Abstract
The aim of monitoring patients is to detect organ dysfunction and guide the restoration and maintenance of tissue oxygen delivery. Monitoring is a crucial part of the care of the critically ill patient in the emergency department as the physiological response to critical illness is linked strongly to outcome. As it is important to appreciate the limitations of monitoring systems and monitored data, and to understand that invasive monitoring may be hazardous, this review concentrates on the techniques used to monitor critically ill patients in the emergency department. End tidal carbon dioxide monitoring, pulse oximetry, arterial blood pressure monitoring, central venous pressure monitoring, continuous central venous oxygenation saturation monitoring, temperature monitoring, and urine output are discussed. Practitioners should be familiar with the physiology and technology underlying these monitoring techniques and be aware of the pitfalls in interpretation of monitored data.
Keywords: monitoring, emergency medicine, critical illness
A 65 year old man is brought into the resuscitation room with a clinical diagnosis of pneumonia. On examination, he is maintaining his airway but has a respiratory rate of 40/min and is cyanosed. Clinical examination reveals atrial fibrillation with a pulse of 140 bpm and blood pressure of 80/40 mm Hg. He is warm peripherally but with a capillary refill time of 5 s. He opens his eyes to speech, obeys commands, and is confused. A tympanic temperature reads 34°C.
The patient receives high flow oxygen through a face mask with a reservoir bag. A large bore cannula is inserted and colloid is given rapidly. The patient deteriorates despite these measures and has a cardiac arrest. He is connected to the ECG monitor, which shows pulseless electrical activity. Chest compressions are started, and fluid and adrenaline (epinephrine) are given intravenously. He is intubated with a size 8 tracheal tube. Clinical assessment and chest radiograph exclude a pneumothorax.
Importance of monitoring the critically ill patient
The verb monitor originates from the Latin word “monere”, which means “to remind, advise, or warn”. The physiological response to critical illness is linked strongly to outcome. The ultimate aim of monitoring in the critically ill is to assist in the prevention or treatment of organ dysfunction and cellular injury by optimising the supply of oxygen to the tissues. Oxygen delivery is the product of cardiac output and blood oxygen content; thus, several commonly monitored variables contribute to the monitoring of oxygen delivery. Cardiac output is the product of stroke volume and heart rate (easily measured and monitored), whilst blood oxygen content is related to haemoglobin content and oxygen saturation, which are both easily measured and monitored. Early goal‐directed therapy applied in the emergency department to critically ill patients reduces mortality. This strategy is dependent on specialised monitoring in order to improve oxygen delivery to the tissues. Organ dysfunction may be monitored by several methods depending on the organ, for example, urine output as a monitor of renal organ function
Monitoring of the critically ill patient enables quantification of physiological reserve, and indicates the effectiveness of interventions. Trends in monitoring may help to indicate the best location for the patient on transfer from the emergency department. It is important that emergency physicians understand the principles of the monitoring so that it can be interpreted correctly, while recognising its limitations. Monitoring is only an adjunct to the careful observation of clinical signs in the critically ill patient. The following discussion is restricted to monitoring that is likely to be used in the emergency department
End tidal carbon dioxide monitoring
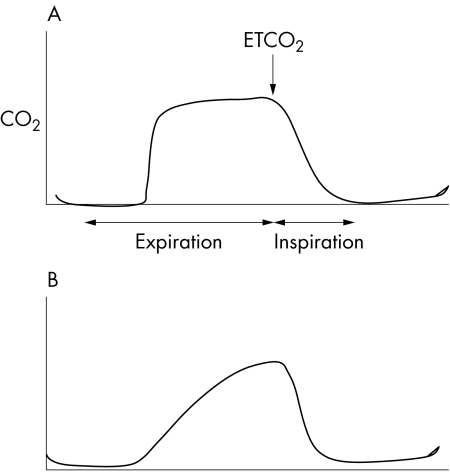
Measurement of end tidal carbon dioxide (ETCO2) is used to confirm placement of the tracheal tube in a major airway. In the presence of a cardiac output, failure to detect carbon dioxide indicates that the tube is in the oesophagus.1 Continuous carbon dioxide monitoring also provides other useful information. Capnometry is defined as the measurement of expired carbon dioxide; this is achieved most simply by attaching a single‐use colourimetric detector to the tracheal tube. A pH‐sensitive indicator strip enables semi‐quantitative detection of exhaled carbon dioxide. The absence of carbon dioxide is indicated by a purple colour, while a yellow colour indicates a CO2 concentration of more than 2%, indicating successful tube placement in the upper airways.
