Abstract
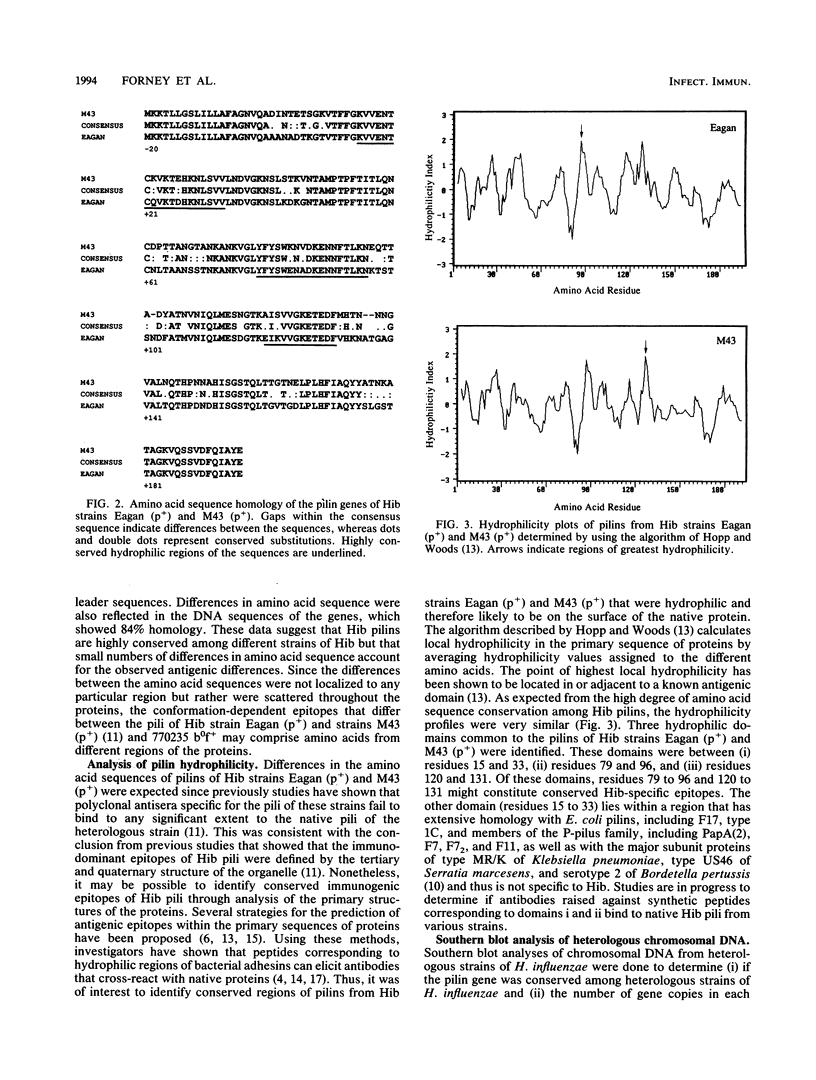
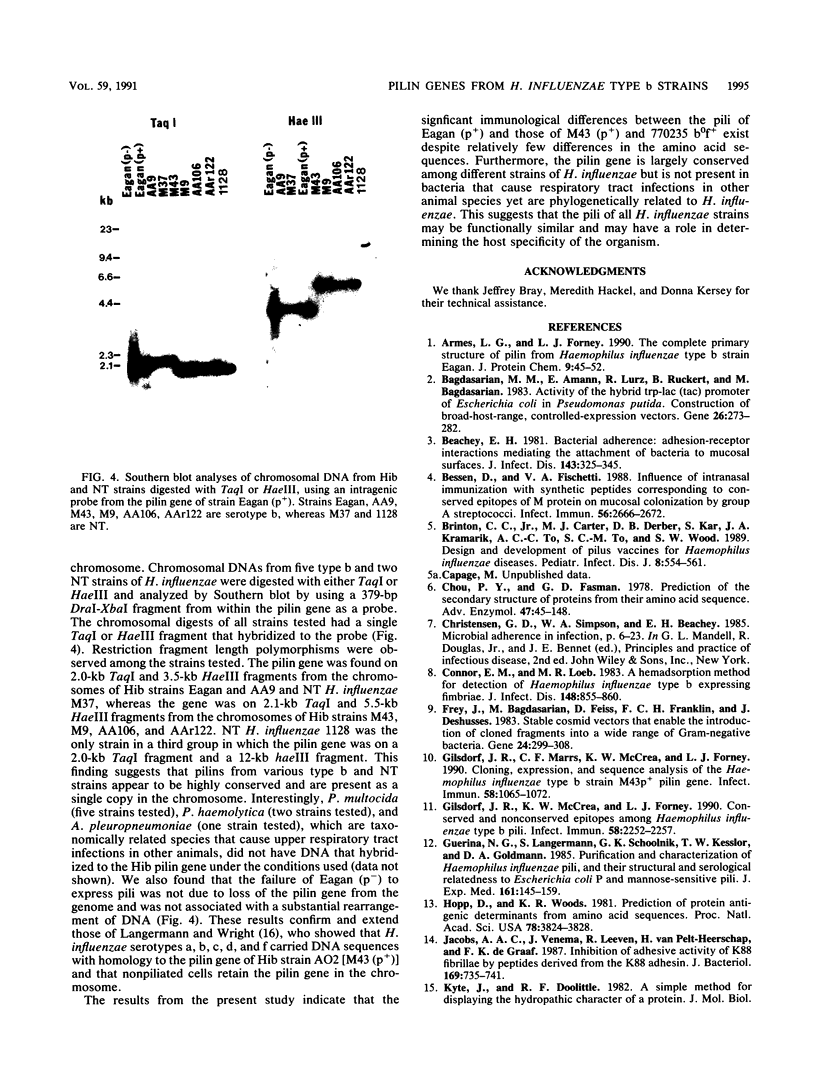
Previous studies have demonstrated antigenic differences among the pili expressed by various strains of Haemophilus influenzae type b (Hib). In order to understand the molecular basis for these differences, the structural gene for pilin was cloned from Hib strain Eagan (p+) and the nucleotide sequence was compared to those of strains M43 (p+) and 770235 b0f+, which had been previously determined. The pilin gene of Hib strain Eagan (p+) had a 648-bp open reading frame that encoded a 20-amino-acid leader sequence followed by the 196 amino acids found in mature pilin. The translated sequence was three amino acids larger than pilins of strains M43 (p+) and 770235 b0f+ and was 78% identical and 95% homologous when conservative amino acid substitutions were considered. Differences between the amino acid sequences were not localized to any one region but rather were distributed throughout the proteins. Comparison of protein hydrophilicity profiles showed several hydrophilic regions with sequences that were conserved between strain Eagan (p+) and pilins of other Hib strains, and these regions represent potentially conserved antigenic domains. Southern blot analyses using an intragenic probe from the pilin gene of strain Eagan (p+) showed that the pilin gene was conserved among all type b and nontypeable strains of H. influenzae examined, and only a single copy was present in these strains. Homologous genes were not present in the phylogenetically related species Pasteurella multocida, Pasteurella haemolytica, and Actinobacillus pleuropneumoniae. These data indicate that the pilin gene was highly conserved among different strains of H. influenzae and that small differences in the pilin amino acid sequences account for the observed antigenic differences of assembled pili from these strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armes L. G., Forney L. J. The complete primary structure of pilin from Haemophilus influenzae type b strain Eagan. J Protein Chem. 1990 Feb;9(1):45–52. doi: 10.1007/BF01024983. