Abstract
The prognosis of patients having a cardiac arrest is generally poor, with a few exceptions. Interventions that aim to improve outcome in cardiac arrest have proved to be disappointing. In particular, no drug has been reliably proved to increase survival to discharge after cardiac arrest. Given that coronary thrombosis in situ and pulmonary thromboembolism are implicated in a large proportion of patients with cardiac arrest, the use of thrombolytic agents has been suggested. Case reports and animal studies have shown favourable results, and have proposed plausible mechanisms to explain them. This is a review of the current literature focusing on the use of thrombolysis during cardiac arrest. A comprehensive literature search was carried out on Medline from 1966 to January 2006, Embase from 1988 to January 2006 and the Cochrane Library, using the Ovid interface. Six articles were selected for review. Although some results are encouraging, all the studies currently available are limited by size and flaws in design.
Retrospective analysis of in‐hospital cardiac arrest shows survival rates of up to 15%.1,2 For out‐of‐hospital cardiac arrest, survival rates of 7% at best have been estimated.3 When functional and neurological recovery are taken into consideration, outcome of resuscitation from cardiac arrest is even less encouraging. With the exception of basic life support, rapid defibrillation and, in selected patients, the administration of therapeutic hypothermia, interventions that aim to improve outcome in cardiac arrest have proved to be disappointing. In particular, no drug has reliably proved to increase survival to discharge after cardiac arrest.4,5,6
Studies that aim to identify causal factors in out‐of‐hospital cardiac arrest show that 50–70% of cases are attributable to either massive pulmonary thromboembolism (PTE) or acute myocardial infarction.7,8 Although cardiac arrest initiated by intracoronary thrombosis in situ is different from the mechanisms associated with pulmonary thromboembolism, thrombolysis has proved to be an effective treatment strategy for both these diseases.9,10 Recent clinical case reports and small case series have suggested that thrombolysis during cardiac arrest can contribute to haemodynamic stability and is associated with improvements in long‐term survival and functional recovery (table 1). The aim of this review was to establish whether thrombolysis during cardiac arrest improves outcome in terms of overall morbidity and neurological outcome.
Table 1 Cases reporting treatment with thrombolysis during cardiopulmonary resuscitation (reproduced with permission from Böttiger and Spöhr24).
| Reference | Thrombolytic agent | Survival |
|---|---|---|
| Renkes‐Hegendorfer and Herrmann | Streptokinase | Y |
| Borst and Wolf | Streptokinase | Y |
| Jester and Langheinrich | Streptokinase | – |
| Kostering et al | Streptokinase | – |
| Unseld et al | Streptokinase | Y |
| Wester et al | Urokinase | Y |
| Schaffer | Streptokinase Alteplase | – |
| Langdon et al | Streptokinase Alteplase | Y |
| Atzinger et al | Urokinase | Y |
| Hopf et al | Urokinase | Y |
| Trenkwalder et al | Streptokinase | Y |
| Böttiger et al | Streptokinase Alteplase | Y |
| Harke | Urokinase | – |
| Klinge et al | Alteplase | – |
| Siebenlist and Gattenlohner | Alteplase | N |
| Böttiger et al | Alteplase | Y |
| Fred and Yang | Alteplase | N |
| Muller and Axthelm | Alteplase | Y |
| Pharo et al | Alteplase | Y |
| Onoyama et al | Urokinase | – |
| Oneglia and Rusconi | Reteplase | – |
| Schluter et al | Alteplase | Y |
| Soltesz et al | Alteplase | Y |
| Kuisma et al | Alteplase | Y |
| Schulte‐Sinkus and Standl | Alteplase | Y |
| Cyrkowicz et al | Alteplase | Y |
| Kehoe and DaCruz | Alteplase | – |
| Meier | Alteplase | Y |
| Wittmann and Dietz | Alteplase | Y |
| Duchateau et al | Alteplase | Y |
| Grabner et al | Alteplase | Y |
| Lapostolle et al | Alteplase | Y |
| Nordmeyer | Alteplase | Y |
| Kosits25 | Tenecteplase | Y |
N, no; Y, yes; −, no survival details available.
Methods
A comprehensive literature search was carried out as outlined below.
