Abstract
Objectives
Evidence on the effect of crystalloid and colloid resuscitation fluids on coagulation is confusing, with contradictory results from previous studies. This study was performed to test the effect on whole blood coagulation of a range of resuscitation fluids in vitro using a single method at a single dilution.
Methods
Seven resuscitation fluids were tested in vitro at a dilution of 40%. Whole blood coagulation was measured using a Sonoclot analyser.
Results
A crystalloid/colloid split of effect on coagulation in vitro was not seen. The time to clot formation with Gelofusine, dextran and hydroxyethyl starch was a greatly increased, whereas saline and Haemaccel had little effect, or were slightly procoagulant.
Conclusions
Some resuscitation fluids have a profound effect on coagulation. The confusion in the literature may result from the effect on coagulation being both fluid and dilution dependent, with no simple crystalloid/colloid split.
Keywords: fluid therapy, resuscitation, whole blood coagulation
Resuscitation fluids are commonly used to treat patients with profuse haemorrhage, and are often given in large volumes. Many different types of resuscitation fluid are available, and there is ongoing controversy about the relative merits of a colloid or a crystalloid resuscitation fluid.1,2,3 Coagulopathy often becomes a problem following large volume fluid resuscitation for profuse haemorrhage. The origin of this coagulopathy is multifactorial,4 and it is usually assumed that resuscitation fluids contribute by cooling the patient and diluting clotting factors. However, there may also be a direct effect due to the interaction of the molecules within the resuscitation fluid with the coagulation system. This is less well known, as data on the direct effects of crystalloid and colloid resuscitation fluids on coagulation are limited and contradictory.
The literature in this area is heterogeneous. Different comparisons between fluids have been used, at varying dilutions. Several measures of coagulation have been employed, which adds to the difficulty in the interpretation of results. Crystalloids have usually been reported to enhance coagulation5,6,7,8 or have no effect,9 whereas colloids have been variously reported to impair coagulation,8,9,10,11,12,13,14,15 to give a weak impairment of coagulation,16,17 to have no effect,18,19,20,21 or to enhance coagulation.22,23 Most of the previous studies have compared only two fluids, and no previous study has used a single method to examine the whole range of commonly used resuscitation fluids. The confusion in the literature has meant that effects on coagulation are rarely considered either in the colloid versus crystalloid debate or in the choice by the emergency physician (EP) of a specific resuscitation fluid for an individual patient.
Using whole blood coagulation analysis we have already found that 0.9% saline has a procoagulant effect at lower dilutions and an anticoagulant effect on higher dilutions, and that Gelofusine has a marked anticoagulant effect.24 Across the world there is considerable variation in the resuscitation fluids used by EPs. If there is a direct effect of a resuscitation fluid on coagulation, this might influence the choice of fluid given in emergency care. This study set out to investigate the in vitro effects on coagulation of a range of resuscitation fluids used worldwide.
METHODS
Approval was obtained from the local research ethics committee. The study was carried out in a teaching hospital with a particular interest in trauma management. Advertising posters and personal contacts obtained the volunteers for the study. All volunteers gave their informed consent.
Coagulation was evaluated using a coagulation and platelet function analyser25 (Sonoclot; Scienco Inc., USA), a viscoelastic test of whole blood coagulation closely related to thromboelastography (TEG).26,27 The Sonoclot has been well validated28 and measures the change in impedance created by the developing blood clot as a small probe is vibrated at an ultrasonic frequency in a coagulating blood sample29 at 37°C. The parameters measured were: (a) the activated clotting time (ACT), which is time to first fibrin formation, and represents the initiation phase of clot formation, being similar to the conventional coagulation screen; (b) the clot rate (CR), which is the initial rate of increase in clot weight and is a measure of the rate of fibrin formation; and (c) the time to peak (TTP), which is the overall time taken for the clot to form and achieve maximum clot weight. TTP was used as the primary outcome variable, with sample sizes calculated assuming a minimum clinically significant change of 300 seconds (derived from normal values30,31 and the use of the Sonoclot in haemodialysis32), with a β value of 0.8 and an α value of 0.05.