More comprehensive information is provided by capnography, in which the ETCO2 waveform is displayed continuously. Electronic capnometers use infrared light absorption to measure the concentration of CO2 in the exhaled gas which relates inversely to quantity of infrared light transmitted. The monitor may be placed directly in line with the patient's breathing system or a constant sample of gas can be diverted to the capnometer (this is known as a sidestream analyser). Sidestream devices are lighter and more flexible, but have a slower response time of 1–2 s, and some gas mixing occurs so that the measured values of CO2 may be slightly less than in mainstream monitors.
Changes in the shape of the graph (fig 1) enable correlation with clinical conditions such as airflow obstruction and changes in dead space ventilation following a pulmonary embolus. For example, in patients with expiratory obstruction of the small airways, such as asthma, late emptying of poorly ventilated alveoli causes an upward‐sloping plateau phase during expiration, and partial obstruction of a mainstem bronchus may be indicated by a “stepladder” appearance at the beginning of expiration. ETCO2 is measured at the end of the expiratory phase (after dead space volume has been exhaled) and approximates the alveolar CO2 concentration. The ETCO2 provides a very indirect indication of PaCO2 (arterial carbon dioxide partial pressure), but this is unreliable because of variation in the alveolar‐arterial CO2 gradient. Arterial blood gas analysis will enable the ETCO2 to be calibrated against the PaCO2; the capnometer can then be used as an indirect, continuous monitor of PaCO2 and therefore ventilation. Decreases in cardiac output due to hypovolaemia or cardiac dysfunction reduce pulmonary perfusion and increase the alveolar dead space, which reduces the ETCO2 independently of any change in ventilation. Thus, the capnometer is not an absolutely reliable monitor of ventilation and requires frequent calibration against arterial blood gases. Nonetheless, the capnometer is used commonly to monitor the adequacy of ventilation, for example during the transfer of head injured patients.
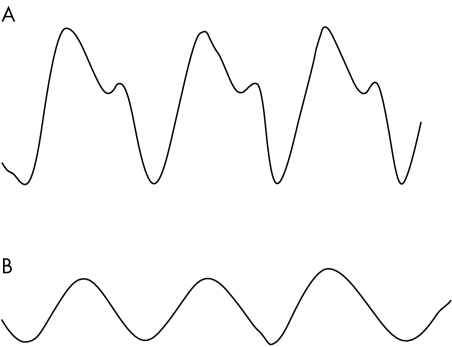
Figure 1 (A) Normal arterial pressure waveform and (B) over‐damped arterial pressure waveform, due to partially blocked arterial cannula or air bubbles in circuit.
False‐positive capnometry after oesophageal intubation has been described in a few circumstances, including following mouth‐to‐mouth resuscitation, ineffective bag‐valve‐mask ventilation (causing gastric distension), and after the ingestion of carbonated beverages. Carbon dioxide is eliminated within 10–15 s of applying ventilation; hence, at least six ventilations should be undertaken before using a colourimetric capnometer. Infrared capnometers are more accurate and reliable than colourimetric capnometers, especially in shocked patients.2 During cardiac arrest there is no delivery of carbon dioxide to the lungs and in 25% of cases carbon dioxide will not be detected despite correct placement of the tracheal tube.3
Pulse oximetry
Pulse oximetry is the continuous non‐invasive measurement of arterial haemoglobin oxygen saturation using a finger or ear probe. The measurement is based on the Beer‐Lambert law: optical absorbency is proportional to the thickness of the medium and the concentration of the substance being measured. Both red and infrared light are transmitted through the tissue with pulsatile blood flow. Oxygenated and deoxygenated haemoglobin differ in their capacity to absorb red (600–750 nm wavelengths) and infrared light (850–1000 nm wavelengths): oxygenated haemoglobin absorbs more infrared light than deoxygenated haemoglobin which, in turn, absorbs more red light than oxygenated haemoglobin. Comparison of absorbances at two different wavelengths (for example, 660 and 940 nm) enables estimation of the relative concentrations of oxygenated and deoxygenated haemoglobin. The oxygen saturation is measured by oximetry at the peak of blood pulsation (SpO2); the non‐pulsatile background absorption is eliminated enabling accurate estimates of arterial saturation (SaO2) to be made. Pulse oximeters will generally measure SpO2 with reasonable accuracy and precision if the SaO2 is above 75%.4
Pulse oximetry is reliable down to haemoglobin concentrations of 5 g/dl,5 but very low cardiac output affects the readings significantly. Pulse oximeters cannot distinguish between carboxyhaemoglobin or methaemoglobin and oxyhaemoglobin because their absorption spectra are similar; in the presence of these dyshaemoglobinaemias the SpO2 overestimates the oxygen saturation.6 Occasionally, dark fingernail polish or very dark skin will cause the oximeter to under‐read by up to 5%. The relationship between SaO2 and PaO2 (arterial oxygen partial pressure) is described by the haemoglobin dissociation curve, but the curve is relatively flat above an SaO2 of 90%. Therefore, hypoxaemia may develop in patients with an initial high PaO2 with relatively little change at first in the SpO2.7
The ECG monitored heart rate should match that of the pulse oximeter; a difference in the readings implies that the pulse oximeter is not detecting arterial pulsation or that an artefact is contaminating the signal. Cardiac arrhythmias do not usually affect the accuracy of pulse oximetry but the use of vasoactive drugs may lead to under‐reading of the SpO2.8 Dependent venous pooling or valvular insufficiency may cause venous pulsation resulting in erroneous readings; this also occurs during cardiac arrest.