Medline from 1966 to January 2006 and Embase from 1988 to January 2006, using the Ovid interface. The search was limited to English language and humans. Keywords used were as follows: exp Heart Arrest/ or cardiac arrest.mp. cardiopulmonary arrest.mp. cardiorespiratory.mp. exp cardiopulmonary resuscitation/ or exp resuscitation/ or resuscitation.mp. and exp Urinary Plasminogen Activator/ or exp Thrombolytic Therapy/ or exp Fibrinolytic Agents/ or exp Tissue Plasminogen Activator/ or thrombolysis.mp. or exp Streptokinase/ exp Tissue Plasminogen Activator/ or exp Fibrinolytic Agents/ or exp Thrombolytic Therapy/ or tenecteplase.mp. exp Tissue Plasminogen Activator/ or alteplase.mp. exp Fibrinolytic Agents/ or exp Tissue Plasminogen Activator/ or reteplase.mp. or exp Plasminogen Activators/ or exp Thrombolytic Therapy/ and exp prognosis/ or prognosis.mp. exp hospital mortality/ or hospital mortality.mp. / or exp mortality/ or mortality.mp. exp morbidity/ or morbidity.mp. / survival.mp. or exp survival/ or exp survival rate/ exp brain injuries/ or neurological function.mp. /neurological outcome.mp.
The Cochrane Library to January 2006
Bibliographical search of papers obtained from the above methods.
Papers were identified by a two‐stage process, in which abstracts were initially screened, followed by retrieval and review of full text documents. Papers were selected if they compared groups of patients (cohort studies or randomised controlled trials) who had received thrombolytic treatment during cardiac arrest (rather than thrombolysis before cardiac arrest or after return of spontaneous circulation (ROSC)) and gave details of survival and functional outcome. DKP screened the papers and WGM checked the selection.
The validity of selected papers was tested using the criteria outlined by the Evidence‐Based Medicine Group (see box 1).11 In addition, a JADAD score12 was calculated for each paper selected. This is included in table 2.13,14,15,16,17,18
Table 2 Selected studies in chronological order.
| Study | Method | Participants | Intervention | Outcomes | Validity | Results |
|---|---|---|---|---|---|---|
| Böttiger et al,13 2001 | Prospective controlled | 90 patients in cardiac arrest; 40 in treatment group; 50 controls | 50 mg rt‐PA and 5000 IU unfractionated heparin repeated if no ROSC in 30 min | CPR‐related bleeding complications, ROSC, ICU admission, 24‐survival, discharge from hospital | Not randomised, not blinded, historical controls, stopped before completion, JADAD score 0 | No bleeding complications due to CPR, improvement in ROSC,OR 2.65 (CI 1.11 to 6.25), ICU admission OR 3.15 (CI 1.32 to 7.69), non‐significant improvement in survival to discharge |
| Lederer et al,14 2001 | Retrospective matched pair controls, case note review | 108 patients who received rt‐PA during cardiac arrest compared with 216 case‐matched controls | Front‐loaded alteplase rt‐PA, heparin at the discretion of treating doctor | CPR‐related bleeding complications, ROSC, 24 h survival, discharge from hospital | Retrospective case note review, not randomised, not blinded, groups not the same at baseline, JADAD score 0 | No difference in CPR‐related bleeding complications, improvement in ROSC 70.4% v 51% (p = 0.001), 24‐h survival 48.1% v 32.9% (p = 0.003), survival to discharge 25% v 15.3% (p = 0.048) |
| Abu‐Laban et al,15 2002 | Prospective RCT | 233 patients in cardiac arrest, 117 in treatment group, 116 controls | 100 mg rt‐PA infused over 15 min | Survival to hospital discharge, ROSC, length of hospital stay, bleeding complications, neurological outcome | Meets EBM group criteria for validity (except criterion 5) heparin given at discretion of treating doctor, delay to treatment, underpowered, JADAD score 5 | 1 patient in treatment group survived to discharge in treatment group compared with none in placebo (absolute difference 0.9, CI 2.6 to 4.8, p = 0.99); ROSC 21.4% in t‐PA group, 23.3% in controls (absolute difference 1.9, CI 12.6 to 8.8, p = 0.85) |
