Abstract
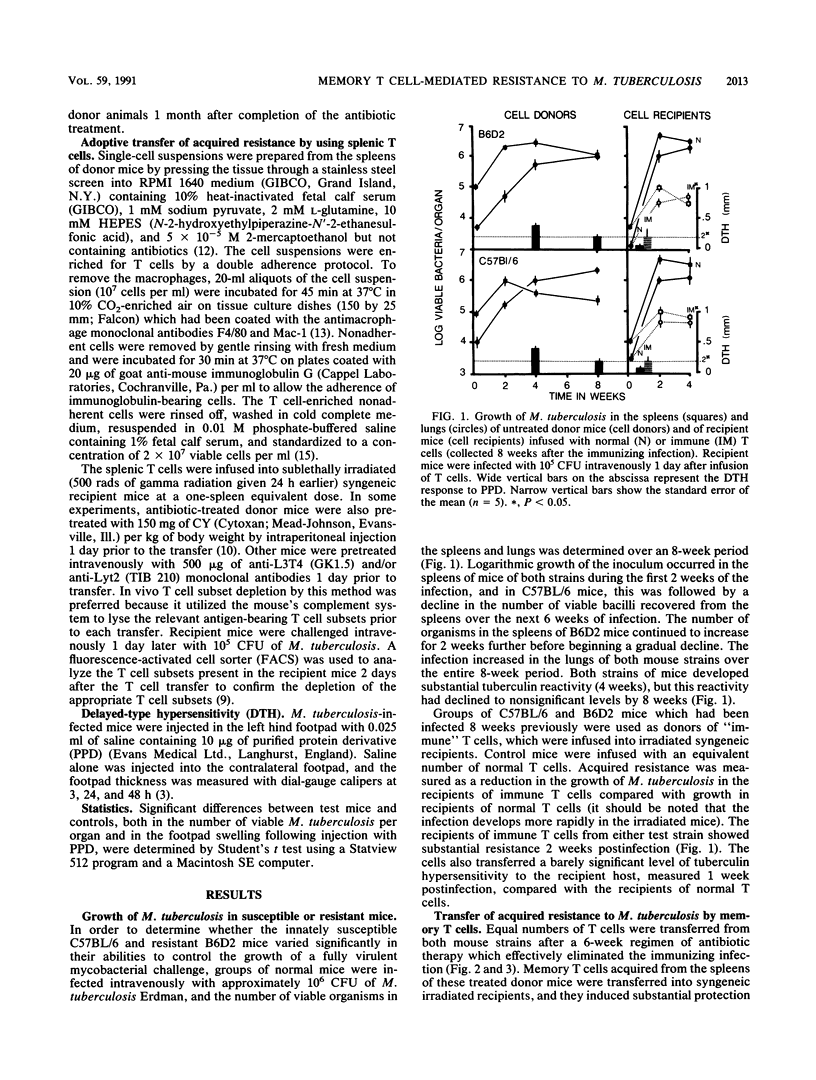
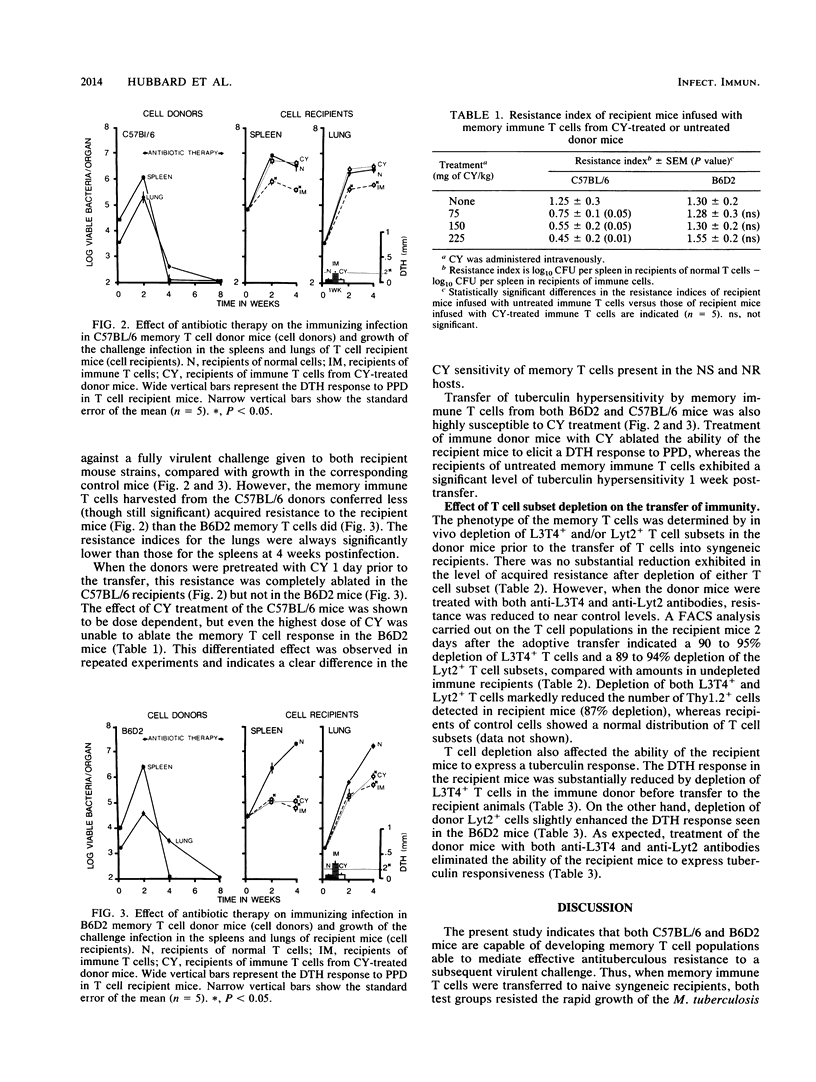
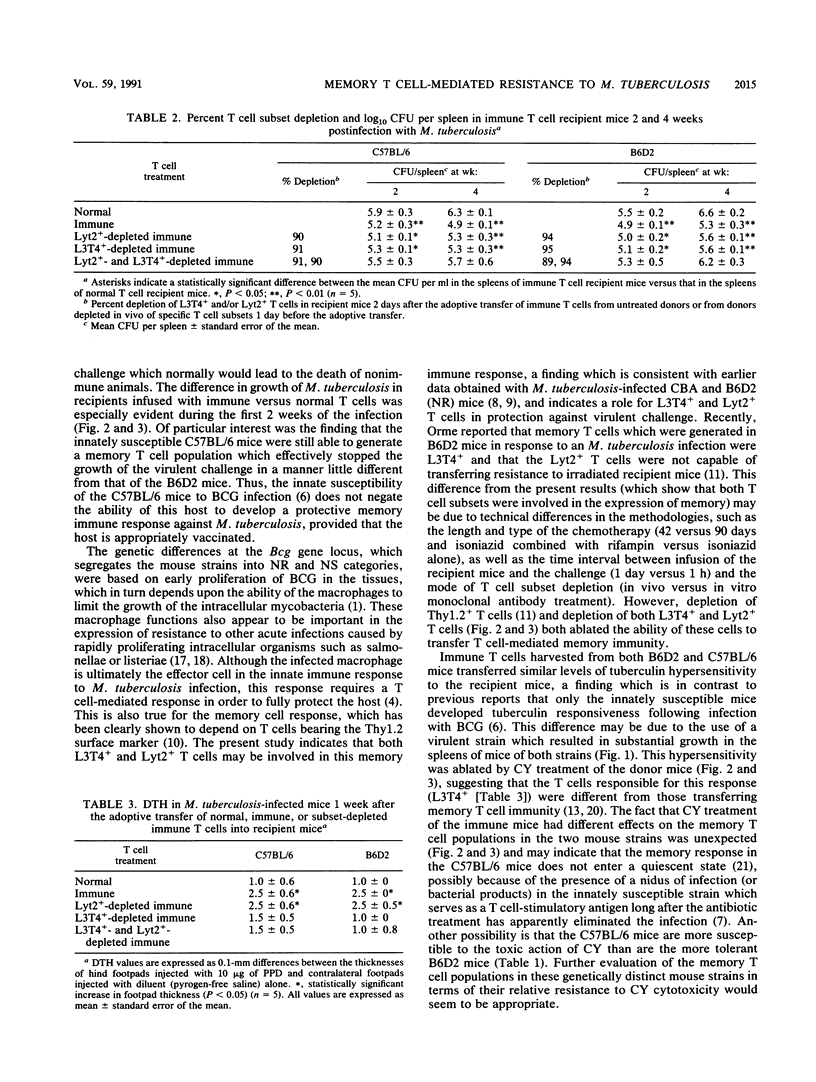
The memory T cell immune response to Mycobacterium tuberculosis infection was examined in strains of mice which vary in their natural susceptibility to Mycobacterium bovis BCG infection. Naturally susceptible (NS) C57BL/6 and naturally resistant (NR) B6D2 F1 hybrid mice were infected with a sublethal dose of M. tuberculosis and then given antibiotic therapy beginning 2 weeks postinfection. T cells from both strains of mice transferred significant levels of resistance to syngeneic mice challenged aerogenically with M. tuberculosis. This memory response was not substantially reduced by depletion of either L3T4+ or Lyt2+ T cells from the donor mice but was ablated by depletion of both T cell subsets. Cyclophosphamide pretreatment of C57BL/6 memory T cell donors also ablated the resistance transferred to recipient mice. In contrast, B6D2 memory T cells were not affected by cyclophosphamide treatment, suggesting that differences may exist in the metabolic state of the memory T cells in the two donor strains, despite the fact that they both develop similar levels of acquired resistance to a subsequent tuberculous challenge.