Blood was taken from a free flowing indwelling cannula throughout, the first 5 ml withdrawn being discarded. The standard Sonoclot technique using cellite activation cuvettes was used, at a temperature of 37°C.33 Three whole blood samples were obtained from 12 human volunteers, the three samples being taken 20 minutes apart. Each sample was divided into three aliquots, and aliquot was diluted to 40% by addition of a resuscitation fluid, one aliquot being undiluted as a control. A Sonoclot profile was immediately obtained, with three Sonoclot analysers being run simultaneously. The order of the different fluids was rotated with each new volunteer to prevent machine bias. The fluids studied were saline 0.9%, Hartmann's solution, Gelofusine, Haemaccel, dextran 40, hydroxyethyl starch (200/0.5), and albumin 4.5%.
The 40% dilution corresponds to that created by resuscitation of a patient with grade IV haemorrhagic shock, and from previous work this level of dilution was likely to reveal differences between the resuscitation solutions.24 The haematocrit of each solution was measured to check that the desired dilution had been achieved, and the pH and platelet numbers were measured.
For each sample the ACT, CR, and TTP were measured by the Sonoclot analyser, and changes from control values were calculated for each resuscitation solution.
RESULTS
The distribution of the differences between test and control values for each resuscitation solution was assessed, and as all were normally distributed, the data were presented as mean and 95% confidence intervals. The percentage changes for each coagulation parameter are shown in table 1.
Table 1 Effects of resuscitation fluids on coagulation parameters expressed as percentage change from control.
| Parameter | Saline | Hartmann's | Gelofusine | Haemaccel | Dextran | HES | Albumin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTP | −6% | +62% | +77% | −19% | +127% | +114% | +41% | |||||||
| CR | −5% | −24% | −43% | −13% | −83% | −63% | −47% | |||||||
| ACT | +12% | −3% | −1% | −3% | +36% | +19% | +2% |
HES, hydroxyethyl starch; TTP, time to peak; CR, clot rate; ACT, activated clotting time.
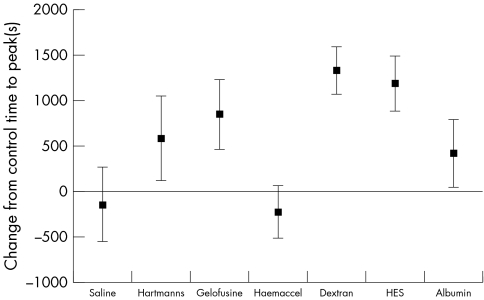
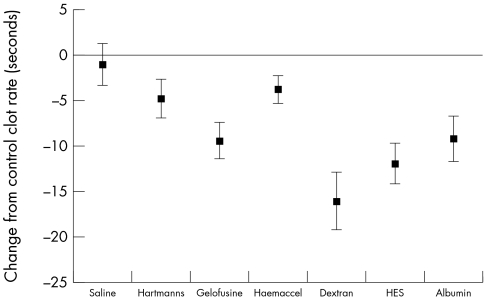
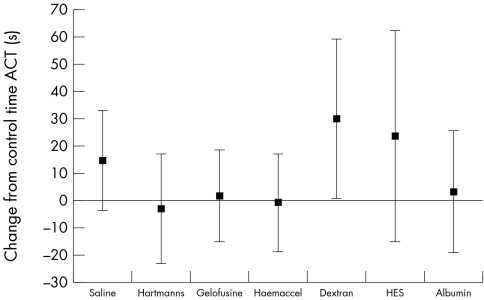
The various resuscitation solutions had different effects on the primary outcome measure of TTP (fig 1). There were also differences in clot rate (fig 2) but in contrast there were no differences (fig 3) in the ACT (analogous to the conventional "coagulation screen").
Figure 1 Change in time to peak clot strength between control and each resuscitation fluid (mean and 95% CI).
Figure 2 Change in clot rate (rate of fibrin formation) between control and each resuscitation fluid (mean and 95% CI).
Figure 3 Change in activated clotting time (analagous to activated partial thromboplastin time) for each resuscitation fluid (mean and 95% CI).
There was no difference in the pH of the mixtures compared with control blood, and the mean dilution achieved, as measured by the haematocrit, was confirmed to be 38.5%. There was also a 39% reduction in the number of platelets in each mixture, as expected with a 40% dilution.