| Janata et al,16 2003 | Retrospective cohort study | 66 patients in cardiac arrest due to PTE, 36 received thrombolysis, compared with 30 controls | Alteplase (rt‐PA) 0.6–1.0 mg/kg maximum dose 100 mg, followed by heparin infusion | Major bleeding complications, complications in relation to duration of CPR, ROSC, survival to discharge | Retrospective case note review, not randomised, not blinded, groups not the same at baseline, small study, JADAD score 0 | Non‐significant trend towards more bleeding complications in rt‐PA group, no effect shown of duration of CPR, ROSC in 24 (67%) v 13 (43%) controls (p = 0.06), 24‐h survival, 19 (53%) v 7 (23%), p = 0.01, survival to discharge 7 (19%) v 2 (7%), p = 0.15 |
| Lederer et al,17 2004 | Retrospective matched‐pair controls, case note review | 108 patients who received rt‐PA during cardiac arrest compared with 216 case‐matched controls, 5‐year follow‐up of survivors in the treatment group | Front loaded alteplase rt‐PA, heparin at the discretion of treating doctor | 5‐year survival, cerebral performance category, NYHA criteria, life satisfaction survey | Retrospective case note review, not randomised, not blinded, groups not the same at baseline, no follow‐up of survivors in control group | 20 (74%) patients survived for 5 years, at discharge, normal CPR scores in 22 patients, moderate disability in 3 patients, severe disability in 2 patients, mean NYHA grade 2.1 (0.9) at discharge 1.5 (0.6) at 5 years, 15 patients attained previous level of activity |
| Fatovich et al,18 2004 | Prospective RCT | 35 patients in cardiac arrest, 19 in treatment group, 16 controls | 50 mg tenecteplase | ROSC, discharged from A&E, discharged from ICU, discharged from hospital | JADAD score 0, differences between groups at baseline, small sample size, JADAD score 5 | Increase in ROSC (42% v 6%, CI 11–61%), 3 patients survived to discharge from A&E 2 from treatment group, 1 control survived to discharge from hospital |
A&E, accident and emergency; CPR, cardiopulmonary resuscitation; EBM, Evidence‐Based Medicine; ICU, intensive care unit; NYHA, New York Heart Association; PTE, pulmonary thromboembolism; RCT, randomised controlled trial; ROSC, return of spontaneous circulation; rt‐PA, recombinant tissue‐type plasminogen activator; t‐Pa, tissue‐type plasminogen activator.
Results
In all, 189 papers were identified using the search strategy outlined above. A review of abstracts resulted in exclusion of 161 irrelevant articles; 11 narrative review articles and 6 letters were also excluded. Eleven papers were examined in more detail. Two papers met the criteria for entry on to the Cochrane database.13,15 Five studies were related to the delivery of thrombolysis after successful resuscitation, and so were excluded.19,20,21,22,23 Bibliographies identified further papers and case reports (table 1, reproduced from Böttiger and Spöhr24). Table 2 details the six papers selected for further evaluation.
Box 1: Questions for assessing the validity of treatment trials
Was the assignment of patients to treatments randomised? Was the randomisation list concealed?
Was the follow‐up of patients sufficiently long and complete?
Were all patients analysed in the groups to which they were randomised?
Were the patients and doctors kept “blind” to the treatment?
Were the groups treated equally, apart from the experimental treatment?
Were the groups similar at the start of the trial?
Discussion
The literature that supports the use of thrombolytic agents as a treatment modality during cardiac arrest is dominated by case reports and small series. Reported cases are marked by an unusually high survival rate, success despite prolonged cardiopulmonary resuscitation (CPR) and exceptional neurological recovery. The first reported case, published in 1974, describes a successful outcome in a patient with an acute PTE.26 Subsequently, 33 single cases and 10 case series documenting a total of 87 patients are recorded (table 2).
Survival without severe neurological disability was documented in 63 (72.4%) patients. The mean time until ROSC was 51.3 min, with six cases documenting survival after >90 min of CPR. Although the unusual record of success may partly be owing to bias in publication and reporting, the encouraging results have led to prospective studies and randomised controlled trials; these are examined in more detail later.
