [Google Scholar]
- 28.Ogino S, Kawasaki T, Brahmandam M, Yan L, Cantor M, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7:413–421. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8:582–588. doi: 10.2353/jmoldx.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Blake C, et al. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogino S, kawasaki T, Brahmandam M, Cantor M, Kirkner GJ, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–217. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, et al. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estecio MR, Gharibyan V, Shen L, Ibrahim AE, Doshi K, et al. LINE-1 hypomethylation in cancer is highly variable and inversely correlated with microsatellite instability. PLoS ONE. 2007;2:e399. doi: 10.1371/journal.pone.0000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogino S, kawasaki T, Kirkner GJ, Yamaji T, Loda M, et al. Loss of nuclear p27 (CDKN1B/KIP1) in colorectal cancer is correlated with microsatellite instability and CIMP. Mod Pathol. 2007;20:15–22. doi: 10.1038/modpathol.3800709. [DOI] [PubMed] [Google Scholar]
- 35.Jass JR, Biden KG, Cummings MC, Simms LA, Walsh M, et al. Characterisation of a subtype of colorectal cancer combining features of the suppressor and mild mutator pathways. J Clin Pathol. 1999;52:455–460. doi: 10.1136/jcp.52.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, et al. TM4 microarray software suite. Methods Enzymol. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- 37.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 38.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 39.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13–27. doi: 10.2353/jmoldx.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popat S, Hubner R, Houlston RS. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 41.Sugai T, Habano W, Jiao YF, Tsukahara M, Takeda Y, et al. Analysis of molecular alterations in left- and right-sided colorectal carcinomas reveals distinct pathways of carcinogenesis: proposal for new molecular profile of colorectal carcinomas. J Mol Diagn. 2006;8:193–201. doi: 10.2353/jmoldx.2006.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sengupta K, Upender MB, Barenboim-Stapleton L, Nguyen QT, Wincovitch SM, Sr, et al. Artificially introduced aneuploid chromosomes assume a conserved position in colon cancer cells. PLoS ONE. 2007;2:e199. doi: 10.1371/journal.pone.0000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abraham JM, Sato F, Cheng Y, Paun B, Kan T, et al. Novel decapeptides that bind avidly and deliver radioisotope to colon cancer cells. PLoS ONE. 2007;2:e964. doi: 10.1371/journal.pone.0000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung W, Kwabi-Addo B, Ittmann M, Jelinek J, Shen L, et al. Identification of novel tumor markers in prostate, colon and breast cancer by unbiased methylation profiling. PLoS ONE. 2008;3:e2079. doi: 10.1371/journal.pone.0002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muleris M, Chalastanis A, Meyer N, Lae M, Dutrillaux B, et al. Chromosomal instability in near-diploid colorectal cancer: a link between numbers and structure. PLoS ONE. 2008;3:e1632. doi: 10.1371/journal.pone.0001632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samowitz WS, Slattery ML, Sweeney C, Herrick J, Wolff RK, et al. APC mutations and other genetic and epigenetic changes in colon cancer. Mol Cancer Res. 2007;5:165–170. doi: 10.1158/1541-7786.MCR-06-0398. [DOI] [PubMed] [Google Scholar]
- 48.Suehiro Y, Wong CW, Chirieac LR, Kondo Y, Shen L, et al. Epigenetic-Genetic Interactions in the APC/WNT, RAS/RAF, and P53 Pathways in Colorectal Carcinoma. Clin Cancer Res. 2008;14:2560–2569. doi: 10.1158/1078-0432.CCR-07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 50.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of colorectal cancers according to the number of methylated markers and KRAS/BRAF mutational status. Note that KRAS mutation is associated with CIMP-low (rather than CIMP-high and CIMP-negative), in agreement with studies using more limited CIMP-specific methylation markers [24], [29].
(0.12 MB TIF)
Markers are listed in the order of the kappa coefficient. * Sensitivity of each marker is defined as “[the number of CIMP-high cases positive for a given marker] / [the number of all CIMP-high cases]”. ∧ Specificity of each marker is defined as “[the number of non-CIMP-high cases negative for a given marker] / [the number of all non-CIMP-high cases]”.
(0.15 MB DOC)