DISCUSSION
These results may point to why there is confusion in the literature about the relative effects of colloid and crystalloid resuscitation fluids on coagulation. Previous work has often assumed that all crystolloids and all colloids have a similar effect. Comparison of varying crystalloid/colloid pairs has given a hetrogenicity that make the systematic reviews in this area difficult to interpret.1,34,35,36,37,38,39 The present study may resolve the apparently contradictory results of previous studies, as it can now be seen that there is not a crystalloid/colloid split in the effects of resuscitation fluids on coagulation. Solutions that might have been expected to give similar results (such as Gelofusine and Haemaccel) have very different effects on coagulation. We have previously shown that the effect of a particular solution also varies depending on the degree of haemodilution.22 Given the current findings it is not surprising that the previous literature gave apparently contradictory results, as previous studies have compared a variety of different solutions at a variety of different dilutions.
Saline 0.9% is known to be procoagulant,6 which may be due to a greater reduction in activity of anticoagulant factors (such as antithrombin III)40 than procoagulant factors,41 with little effect on platelets.42 Many previous studies have used saline 0.9% as the control solution, which may give rise to additional confusion in the interpretation of results, as the "control" is in fact having its own effect on coagulation.
From our results, commonly used colloid solutions (Gelofusine in the UK and Europe, albumin in the USA and Australia) give significant impairment of coagulation. This might be a disadvantage in patients with hypovolaemia due to uncontrolled bleeding. In a recent meta‐analysis of survival following colloid/crystalloid resuscitation, survival seemed to be worse following the colloid resuscitation.39 This effect is particularly seen in the subgroup of injured patients,34,43 and could be coagulation related.
Our findings give some indication of the part of the coagulation system that is being influenced by resuscitation fluids. The ACT is a measure of the speed of initiation of fibrin formation in the "plasma" phase of coagulation, and seems to be little affected by resuscitation fluids. The rate of fibrin formation in the evolving clot (measured by CR) is somewhat affected; however the different resuscitation fluids seemed to give greatest change in the TTP. This measure has previously been related to platelet function, fibrinogen levels, and the interaction between platelets and thrombin,44 and represents the "cellular" phase of coagulation.
The cause of the difference between the effect of Haemaccel and Gelofusine on coagulation is uncertain. The most obvious difference between these fluids is the calcium concentration,45 but this seems to account for only a small effect.46 The molecules are similar, but Gelofusine has a much higher negative charge. As highly negatively charged phospholipids in the platelet wall play a pivotal role in platelet activation and in the interaction of platelets with the plasma coagulation cascades, this could be the site of the interference with coagulation. Both Gelofusine and Haemaccel are known to impair ristocetin induced platelet aggregation, implying an interference with the Von Willebrand/glycoprotein Ib interaction. Gelofusine had little effect on platelet aggregation induced by other pathways, whereas Haemaccel also showed significant inhibition of platelet aggregation through the glycoprotein IIb/IIIa receptor. A previous research letter has reported inhibition of ristocetin induced platelet aggregation by Haemaccel,47 and in patients undergoing elective orthopaedic surgery, platelet aggregation seems to be inhibited by both Haemaccel and Gelofusine.48
We do not yet know if the effects found are important in patients. It was particularly interesting that there was little change in the Sonoclot ACT (which measures the same aspects of coagulation as the conventional coagulation screen, the international normalised ratio and activated partial thromboplastin time), but there was a large change in TTP (which is not measured in the conventional coagulation screen). This raises the possibility that clinicians are missing an effect of resuscitation fluids on coagulation, as the tests that are commonly available in clinical practice do not measure the part of the coagulation process that is affected by the resuscitation fluid. This could lead to the false impression that coagulation is unaffected by fluid therapy.
The commonly used tests of coagulation stop at a relatively early stage of clot formation (the end of the initiation phase, corresponding to the creation of the first fibrin strands). Many clinicians resuscitating the bleeding patient will be unaware that the coagulation screen only assesses about 4% of total thrombin generation,49,50 and that the conventional coagulation screen does not evaluate the important cellular part of the coagulation process.28
The later cellular or propagation phase is the time that many pharmacological agents or blood abnormalities have their most dramatic effect,49 so it is not surprising that the TTP, which analyses the whole coagulation process, reveals the greatest differences between resuscitation fluids. Changing to a whole blood functional analysis (such as TEG or Sonoclot) might allow clinicians to appreciate these abnormalities. A realisation of the limitations of the conventional coagulation screen in acute care has previously led to this strategy being suggested in surgical haemorrhage28,51,52,53 and for procedures under heparinisation, such as haemodialysis.32
The implication of these results for clinical management is unclear, as the potential for adverse effects in patients has not yet been investigated. The optimum timing, type, and volume of resuscitation fluid has not yet been determined,54 and clinical studies are needed to establish whether the type of resuscitation fluid can lead to increased bleeding. However, the magnitude of the change in the whole blood coagulation profile with a 40% dilution of blood with Gelofusine is similar to that seen with heparinisation for haemodialysis.32 In a patient with grade 4 shock, this order of dilution would be created by the infusion of about 1500 ml of Gelofusine. It would be unthinkable to heparinise a bleeding hypovolaemic patient, yet such patients are routinely given a volume of a fluid that produces an equivalent in vitro effect on coagulation. Until in vivo evidence is obtained, we would suggest that when large volume fluid resuscitation is required for bleeding, clinicians should avoid prescribing the fluids that give an in vitro impairment of coagulation.