Proposed mechanism of action
Although the available literature suggests that a survival advantage is conferred by thrombolysis, the mechanism of action is not entirely clear. The Thrombolysis in Myocardial Infarction Trial shows a median time of 60 min from administration to the establishment of grade 2 or 3 coronary flow in patients successfully reperfused using thrombolytic agents.27 The literature in support of thrombolysis during CPR suggests a much more rapid clinical effect. It has been suggested that only a small fraction of Thrombolysis in Myocardial Infarction Trial grade 3 flow may be all that is required for ROSC.28 An alternative or synergistic mechanism may be that emboli in myocardial microcirculation may be more amenable to lysis than a larger occlusive thrombus. Gando et al29 measured markers of fibrin formation and fibrinolysis in 63 patients in cardiac arrest. In all patients, markers of fibrin formation were markedly raised, without a similar rise in markers of fibrinolysis. Böttiger et al30 used different markers and closer spacing of blood samples to demonstrate similar findings in 23 patients. These results, together with similar experimental data in animal models, suggest that cardiac arrest is associated with a disseminated intravascular activation of blood coagulation without adequate endogenous fibrinolysis. Thus, thrombolysis during CPR may result in a general improvement in microcirculatory flow. In keeping with this theory, fibrin‐specific bolus thrombolytics has been shown to have a rapid generalised action even during CPR.31
Neurological recovery
In addition to hypoxic injury, cerebral reperfusion has been implicated as a major determinant of neurological recovery after resuscitation from cardiac arrest. Using a feline model, Fischer and Hossmann32 used labelled circulating blood and fixation of the brain post mortem to directly visualise perfusion immediately after cardiac arrest. Their results suggest that microvascular thrombi result in areas that fail to reperfuse, and this may impede recovery after resuscitation. A similar study in which a thrombolytic bolus was given during CPR showed a considerable difference in cerebral reperfusion between the treatment group and controls.33 Other studies on animal models suggest that recombinant tissue‐type plasminogen activator may improve the resistance of neurones to oxidative stress in vitro34; similarly in stroke models, it may reduce infarct size independent of reperfusion.35 A demonstrable decline in cognitive function has been described after coronary artery bypass graft surgery.36 Similar mechanisms may operate during the low‐output state associated with resuscitation from cardiac arrest. Lederer et al17 investigated cerebral performance in survivors who had received thrombolysis during cardiac arrest. However, the scoring system considered mainly functional capacity rather than more subtle deficits. A battery of neuropsychological tests is required to evaluate cognitive and neurobehavioural outcome,37 which, to date, has not been considered in patients receiving thrombolysis during cardiac arrest.
The experimental evidence of microvascular coagulation that can be prevented or treated by thrombolysis, and the observation that thrombolytic agents may increase tolerance to cerebral ischaemic insult, suggests that thrombolysis may have neuroprotective benefits in cardiac arrest. The evidence also implies potential beneficial effects beyond localised lysis of occlusive thrombi in acute myocardial infarction and PTE.
Safety and side effects
When considering the potential benefits of thrombolysis in cardiac arrest, it is equally important to explore the risk. Many clinical guidelines list prolonged CPR as a contraindication for thrombolysis.38 The meta‐analysis carried out by the Fibrinolytic Therapy Trialists' Collaborative Group9 is the evidence most often quoted outlining the risks of thrombolysis. However, this study did not identify a subgroup of patients who received CPR; instead, it listed the overall incidence of bleeding complications. Clinical studies and case series dealing with thrombolysis before, during or soon after CPR do not suggest additional bleeding risk.19,20,21,22,39,40,41 On the contrary, large studies show that when thrombolysis is withheld in survivors of cardiac arrest, mortality is increased.42,24
Use of thrombolytic agents in cardiac arrest
Although first reported 30 years ago,26 the first study designed to consider the efficacy of thrombolysis during cardiac arrest was not published until 2001.13 The papers presented represent the best available evidence (table 2). Five papers relating to treatment with a thrombolytic agent after successful resuscitation were excluded, because they did not directly deal with the question.19,20,21,22,23 One of these papers was on neurological outcome, suggesting a non‐significant improvement in cerebral performance category score in patients with thrombolysis.19 The principal interest of the other papers was safety of thrombolysis in this context. All were relatively small, non‐randomised and retrospective case note studies and so were of limited value.
Böttiger et al13 were the first to report a prospective intervention trial of patients with thrombolysis in cardiac arrest. The controls were 50 patients who had had an out‐of‐hospital cardiac arrest in the year preceding the trial. After this year, 40 patients believed to have a cardiac arrest owing to a primary cardiac cause in which ROSC was not achieved in the first 15 min of resuscitation were given 50 mg recombinant tissue‐type plasminogen activator over 2 min and a bolus of 5000 IU heparin. Patients were excluded if there were any indications of internal or external bleeding; inclusion was limited to patients aged 18–75 years. The intervention was repeated if ROSC was not achieved in another 30 min.
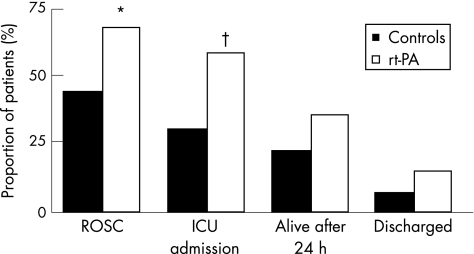
No significant differences were observed between the groups with respect to factors that might influence outcome in cardiac arrest. The authors report a significant increase in the numbers of patients in whom ROSC was achieved in the treatment group versus number of controls (27 (68%) v 22 (44%), p = 0.026). Similarly, more patients were admitted to the intensive care unit (23 (58%) v 15 (30%), p = 0.009), survived 24 h (14 (35%) v 11 (22%), p = 0.171) and were discharged from hospital (6 (15%) v 4 (8%); fig 1).