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M. M., Amann E., Lurz R., Rückert B., Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983 Dec;26(2-3):273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bessen D., Fischetti V. A. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988 Oct;56(10):2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Connor E. M., Loeb M. R. A hemadsorption method for detection of colonies of Haemophilus influenzae type b expressing fimbriae. J Infect Dis. 1983 Nov;148(5):855–860. doi: 10.1093/infdis/148.5.855. [DOI] [PubMed] [Google Scholar]
- Frey J., Bagdasarian M., Feiss D., Franklin F. C., Deshusses J. Stable cosmid vectors that enable the introduction of cloned fragments into a wide range of gram-negative bacteria. Gene. 1983 Oct;24(2-3):299–308. doi: 10.1016/0378-1119(83)90090-2. [DOI] [PubMed] [Google Scholar]
- Gilsdorf J. R., Marrs C. F., McCrea K. W., Forney L. J. Cloning, expression, and sequence analysis of the Haemophilus influenzae type b strain M43p+ pilin gene. Infect Immun. 1990 Apr;58(4):1065–1072. doi: 10.1128/iai.58.4.1065-1072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsdorf J. R., McCrea K., Forney L. Conserved and nonconserved epitopes among Haemophilus influenzae type b pili. Infect Immun. 1990 Jul;58(7):2252–2257. doi: 10.1128/iai.58.7.2252-2257.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Schoolnik G. K., Kessler T. W., Goldmann D. A. Purification and characterization of Haemophilus influenzae pili, and their structural and serological relatedness to Escherichia coli P and mannose-sensitive pili. J Exp Med. 1985 Jan 1;161(1):145–159. doi: 10.1084/jem.161.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., Venema J., Leeven R., van Pelt-Heerschap H., de Graaf F. K. Inhibition of adhesive activity of K88 fibrillae by peptides derived from the K88 adhesin. J Bacteriol. 1987 Feb;169(2):735–741. doi: 10.1128/jb.169.2.735-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langermann S., Wright A. Molecular analysis of the Haemophilus influenzae type b pilin gene. Mol Microbiol. 1990 Feb;4(2):221–230. doi: 10.1111/j.1365-2958.1990.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Lee K. K., Paranchych W., Hodges R. S. Cross-reactive and strain-specific antipeptide antibodies to Pseudomonas aeruginosa PAK and PAO pili. Infect Immun. 