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buschman E., Apt A. S., Nickonenko B. V., Moroz A. M., Averbakh M. H., Skamene E. Genetic aspects of innate resistance and acquired immunity to mycobacteria in inbred mice. Springer Semin Immunopathol. 1988;10(4):319–336. doi: 10.1007/BF02053844. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Effect of chemotherapy on suppressor T cells in BCG-infected mice. Immunology. 1980 Aug;40(4):529–537. [PMC free article] [PubMed] [Google Scholar]
- Edwards D., Kirkpatrick C. H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986 Nov;134(5):1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981 Dec;127(6):2417–2421. [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D. Immunological memory in tuberculosis. I. Influence of persisting viable organisms. Cell Immunol. 1974 Dec;14(3):417–428. doi: 10.1016/0008-8749(74)90192-0. [DOI] [PubMed] [Google Scholar]
- Leveton C., Barnass S., Champion B., Lucas S., De Souza B., Nicol M., Banerjee D., Rook G. T-cell-mediated protection of mice against virulent Mycobacterium tuberculosis. Infect Immun. 1989 Feb;57(2):390–395. doi: 10.1128/iai.57.2.390-395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I., Cobbold S. P., Waldmann H., Kaufmann S. H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987 Sep;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Crossprotection against nontuberculous mycobacterial infections by Mycobacterium tuberculosis memory immune T lymphocytes. J Exp Med. 1986 Jan 1;163(1):203–208. doi: 10.1084/jem.163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Demonstration of acquired resistance in Bcgr inbred mouse strains infected with a low dose of BCG montreal. Clin Exp Immunol. 1984 Apr;56(1):81–88. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Stokes R. W., Collins F. M. Growth of Mycobacterium avium in activated macrophages harvested from inbred mice with differing innate susceptibilities to mycobacterial infection. Infect Immun. 1988 Sep;56(9):2250–2254. doi: 10.1128/iai.56.9.2250-2254.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Parker D. Effect of cyclophosphamide on immunological control mechanisms. Immunol Rev. 1982;65:99–113. doi: 10.1111/j.1600-065x.1982.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Paetkau V., Rawls W. E., Rosenthal K. L. Abrogation of anti-Pichinde virus cytotoxic T cell memory by cyclophosphamide and restoration by coinfection or interleukin 2. J Immunol. 1985 Aug;135(2):1401–1407. [PubMed] [Google Scholar]


