Abstract
Objective
To assess if the combination of cardiac troponin (cTn) and Ischemia Modified Albumin (IMA) can be used for early exclusion of acute myocardial infarction (AMI).
Methods
Prospective consecutive admissions to the emergency department (ED) with undifferentiated chest pain were assessed clinically and by electrocardiography. A total of 539 patients (335 men, 204 women; median age 51.9 years) considered at low risk of AMI had blood drawn on admission. If the first sample was less than 12 hours from onset of chest pain, a second sample was drawn two hours later, at least six hours from onset of chest pain. Creatine kinase MB isoenzyme (CKMB) mass was measured on the first sample and CKMB mass and cTnT on the second sample. An aliquot from the first available sample was frozen and subsequently analysed for IMA. If cTnT had not been measured on the original sample cTnI was measured (n = 189).
Results
Complete data were available for 538/539 patients. IMA or cTn was elevated in the admission sample of all patients with a final diagnosis of AMI (n = 37) with IMA alone elevated in 2/37, cTn alone in 19/37, and both in 16/37. In 173/501 patients in whom AMI was excluded both tests were negative. In the non‐AMI group 22 patients had elevation of both IMA and cTn in the initial sample, suggesting ischaemic disease.
Conclusion
Admission measurement of cardiac troponin plus IMA can be used for early classification of patients presenting to the ED to assist in patient triage.
Keywords: troponin, Ischaemia Modified Albumin, chest pain, myocardial infarction
The effective triage of patients presenting with undifferentiated chest pain to the emergency department (ED) remains a challenge for the clinician. In the UK, 6% of patients with prognostically significant myocardial damage are discharged,1 similar to the rate of 2–8% reported in studies from the USA,2 whereas 70% of hospital admissions are of low risk individuals.3 Similar figures are seen in other healthcare systems.
A range of approaches have been used to address this problem including computerised decision support risk algorithms such as the Goldman algorithm4 and Thrombolysis In Myocardial Ischaemia (TIMI) risk score.5 These can then be combined with rapid rule out protocols based on serial measurements of one or more cardiac biomarkers. Appropriate patient selection and a rapid rule out acute myocardial infarction (AMI) pathway have been successfully developed as part of chest pain evaluation units. These units are clinically and cost effective6,7 but require the patient to be retained for 6–12 hours for investigation, depending on the protocol used.
The development of myocardial ischaemia precedes myocyte necrosis, and biomarkers of myocardial ischaemia will show a rise before necrosis. They will have a clinical role for early differentiation of those patients who will subsequently become positive for biomarkers of cardiac myonecrosis from those in whom myocyte damage does not occur. When used in combination with appropriate patient selection and other investigations, a combined strategy including ischaemia markers could be used to improve initial patient risk stratification. We have previously evaluated the kinetics8 and clinical application of one of these potential markers, Ischemia Modified Albumin (IMA) in combination with cardiac troponin T (cTnT) and the electrocardiogram (ECG), in the acute coronary syndrome (ACS) population.9 We now report the results of a similar study assessing the value of the combination of IMA with conventional cardiac biomarkers for the assessment and diagnosis of patients with chest pain in a low risk ED cohort.
Methods
Patient selection
We aimed to assess if the combination of cTn and IMA can be used for early exclusion of AMI in a low prevalence chest pain population. The local ethics committee approved the study, which was conducted in accordance with the declaration of Helsinki. We conducted a prospective observational study of consecutive admissions to the ED with undifferentiated chest pain. Patients were initially assessed by clinical history and a 12‐lead ECG. They were then considered for the rapid rule out protocol. Exclusion criteria for the protocol were:
significant ECG changes (unless known to be old): ST segment elevation >1 mm or T wave inversion in two continuous leads; atrial fibrillation; tachydysrhythmia >120 beats/min; bradycardia <40 beats/min; second or third degree heart block or left bundle branch block
comorbidity requiring hospital admission
suspected or proved alternative source of chest pain requiring hospital admission
known coronary artery disease (CAD) with unstable angina
atypical clearly non‐cardiac chest pain (stabbing, pleuritic, positional, or reproduced by palpation) in a patient with no history of and few risk factors for CAD.