ACKNOWLEDGEMENTS
This work was supported by the Anthony Hopkins Memorial Fund and Barts and the London Charitable Foundation. The Sonoclot Analyser was loaned by Scienco Inc.
Abbreviations
ACT - activated clotting time
CR - clot rate
ED - emergency department
EP - emergency physician
TEG - thromboelastography
TTP - time to peak
Footnotes
Competing interestst: there are no competing interests.
References
- 1.Alderson P, Schierhout G, Roberts I.et al Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev 2002(2)CD000567. [DOI] [PubMed]
- 2.Moore F, McKinley B, Moore E. The next generation in shock resuscitation. Lancet 20043631988–1996. [DOI] [PubMed] [Google Scholar]
- 3.Boldt J. Fluid choice for resuscitation of the trauma patient: a review of the physiological, pharmacological, and clinical evidence. Can J Anaesth 200451500–513. [DOI] [PubMed] [Google Scholar]
- 4.Martinowitz U, Kenet G, Segal E.et al recombinant activated Factor VII for adjunctive haemorrhage control in trauma. J Trauma 200151431–438. [DOI] [PubMed] [Google Scholar]
- 5.Tocantins L M. The clot accelerating effect of dilution on blood and plasma. Relation to the mechanism of coagulation of normal and haemophiliac blood. Blood 19516720–739. [PubMed] [Google Scholar]
- 6.Ruttmann T G, James M F, Viljoen J F. Haemodilution induces a hypercoagulable state. Br J Anaesth 199676412–414. [DOI] [PubMed] [Google Scholar]
- 7.Jamnicki M, Zollinger A, Seifert B.et al The effect of potato starch derived and corn starch derived hydroxyethyl starch on in vitro blood coagulation. Anaesthesia 199853638–644. [DOI] [PubMed] [Google Scholar]
- 8.Roche A M, James M F M, Grocott M P W.et al Coagulation effects of in vitro haemodilution with a balanced electrolyte hetastarch solution and lactated Ringer's solution. Anaesthesia 200257950–955. [DOI] [PubMed] [Google Scholar]
- 9.Konrad C, Markl T, Schuepfer G.et al In vitro effects of different medium molecular hydroxyethyl starch solutions and lactated Ringer's solution on coagulation using SONOCLOT. Anesth Analg 200090274–284. [DOI] [PubMed] [Google Scholar]
- 10.Fries D, Innerhofer P, Klingler A.et al The effect of the combined administration of colloids and lactated Ringer's solution on the coagualtion system: An in vitro study using thromboelastography coagulation analysis (ROTEG). Anesth Analg 2002941280–1287. [DOI] [PubMed] [Google Scholar]
- 11.Mardel S, Saunders F, Ollerenshaw L.et al Reduced quality of in‐vitro clot formation with gelatin‐based plasma substitutes. Lancet 1996347825. [DOI] [PubMed] [Google Scholar]
- 12.Mardel S, Saunders F, Allen H.et al Reduced quality of clot formation with gelatin‐based plasma substitutes. Br J Anaesth 199880204–207. [DOI] [PubMed] [Google Scholar]
- 13.Egli G A, Zollinger A, Seifert B.et al Effect of progressive haemodilution with hydroxyethyl starch, gelatin and albumin on blood coagulation. Br J Anaesth 199778684–689. [DOI] [PubMed] [Google Scholar]
- 14.de Jonge E, Levi M, Berends F.et al Impaired haemostasis by intravenous administration of a gelatin‐based plasma expander in human subjects. Thromb Haemost 199879286–290. [PubMed] [Google Scholar]
- 15.Cogbill T H, Moore E E, Dunn E L.et al Coagulation changes after albumin resuscitation. Crit Care Med 1981922–26. [DOI] [PubMed] [Google Scholar]
- 16.Petroianu G A, Liu J, Malek W H.et al The effect of in vitro hemodilution with gelatin, dextran, hydroxyethyl starch, or Ringer's solution on thromboelastograph. Anesth Analg 200090795–800. [DOI] [PubMed] [Google Scholar]