1990 Sep;58(9):2727–2732. doi: 10.1128/iai.58.9.2727-2732.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiPuma J. J., Gilsdorf J. R. Structural and serological relatedness of Haemophilus influenzae type b pili. Infect Immun. 1988 May;56(5):1051–1056. doi: 10.1128/iai.56.5.1051-1056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Connor E., Penney D. A comparison of the adherence of fimbriated and nonfimbriated Haemophilus influenzae type b to human adenoids in organ culture. Infect Immun. 1988 Feb;56(2):484–489. doi: 10.1128/iai.56.2.484-489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. Practical aspects of preparing phage and plasmid DNA: growth, maintenance, and storage of bacteria and bacteriophage. Methods Enzymol. 1987;152:145–170. doi: 10.1016/0076-6879(87)52016-x. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Smith A. L., Averill D. R., Smith D. H. Haemophilus influenzae meningitis in infant rats after intranasal inoculation. J Infect Dis. 1974 Feb;129(2):154–162. doi: 10.1093/infdis/129.2.154. [DOI] [PubMed] [Google Scholar]
- Pichichero M. E., Loeb M., Anderson, Smith D. H. Do pili play a role in pathogenicity of Haemophilus influenzae type B? Lancet. 1982 Oct 30;2(8305):960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., Mendelman P. M., Haas J. E., Schoenborn M. A., Mack K. D., Smith A. L. Characterization of Haemophilus influenzae type b fimbriae. Infect Immun. 1984 Dec;46(3):787–796. doi: 10.1128/iai.46.3.787-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- van Alphen L., van den Berghe N., Geelen-van den Broek L. Interaction of Haemophilus influenzae with human erythrocytes and oropharyngeal epithelial cells is mediated by a common fimbrial epitope. Infect Immun. 1988 Jul;56(7):1800–1806. doi: 10.1128/iai.56.7.1800-1806.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham S. M., Mooi F. R., Sindhunata M. G., Maris W. R., van Alphen L. Cloning and expression in Escherichia coli of Haemophilus influenzae fimbrial genes establishes adherence to oropharyngeal epithelial cells. EMBO J. 1989 Nov;8(11):3535–3540. doi: 10.1002/j.1460-2075.1989.tb08519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]