Rule out was carried out by admission either to the chest pain observation unit (CPOU) or to the medical admissions unit (MAU). The CPOU is open from 9:00 am to 9:00 pm Monday to Friday. It has been described in detail previously6 and took the majority of the admissions. All patients had a sample drawn on admission. If the most significant episode of chest pain was <12 hours from presentation, the sample was analysed for creatine kinase MB isoenzyme (CKMB). A second sample for CKMB and cTnT was then drawn at least two hours later and six hours from onset of pain. If the most significant episode of pain was >12 hours, the sample was analysed for CKMB and cTnT and no further samples were drawn. Following analysis, a serum aliquot from the first sample, taken according to the protocol, was frozen and subsequently analysed for IMA (Ischemia Technologies, Denver, Colorado, USA). If cTnT (Roche Diagnostics, Lewes, Sussex, UK) had not been measured on the original sample, cTnI (Beckman Coulter, High Wycombe, Buckinghamshire, UK) was also measured.
The patient was admitted when the CKMB exceeded 5 μg/l in any sample, the cTnT exceeded 0.1 μg/l in any sample, or difference in CKMB values between the first and second samples exceeded 1.6 μg/l. All patients who were ruled out proceeded to stress testing either directly in the CPOU or were subsequently referred from the MAU for investigation in the CPOU if appropriate. Patients with negative tests were discharged and followed up with a review visit and repeat blood draw for cTnT testing at 72 hours. Appropriate follow up was arranged for patients with positive or inconclusive tests, in whom stress testing was not possible, or for patients with known CAD.
Patients were followed up for survival, readmissions, and mortality using the patient administration system and by direct follow up. In all cases where there were readmissions and deaths we examined the hospital records, postmortem results if available, and death certificates. A follow up letter was sent to the patient's general practitioner at six months to confirm survival status if this was not known. Particular care was taken to determine if revascularisation procedures, coronary artery bypass graft, or percutaneous transluminal coronary angioplasty were performed during the six month follow up period. Readmissions were recorded for all patients and characterised on the basis of nature and relation to the primary presentation. Survival status and cause of death were established for all patients.
Final diagnostic categorisation was on the basis of the most senior medical opinion, taking into account the results of all available diagnostic tests. To allow comparison with current recommendations, we used the European Society of Cardiology/American College of Cardiology (ESC/ACC) criteria to diagnose AMI, with cTnT as the diagnostic cardiac biomarker (cut‐off of ⩾0.05 μg/l, the optimised 10% coefficient of variation (CV) cut‐off from a large multicentre study10) in the admission, 6 hour, or 72 hour samples as appropriate.
Analytical methods
Measurement of cTnT and CKMB was done using an Elecsys 2010 (Roche Diagnostics). Assay %CV for cTnT was 5.8–5.7 (0.47–11.5 μg/l), range 0.01–25.0 μg/l, functional sensitivity 0.03 μg/l and for CKMB 4.0–4.1 (5.89–60.5 μg/l), range 0.1–500 μg/l. Measurement of cTnI was by the Access AccuTnI assay (Beckman Coulter), %CV was 4.4–6.9% (1.34–30.55 μg/l), range 0.01–100 μg/l, functional sensitivity 0.03 μg/l. IMA was measured by the ACB assay (Ischemia Technologies, Inc, Denver, Colorado, USA) on the Cobas Mira plus (Roche Diagnostics). The %CV was 7.5–4.9 (72.5–140.2 kU/l), range 14–216 kU/l, 97.5th centile 85 kU/l (range 46–90 kU/l).
The ability of combination of IMA and cTn in the admission sample to predict a final diagnosis of AMI and to predict outcomes was examined and compared with that obtained for CKMB, the current standard of care. Diagnostic efficiency was compared by classification of patients as biomarker positive or negative using decision thresholds maximised for sensitivity. Troponin positive was defined using a decision threshold of ⩾0.03 μg/l (the 10% CV point for both assays, which are clinically equivalent). IMA positive was defined using a decision threshold of >85 kU/l (the 97.5th centile). CKMB positive was defined as greater or equal to 5 µg/l, as per the protocol described above. These results were then dichotomously tabulated against final diagnosis and against outcome.
We analysed the data using the Analyse‐It (www.analyse‐it.com) add in for Excel. Patient demographics were expressed non‐parametrically. Data were tabulated and individual diagnostic pairs compared in 2×2 table for calculation of sensitivity and specificity and for significance testing by Fisher's exact test. Tabulated data were compared by χ2. A p value of ⩽0.05 was considered significant by two tailed testing. Initial examination confirmed there was no differences if cTnT or cTnI results were separately analysed so the results were pooled for the final analysis.