- 17.Konrad C, Markl T, Schuepfer G.et al The effects of in vitro hemodilution with Gelatin, hydroxyethyl starch, and lactated Ringers solution on markers of coagulation: An analysis using SONOCLOT. Anesth Analg 199988483–488. [DOI] [PubMed] [Google Scholar]
- 18.Mortier E, Ongenae M, De Baerdemaeker L.et al In vitro evaluation of the effect of profound haemodilution with hydroxyethyl starch 6%, modified fluid gelatin 4% and dextran 40 10% on coagulation profile measured by thromboelastography. Anaesthesia 1997521061–1064. [DOI] [PubMed] [Google Scholar]
- 19.Tigchelaar R C G, Huet G, Korsten J.et al Hemostatic effects of three colloid plasma substitutes for priming solution in cardiopulmonary bypass. Eur J Cardiothorac Surg 199711626–632. [DOI] [PubMed] [Google Scholar]
- 20.London M J, Ho J S, Triedman J K.et al A randomized clinical trial of 10% pentastarch (low molecular weight hydroxyethyl starch) versus 5% albumin for plasma volume expansion afetr cardiac operations. J Thorac Cardiovasc Surg 198997785–797. [PubMed] [Google Scholar]
- 21.Nagy K K, Davis J, Duda J.et al A comparison of Pentastarch and Lactated Ringer's Solution in the resuscitation of patients with hemorrhagic shock. Circ Shock 199340289–294. [PubMed] [Google Scholar]
- 22.Gorton H, Lyons G, Manraj P. Preparation for regional anaesthesia induces changes in thromboelastography. Br J Anaesth 200084403–404. [DOI] [PubMed] [Google Scholar]
- 23.Karoutsos S, Nathan N, Lahrimi A.et al Thrombelastogram reveals hypercoagulability after administration of gelatin solution. Br J Anaesth 199982175–177. [DOI] [PubMed] [Google Scholar]
- 24.Brazil E V, Coats T J. Sonoclot coagulation analysis of in‐vitro haemodilution with resuscitation solutions. J R Soc Med 200093507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hett D A, Walker D, Pilkington S N.et al Sonoclot analysis. Br J Anaesth 199575771–776. [DOI] [PubMed] [Google Scholar]
- 26.Mallett S V, Cox D J A. Thromboelastography. Br J Anaesth 199269307–313. [DOI] [PubMed] [Google Scholar]
- 27.Shih R, Cherng Y, Chao A.et al Prediction of bleeding diathesis in patients undergoing cardiopulmonary bypass during cardiac surgery: viscoelastic measures versus routine coagulation test. Acta Anaesthesiol Sin 199735133–139. [PubMed] [Google Scholar]
- 28.Tuman K.et al Comparison of viscoelastic measures of coagulation after cardio‐pulmonary bypass. Anesth Analg 19896969–75. [PubMed] [Google Scholar]
- 29.Liszka‐Hackzell J J, Ekback G. Analysis of the information content in Sonoclot data and reconstruction of coagulation test variables. J Med Syst 2002261–8. [DOI] [PubMed] [Google Scholar]
- 30.Pivalizza E G, Pivalizza P J, Kee S.et al Sonoclot analysis in healthy children. Anesth Analg 200192904–906. [DOI] [PubMed] [Google Scholar]
- 31.Horlocker T, Schroeder D. Effect of age, gender, and platelet count on Sonoclot coagulation analysis in patients undergoing orthopaedic operations. Mayo Clin Proc 199772214–219. [DOI] [PubMed] [Google Scholar]
- 32.Furuhashi M, Ura N, Hasegawa K.et al Sonoclot coagulation analysis: new bedside monitoring for determination of the appropriate heparin dose during haemodialysis. Nephrol Dial Transplant 2002171457–1462. [DOI] [PubMed] [Google Scholar]
- 33.Scienco Operator's manual. Sonoclol coagulation platelet function analvzer with graphics printer. http://www.sienco.com/documents/313manualchapter1.pdf . Accessed 9th April 2003
- 34.Velanovitch V. Crystalloid versus colloid fluid resuscitation: a meta‐analysis of mortality. Surgery 198910565–71. [PubMed] [Google Scholar]
- 35.Bisonni R, Holtgrave D, Lawler F.et al Colloids versus crystalloids in fluid resuscitation: an analysis of randomised controlled trials. J Fam Pract 199132387–390. [PubMed] [Google Scholar]