Arterial blood pressure monitoring
Arterial blood pressure can be measured directly, accurately, and continuously by inserting a cannula in the radial, brachial, femoral, or dorsalis paedis artery and connecting it to a calibrated transducer, which converts pressure into an electrical signal (fig 2). The arterial cannula also enables repeated blood sampling. A pressurised flushing circuit maintains a low flow of saline to keep the cannula patent. The system is calibrated against zero by opening the transducer to air.
Figure 2 (A) Normal capnograph pattern from an intubated patient and (B) capnograph pattern from a patient with bronchcospasm or obstructed endotracheal tube.
Arterial blood pressure is an unreliable marker of cardiac output because the latter is influenced significantly by systemic vascular resistance (SVR). Critically ill patients have a very variable SVR, ranging from very low in septic shock to very high in cardiogenic or hypovolaemic shock. Arterial pressure is also affected by changes in intravascular volume and cardiac output. A significant decrease in mean arterial pressure (MAP) in the critically ill patient often signifies inadequate blood flow to vital tissue. Autoregulatory mechanisms in the vasculature of the brain and kidney may fail because of this impaired oxygen delivery and the perfusion of these organs is then a direct function of the blood pressure. MAP is the main determinant of blood flow to tissues but is not simply the average of the systolic and diastolic pressure. Electronic arterial pressure monitors derive MAP from the area under the pressure waveform and the duration of the cardiac cycle.
Insertion of arterial catheters can cause several complications including thrombosis, embolism, haematoma, pseudoaneurysm, ischaemic necrosis, and infection. Despite these potential complications, invasive direct blood pressure monitoring is a very valuable tool and is essential for monitoring when inotropic and vasopressor drugs are being used and when anaesthesia is induced in a critically ill patient.
Central venous pressure monitoring
Central venous pressure (CVP) is a potentially useful but often misinterpreted tool for assessing the intravascular volume status of the critically ill patient. Although the CVP is useful for guiding fluid therapy in the critically ill, single CVP measurements correlate poorly with intravascular volume. Central venous catheters are inserted usually into the internal jugular or subclavian vein. Recent National Institute of Clinical Excellence (NICE) guidelines recommend the use of ultrasound to guide insertion.9 The Seldinger technique is used for insertion and the CVP measured using a pressure transducer. Normal CVP is very variable and depends not only on intravascular volume, but also on patient position, venous tone, intrathoracic pressure, and cardiac valvular disease. Depending on the circumstances, a value anywhere between 6 and 20 mm Hg could be compatible with normovolaemia.
The ventricular preload is determined by the transmural vascular pressure (intravascular minus extravascular pressure), but the CVP transducer measures only intravascular pressure. Changes in the capnograph waveform occur in significant clinical conditions, such as airflow obstruction or increased dead space ventilation (as seen with pulmonary embolism). During spontaneous breathing, the intrathoracic pressure is usually close to zero at the end of expiration and therefore the CVP should be measured at the end of expiration. The measured CVP may fluctuate spontaneously by a few mm Hg without any change in the clinical condition of the patient. The adequacy of intravascular volume is determined by watching the response of the CVP after a fluid bolus of 250–500 ml: no change or a small temporary increase in CVP implies hypovolaemia, a sustained small rise implies normovolaemia, and a significant and rapid rise may indicate hypervolaemia.