Results
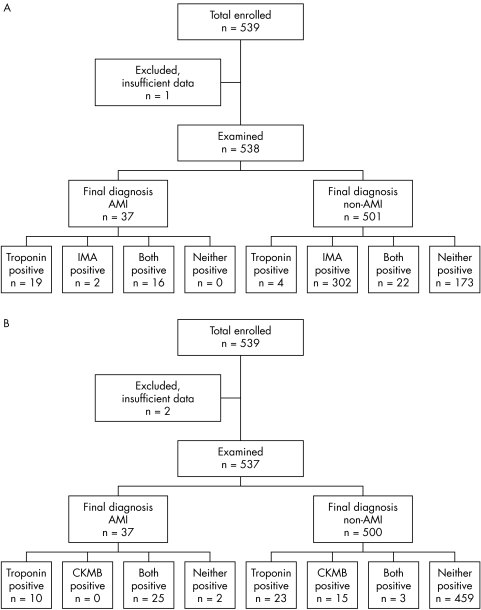
Our study included a total of 539 patients (335 men, 204 women, median age 51.9 years, range 26.4–94.4 years). Sufficient data were available for 538 patients (99%) for a final diagnosis to confirm or exclude AMI. The final diagnostic classification according to ESC/ACC criteria was 37 AMI (6.9%) and 501 non‐AMI (93.1%). Median duration from symptom onset to the first sample was six hours (range 1–24, interquartile range 3–12). The breakdown of patients according to IMA and cTn compared with final diagnosis is summarised in fig 1. IMA and cTn were both negative on admission in 173/501 (34.5%) patients with a final diagnosis of non‐AMI. IMA or cTn or both were positive in all cases of AMI. A total of 26 patients had an admission cTn in the range 0.03–0.05 μg/l of whom 6 had detectable values (⩾0.01 and <0.05 μg/l) at six hours and 25/26 had undetectable values at 72 hours. There was no correlation from onset of symptoms to degree of IMA or cTnT elevation.
Figure 1 Flow chart showing final diagnosis of patients with acute myocardial infarction (AMI) according to test results and final diagnosis for (A) Ischemia Modified Albumin (IMA) and troponin and (B) creatine kinase MB isoenzyme (CKMB) and troponin.
The sensitivity of the strategy cTn positive or IMA positive or both positive was 100% (90.5–100.0%) but with a specificity of 34.5% (30.4–38.7%). The rule out sensitivity (negative predictive value) of a negative IMA plus negative troponin was 100% (97.9–100.0%). This means that the test has a role for exclusion but not confirmation of a final diagnosis of AMI. The admission cTn had a diagnostic sensitivity of 94.6% (81.8–99.3%). Neither a positive IMA nor a positive admission cTn in the non‐AMI group was associated with a significantly greater chance of a positive stress ECG. In contrast, the strategy of CKMB plus cTn did not have as high sensitivity, missing two patients with AMI at a diagnostic sensitivity of 94.6% (81.8–91.3%) although the specificity was significantly higher at 91.8% (89.0–94.0%). The measurement of CKMB plus cTn did not improve the diagnostic sensitivity above that of measurement of cTn alone, 94.6% (81.8–91.3%). The measurement of cTn alone (35/37) was significantly more sensitive than the measurement of CKMB alone (25/37) for the final diagnosis of AMI (p = 0.006).