- 36.Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ 1998316961–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkes M, Navickis R. Patient survival after human albumin administration. A meta‐analysis of randomised, controlled trials. Ann Intern Med 2001135149–164. [DOI] [PubMed] [Google Scholar]
- 38.Wade C, Kramer G, Grady J.et al Efficacy of hypertonic 7. 5% saline and 6% dextran‐70 in treating trauma: A meta‐analysis of controlled clinical studies, Surgery 1997122609–616. [DOI] [PubMed] [Google Scholar]
- 39.Bunn F, Alderson P, Hawkins V. Colloid solutions for fluid resuscitation. Cochrane Database Syst Rev 2003(2)CD001319. [DOI] [PubMed]
- 40.Ruttmann T G, James M F M, Aronson I. In vivo investigation into the effects of haemodilution with hydroxyethyl starch (200/0.5) and normal saline on coagulation. Br J Anaesth 199880612–616. [DOI] [PubMed] [Google Scholar]
- 41.Fletcher J E, Heard C M B. Possible mechanism to explain increased coagulability of blood after haemodilution. Br J Anaesth 199778478. [DOI] [PubMed] [Google Scholar]
- 42.Ruttmann T G, James M F M. Pro‐coagulant effect of in vitro haemodilution is not inhibited by asprin. Br J Anaesth 199983330–332. [DOI] [PubMed] [Google Scholar]
- 43.Choi P, Yip G, Quinonez L.et al Crystalloids vs colloids in fluid resuscitation: A systematic review. Crit Care Med 199927200–210. [DOI] [PubMed] [Google Scholar]
- 44.Miyashita T, Kuro M. Evaluation of platelet function by sonoclot analysis compared with other hemostatic variables in cardiac surgery. Anesth Analg 1998871228–1233. [DOI] [PubMed] [Google Scholar]
- 45.Evans P A, Glenn J R, Heptinstall S.et al Effects of gelatin‐based resuscitation fluids on platelet aggregation. Br J Anaesth 199881198–202. [DOI] [PubMed] [Google Scholar]
- 46.Coats T J, Heron M. Does calcium cause the different effects of Gelofusine and Haemaccel on coagulation? Emerg Med J 200623193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stibbe J, Kirby E. Inhibition of ristocetin‐induced platelet aggregation by Haemaccel. BMJ 19752750–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans P A, Heptinstall S, Crowhurst E C.et al Prospective double‐blind randomized study of the effects of four intravenous fluids on platelet function and hemostasis in elective hip surgery. J Thromb Haemost 200312140–2148. [DOI] [PubMed] [Google Scholar]
- 49.Mann K G, Butenas S, Brumme l K. The dynamics of thrombin formation. Arterioscler Tromb Vasc Biol 20032317–25. [DOI] [PubMed] [Google Scholar]
- 50.Brummel K E, Paradis S G, Butenas S.et al Thrombin functions during tissue factor‐induced blood coagulation. Blood 2002100148–153. [DOI] [PubMed] [Google Scholar]
- 51.Ekback G, Schott U, Axelsson K.et al Perioperative autotransfusioon and functional coagulation analysis in total hip replacement. Acta Anaesthesiol Scand 199539390–395. [DOI] [PubMed] [Google Scholar]
- 52.Chapin J, Becker G, Hurlbert J. Comparison of thromboelastograph and Sonoclot coagulation analyser for assessing coagulation status during orthotopic liver transplantation. Transplant Proc 19892135–39. [PubMed] [Google Scholar]
- 53.Nuttall G A, Oliver W C, Ereth M H.et al Coagulation tests predict bleeding after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 199711815–823. [DOI] [PubMed] [Google Scholar]
- 54.Kwan I, Bunn F, Roberts I, on behalf of the WHO Pre‐Hospital Trauma Care Steering Committee Timing and volume of fluid administration for patients with bleeding following trauma. Cochrane Database Syst Rev 2003(3)CD002245. [DOI] [PubMed]