There will be a discrepancy between the CVP and left side heart filling pressures in patients with chronic obstructive pulmonary disease, pulmonary hypertension, or mitral valve disease. Complications of insertion include pneumothorax, haemothorax, and arterial puncture, as well as later infective complications.
Continuous central venous oxygenation saturation monitoring
Measurement of pulmonary artery mixed venous oxygen saturation (SvO2) has been advocated as a useful index of tissue oxygenation and it has been demonstrated that patients with a wide variety of critical conditions such as cardiac failure and sepsis have a poorer outcome when the SvO2 is decreased. However, this measurement requires the insertion of a pulmonary artery catheter (PAC), which is relatively invasive and associated with several complications. The use of PACs is on the decline and they are being replaced gradually by non‐invasive cardiac output monitors. The monitoring of central venous oxygen saturation (ScvO2) has been advocated as a simple method of assessing changes in the global oxygen supply‐to‐demand ratio in various clinical settings.10 The advantage of ScvO2 measurement is that it requires only the insertion of a central venous catheter rather than a PAC.
A recent prospective randomised study conducted in an emergency department comparing two algorithms for early goal‐directed therapy in patients with severe sepsis and septic shock has demonstrated the utility of ScvO2 monitoring in improving the outcome from early sepsis.11 Maintenance of ScvO2 above 70% (in addition to maintaining CVP above 8–12 mm Hg, MAP above 65 mm Hg, and urine output above 0.5 ml/kg/h) resulted in a 15% absolute reduction in mortality compared to the same treatment without ScvO2 monitoring. These findings have rekindled interest in ScvO2 measurements in critically ill patients.
In healthy individuals, ScvO2 is slightly less than SvO2; however, for patients with shock, ScvO2 becomes greater than SvO2, particularly in sepsis. This is because of the redistribution of blood towards the cerebral and coronary circulation, away from the splenic, renal, and mesenteric vascular system. In critically ill patients, changes in ScvO2 paralleled changes in SvO2 and therefore the absolute differences between the two measurements are less important.12 Measurements are not affected by any of the variables that might influence reflection spectrophotometry, such as dysfunctional haemoglobins, abnormal pH, and blood temperature.
Temperature monitoring
Temperature monitoring is a vital but often neglected part of the management of a critically ill patient. Hypothermia causes coagulopathy and increases blood loss as well as depressing the functions of all organs. Rewarming increases cardiovascular instability because of vasodilatation and shivering. These patients often have substantial oxygen debt and this is exacerbated by shivering. Adrenergic responses are increased in hypothermia and this increases cardiac morbidity.
Peripheral temperature reflects tissue perfusion and is affected by vasoconstriction and low cardiac output. Core temperature can be monitored at the tympanic membrane, oesophagus, bladder, or rectum. In shock there is an increased gradient between core and peripheral temperature and this provides a useful non‐specific monitor of the effectiveness of resuscitation. Thermistors are used commonly for monitoring core temperatures and are based on the principle of changing resistance with temperature. Core temperature can be assessed rapidly using non‐invasive tympanic thermometers, but these are not always reliable in critically ill patients.13 Oesophageal and urinary bladder methods measure temperature continuously but have the disadvantage of being suitable only for intubated and sedated patients, or patients with a urinary catheter, respectively.14 Studies have shown that oesophageal and urinary bladder methods are highly reliable and superior to rectal methods.15
Urine output
Hourly urine output is a very useful guide to the adequacy of cardiac output, splanchnic perfusion, and renal function. A commonly used urine flow threshold of 0.5 ml/kg/h is used to aid monitoring during resuscitation; a urine output of less than this indicates inadequate resuscitation.
Case progression
After 3 min of CPR, and intravenous adrenaline and colloids, a cardiac output is restored with a blood pressure of 90/50 mm Hg; but the patient remains unresponsive and requires on‐going positive pressure ventilation, using a portable ventilator. A left radial arterial line is inserted and a central line inserted via the right internal jugular vein, under ultrasound control. A central venous oximetry catheter is placed and, following calibration, the ScvO2 reading is 52%. The CVP reading is 12 mm Hg. A fluid challenge does not increase the CVP significantly and further fluid is given until normovolaemia is restored. At this stage, persisting hypotension is treated with an infusion of noradrenaline (norepinephrine) and the patient is transferred to the intensive care unit.
Questions
How accurate is pulse oximetry in hypotensive patients?