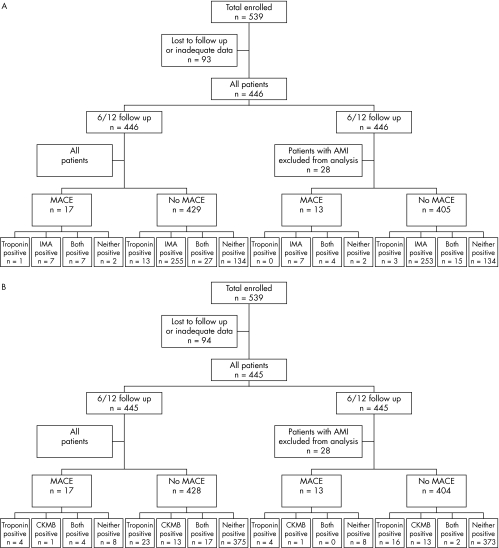
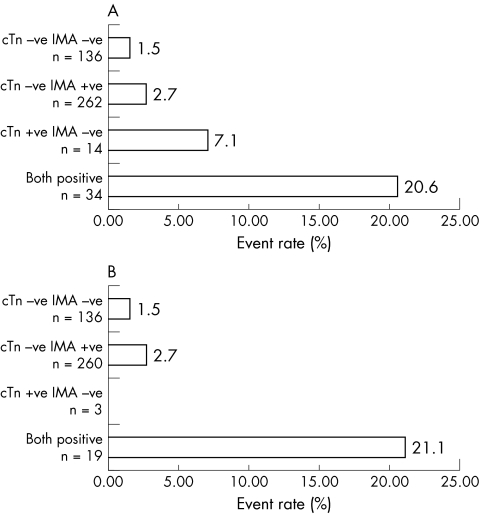
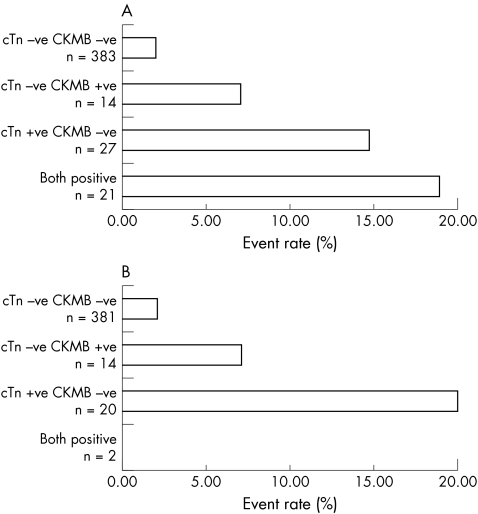
Outcome data were available for 446/539 patients (82.7%) (fig 2). There was no difference in demographics between the two groups. There were 17 follow up events, 6 cardiac deaths, 1 AMI, and 10 revascularisations. The event rate was 7/34 (20.6%) in those who were positive for both markers, 1/14 (7.1%) in those who were only cTn positive and 7/262 (2.7%) in those who were IMA positive alone. The event rate in those who were negative for both was 2/136 (1.5%). Only those who were positive for both markers had a significant increase in event rate compared with the negative group (relative risk (RR) 4.6, 95% CI 2.8 to 7.6, p = 0.0004). In those patients where a final diagnosis excluded AMI (n = 418) there were 13 events. In this group, only an elevated cTn plus an elevated IMA predicted an adverse event with a relative risk of 6.6 (95% CI 3.2 to 13.9, p = 0.004) (fig 3). Elevation of cTn without IMA elevation or IMA without cTn elevation was not associated A different situation was seen when CKMB was combined with cTn. The event rate was 4/21 (19.0%) in those who were positive for both CKMB and cTn, 4/27 (14.8%) in those who were only cTn positive, and 1/14 (7.1%) in those who were CKMB positive alone. The event rate in those who were negative for both markers was 8/383 (2.1%). Those who were positive for both markers or cTn alone had a significant increase in event rate compared with the negative group (cTn and CKMB positive: RR 7.7, 95% CI 3.0 to 19.4, p = 0.004; cTn positive CKMB negative RR 5.7 95% CI 2.4–19.1, p = 0.01). In those patients in whom a final diagnosis excluded AMI (n = 418) there were 13 events. In this group, only an elevated cTn predicted an adverse event with a relative risk of 8.1 (95% CI 3.2 to 20.6, p = 0.0035) (fig 4). Elevation of cTn with CKMB elevation or CKMB without cTn elevation was not associated with a significantly increased risk. The positive event rates for the combination cTn and IMA were compared with those for the combination of cTn plus CKMB and found to be significantly different for both the entire group (χ2 11.6, p = 0.009) and the subset excluding AMI (χ2 13.0, p = 0.0046).
Figure 2 Six month outcome of acute myocardial infarction (AMI) according to test results for (A) Ischemia Modified Albumin (IMA) and troponin (B) and creatine kinase MB isoenzyme (CKMB) and troponin. MACE, major adverse cardiac events.
Figure 3 Event rate according to biomarker classification for (A) the combination of troponin (cTn) and Ischaemia Modified Albumin (IMA) in all patients and (B) those in whom myocardial infarction was excluded.
Figure 4 Event rate according to biomarker classification for (A) the combination of troponin and CKMB in all patients and (B) those in whom myocardial infarction was excluded.