Is measurement of the tympanic temperature the best way to determine the core temperature in the critically ill?
Is ETCO2 monitoring of value after intubation of a patient in cardiac arrest?
Can the measurement of blood pressure from an arterial line assess accurately the cardiac output?
How does ScvO2 relate to SvO2 in the critically ill patient with sepsis?
Summary and discussion
Monitoring the critically ill patient in the emergency room is important, especially in the face of increasing evidence that early aggressive intervention in the emergency room improves outcome. However, there are few clinical trial data to demonstrate a beneficial effect for most forms of monitoring.16 Practitioners should be familiar with the physiology and technology underlying these monitoring techniques and be aware of the pitfalls in interpretation of monitored data. Monitoring is not infallible; it should be used as an adjunct to clinical examination.
Abbreviations
CVP - central venous pressure
ETCO2 - end tidal carbon dioxide
MAP - mean arterial pressure
NICE - National Institute of Clinical Excellence
PAC - pulmonary artery catheter
PaO2 - arterial oxygen partial pressure
PaCO2 - arterial carbon dioxide partial pressure
PEEP - positive end‐expiratory pressure
SaO2 - arterial oxygen saturation
ScvO2 - central venous oxygen saturation
SpO2 - oxygen saturation at the peak of blood pulsation
SvO2 - mixed venous oxygen saturation
SVR - systemic vascular resistance
Footnotes
Funding: none
Competing interests: none declared
References
- 1.Holland R, Webb R K, Runciman W B. Oesophageal intubation: an analysis of 2000 incident reports. Anaesth Intensive Care 199321608–610. [DOI] [PubMed] [Google Scholar]
- 2.Salem M R. Verification of endotracheal tube position. Anesthesiol Clin North America 200119813–839. [DOI] [PubMed] [Google Scholar]
- 3.Takeda T, Tanigawa K, Tanaka H.et al The assessment of three methods to identify tracheal tube placement in the emergency setting. Resuscitation 200356153–156. [DOI] [PubMed] [Google Scholar]
- 4.Wouters P F, Gehring H, Meyfroidt G.et al Accuracy of pulse oximeters: the European Multi‐Center Trial. Anesth Analg 200294S13–S16. [PubMed] [Google Scholar]
- 5.Jay G D, Hughes L, Renzi F P. Pulse oximetry is accurate in acute anemia from hemorrhage. Ann Emerg Med 19942432–35. [DOI] [PubMed] [Google Scholar]
- 6.Jubran A. Pulse oximetry. In: Tobin MJ, ed. Principles and practice of intensive care monitoring. New York: McGraw‐Hill, 1998261–287.
- 7.Jubran A. Pulse oximetry. Intensive Care Med 2004302017–2020. [DOI] [PubMed] [Google Scholar]
- 8.Wong D H, Tremper K K, Davidson J. Pulse oximetry is accurate in patients with dysrrythmias and a pulse deficit. Anesthesiology 1989701024–1025. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Clinical Excellence Guidance on the use of ultrasound locating devices for placing central venous catheters. Technology appraisal guidance No. 49, September 2002. www.nice.org.uk
- 10.Rivers P R, Ander D S, Powell D. Central venous oxygenation saturation monitoring in the critically ill patient. Curr Opin Crit Care Med 20017204–211. [DOI] [PubMed] [Google Scholar]
- 11.Rivers E, Nguyen B, Havstad S.et al Early goal directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 20013451368–1377. [DOI] [PubMed] [Google Scholar]
- 12.Reinhart K, Kuhn H J, Hartog C.et al Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med 2004301572–1578. [DOI] [PubMed] [Google Scholar]
- 13.Staven K, Saxholm H, Smith‐Erichsen N. Accuracy of infrared ear thermometry in adult patients. Intensive Care Med 199723100–105. [DOI] [PubMed] [Google Scholar]
- 14.Giuliano K K, Scott S S, Elliot S.et al Temperature measurement in critically ill orally intubated adults: a comparison of pulmonary artery core, tympanic, and oral methods. Crit Care Med 1999272188–2193. [DOI] [PubMed] [Google Scholar]
- 15.Lefrant J Y, Muller L, Emmanuel Coussaye J.et al Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med 200329414–418. [DOI] [PubMed] [Google Scholar]
- 16.Bellomo R, Uchino S. Cardiovascular monitoring tools: use and misuse. Curr Opin Crit Care 20039225–229. [DOI] [PubMed] [Google Scholar]