Discussion
In this study we found that the combination of cTn and IMA at presentation could be used to both confirm and exclude a final diagnosis of myocardial infarction. It is apparent from the data shown above that for the emergency medicine physician, the role of this test will be to exclude those likely to have AMI at presentation. A negative test allows exclusion but a positive IMA alone will need a follow up cTn measurement to confirm AMI. The diagnostic sensitivity of the strategy of either positive was 100% but the specificity was low at 34.5%. There may be a number of reasons for this. Firstly, IMA is a test for ischaemia not infarction. Myocardial ischaemia may occur without proceeding to infarction. Previous studies of the ability of IMA to predict a positive cTn have shown good performance with an area under the receiver operator characteristic (ROC) curve of 0.78 but did not show 100% concordance in patients admitted with chest pain.11 Secondly, the study used as diagnostic “gold standard” definite AMI with elevated cTn according to ESC/ACC criteria rather than myocardial ischaemia. There is currently no method of reliably detecting myocardial ischaemia. Previous studies of IMA have shown it is possible to distinguish reliably between the ACS and non‐ACS populations (area under the ROC curve 0.95) but there is overlap when attempting to distinguish between AMI and unstable angina (area under the ROC curve 0.66).12
The diagnostic sensitivity of the admission cTn measurement was high, probably due to the relatively long time from symptom onset to sampling (six hours), a deliberate characteristic of the way the chest pain assessment pathway is structured. This may also be due to the poor agreement between reported symptom onset and incidence of biomarker elevation which has led to recommendations that time of presentation is preferred to time of symptom onset as a reliable time point.13 Minor elevations of cTn (0.03–0.05) were seen in patients on first presentation, which did not reach the diagnostic threshold during the period of admission and were undetectable in 96% at 72 hours. These patients had a final diagnosis of non‐AMI. Minor elevations of cTn are known to indicate an adverse prognosis in both the high prevalence14 and low prevalence ACS populations.15 In addition, low level cTn elevations occur in a range of non‐ACS populations and can be a cause of confusion if not interpreted in the context of other clinical features.16 In this study, elevation of cTn in the non‐AMI group was only significant when accompanied by elevation of IMA where it was associated with an event rate of 21%. This was in a small number of patients overall but indicates that the combination of a sensitive troponin cut‐off plus IMA at admission may be superior to the conventional strategy. In the study that examined the clinical significance of low level positive cTn values the combination of cTn elevation plus other evidence of myocardial ischaemia in the form of ECG changes was found to be the only discriminant of higher risk.15 Measurement of CKMB offered little additional information when compared with measurement of cTn alone both diagnostically and prognostically. This is consistent with the original studies on troponin as an outcome predictor as well as studies on the new cTn assays.10,17,18,19
The clinical dilemma with the low risk chest pain population is to achieve reliable early diagnosis of those with have a final diagnosis of AMI and to identify those at high risk of subsequent cardiac events. The objective is efficient early exclusion of low risk individuals. The main value of the measuring the combination of IMA and cTnT on admission in a population with a non‐diagnostic ECG was to rule out a final diagnosis of AMI, with a sensitivity of 100%. This is in agreement with the claims of the manufacturer for the test. In addition to exclusion of AMI with high sensitivity, a negative IMA plus cTn was associated with a low event rate (1.5%). There was only one cardiac death in this group, which occurred in a patient with a previous history of congestive heart disease.
The major impact of a combination strategy based on rapid diagnosis on first presentation will be to improve initial risk stratification and clinical management decisions. Current strategy uses ECG and initial risk assessment to divide patients into high, medium, and low risk groups. The medium or low risk groups then require a further periods of observation with serial measurement of cardiac biomarkers. Patients who rule out for myocardial damage may then progress to non‐invasive assessment of myocardial perfusion. Methods include stress electrocardiography, stress echocardiography, or myocardial perfusion imaging according to the physical capability of the patient and the local availability of facilities. The objective is to produce a final categorisation into those at significant risk of future cardiac events who require invasive imaging and consideration for revascularisation procedures. A triple negative test (IMA <85 kU/l, cTn <0.03 μg/l, normal ECG) will identify a third of patients who can be assigned to a very low risk category in which retention in the CPOU for further serial marker testing is not required. This group can proceed immediately to non‐invasive assessment either in the CPOU or as an out‐patient procedure. In addition, it will raise the prior probability of cardiac disease in the remaining group. This has great potential for providing both clinical and cost effective care by allowing exclusion of a low risk category and immediate further investigation of a higher risk group.
The limitations of this study are the delay from first presentation to initial sampling, which will reduce the diagnostic utility of IMA measurement, and that it was not a prospective randomised study. However, the results have such potential impact that further studies should be performed.
Conclusion
In the present study a positive IMA and cTn on admission predicted a final diagnosis of AMI. When both were negative on admission, the final diagnosis excluded AMI and had a low probability of cardiac events.
Abbreviations
ACS - acute coronary syndrome
AMI - acute myocardial infarction
CAD - coronary artery disease
CKMB - creatine kinase MB isoenzyme
CPOU - chest pain observation unit
cTn - cardiac troponin
ECG - electrocardiogram
ED - emergency department
IMA - Ischemia Modified Albumin
MAU - medical admissions unit
Footnotes
Competing interest: Dr P O Collinson is a member of the Ischemia Technologies Scientific Advisory Board.
References
- 1.Collinson P O, Premachandram S, Hashemi K. Prospective audit of incidence of prognostically important myocardial damage in patients discharged from emergency department. BMJ 20003201702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta R H, Eagle K A. Missed diagnoses of acute coronary syndromes in the emergency room—continuing challenges. N Engl J Med 20003421207–1210. [DOI] [PubMed] [Google Scholar]
- 3.Collinson P O, Rao A C, Canepa‐Anson R.et al Impact of European Society of Cardiology/American College of Cardiology guidelines on diagnostic classification of patients with suspected acute coronary syndromes. Ann Clin Biochem 200340156–160. [DOI] [PubMed] [Google Scholar]
- 4.Nichol G, Walls R, Goldman L.et al A critical pathway for management of patients with acute chest pain who are at low risk for myocardial ischemia: recommendations and potential impact [see comments]. Ann Intern Med 1997127996–1005. [DOI] [PubMed] [Google Scholar]
- 5.Antman E M, Cohen M, Bernink P J.et al The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000284835–842. [DOI] [PubMed] [Google Scholar]
- 6.Goodacre S W, Morris F M, Campbell S.et al A prospective, observational study of a chest pain observation unit in a British hospital. Emerg Med J 200219117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodacre S, Nicholl J, Dixon S.et al Randomised controlled trial and economic evaluation of a chest pain observation unit compared with routine care. BMJ 2004328254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha M K, Gaze D C, Tippins J R.et al Ischemia modified albumin is a sensitive marker of myocardial ischemia after percutaneous coronary intervention. Circulation 20031072403–2405. [DOI] [PubMed] [Google Scholar]
- 9.Sinha M K, Roy D, Gaze D C.et al Role of “Ischemia Modified Albumin”, a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J 20042129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collinson P O, Stubbs P J, Kessler A C. Multicentre evaluation of the diagnostic value of cardiac troponin T, CK‐MB mass, and myoglobin for assessing patients with suspected acute coronary syndromes in routine clinical practice. Heart 200389280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christenson R H, Duh S H, Sanhai W R.et al Characteristics of an albumin cobalt binding test for assessment of acute coronary syndrome patients: a multicenter study. Clin Chem 200147464–470. [PubMed] [Google Scholar]
- 12.Bhagavan N V, Lai E M, Rios P A.et al Evaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem 200349581–585. [DOI] [PubMed] [Google Scholar]
- 13.Wu A H, Apple F S, Gibler W B.et al National Academy of Clinical Biochemistry Standards of Laboratory Practice: recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem 1999451104–1121. [PubMed] [Google Scholar]
- 14.Bertrand M E, Simoons M L, Fox K A.et al Management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J 2002231809–1840. [DOI] [PubMed] [Google Scholar]
- 15.Kontos M C, Shah R, Fritz L M.et al Implication of different cardiac troponin I levels for clinical outcomes and prognosis of acute chest pain patients. J Am Coll Cardiol 200443958–965. [DOI] [PubMed] [Google Scholar]
- 16.Collinson P O, Stubbs P J. Are troponins confusing? Heart 2003891285–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerhardt W, Katus H, Ravkilde J.et al S‐troponin T in suspected ischemic myocardial injury compared with mass and catalytic concentrations of S‐creatine kinase isoenzyme MB [see comments]. Clin Chem 1991371405–1411. [PubMed] [Google Scholar]
- 18.Hamm C W, Ravkilde J, Gerhardt W.et al The prognostic value of serum troponin T in unstable angina [see comments]. N Engl J Med 1992327146–150. [DOI] [PubMed] [Google Scholar]
- 19.Ravkilde J, Horder M, Gerhardt W.et al Diagnostic performance and prognostic value of serum troponin T in suspected acute myocardial infarction. Scand J Clin Lab Invest 199353677–685. [DOI] [PubMed] [Google Scholar]