Figure 1 Outcomes in patients treated with recombinant tissue‐type plasminogen activator (rt‐PA) and in controls. ICU, intensive care unit; ROSC, return of spontaneous circulation (reproduced from Böttiger et al13). *p = 0.026; †p = 0.009.
A lack of randomisation, small numbers and the use of historical controls limited this study. The authors indicate that the ethics committee was unwilling to approve the random assignment of unconscious patients. The ethics committee also requested an interim analysis and stopped the study after 40 patients had been treated. No power calculation was included and so it was difficult to assess how limited numbers might have affected the findings. Differences between the groups in ROSC and admission to the intensive care unit achieve statistical significance, but this significance is lost when survival for 24 h or discharge from hospital is considered. No assessment of functional outcome in survivors is reported. The decision to recruit or exclude patients was the responsibility of the treating doctor; basing this on the definition of “cardiac arrest for cardiological reasons” may have led to a selection bias. This might account for the remarkable success for ROSC in the patients with thrombolysis and controls. The authors believed that the primary end point of safety (lack of CPR‐related bleeding complications) was met. Although two episodes of gastrointestinal bleeding are reported, these were considered not be related to CPR. The authors suggest that although the number of patients in this study was too small to draw conclusions about survival outcome, the study protocol was feasible and safe, and that a randomised controlled trial might now be considered ethical.
In a retrospective case‐matched study, Lederer et al14 detail the outcome of 108 patients who were given thrombolysis during prehospital cardiac arrest. The study compared case notes of patients aged 30–80 years who had been given a front‐loaded t‐PA regimen with those of 216 matched controls. ROSC was documented in 70.4% of patients treated with thrombolysis compared with 51% in the control group (p = 0.001). Survival for >24 h was achieved by 48.1% of patients in the treatment group and by 32.9% of controls (p = 0.003), and 25% of the treatment group was discharged from hospital compared with 15% of the controls (p = 0.048). This study was limited by a lack of randomisation. The decision to treat with thrombolysis was made by the doctor on duty, and so patient selection and enrolment was at risk of bias. The design of the study was observational, based on a retrospective review of charts. Although attempts were made to match patients for certain characteristics, there were still marked differences between the groups at baseline.
A subsequent paper,17 by the same authors, details the functional outcome of survivors at 5 years. In all, 22 of 27 (81%) patients were discharged from hospital without neurological deficit and 18 (67%) patients were alive at 5 years. Unfortunately, only patients from the treatment group were followed up, and so no comparison with outcome in controls could be made, rendering this paper of limited value.
Abu‐Laban et al15 conducted a randomised controlled trial on the prehospital thrombolysis in patients presenting with pulseless electrical activity (PEA). Patients aged >16 years were randomised to receive 100 mg t‐PA as an intravenous infusion over 15 min or a placebo. Before enrolment all patients underwent endotracheal intubation, and were given at least 500 ml normal saline and 1 mg epinephrine. Patients were excluded if cardiac arrest was thought to be due to hypothermia, tension pneumothorax, cardiac tamponade, and haemorrhage or electrolyte imbalance. Over the 1‐year period, 233 patients were recruited (117 in the t‐PA group and 116 in the placebo group). Baseline characteristics were similar in both groups, particularly with respect to variables predictive of survival. The main findings were that the study showed no beneficial effects of fibrinolysis. ROSC was achieved in 25 (21.4%) patients from the treatment group compared with 27 (23.3%) from the placebo group (p = 0.85). Four patients, all from the treatment group, survived >24 h. One of these survivors was eventually discharged from hospital. Thus the absolute difference in survival between the t‐PA and placebo groups was 0.9% (confidence interval (CI) −2.6 to 4.8). This study fulfils the EBM group validity criteria in all but one aspect, in that the treatment of patients with heparin was at the discretion of the treating doctor. Several factors may have led to an inaccurate result. Firstly, owing to the strict enrolment protocol, treatment with thrombolytic agents was considerably delayed. The authors document initiation of treatment a mean of 36 min from collapse. At this stage, without success, most resuscitation attempts would normally have been terminated. The group studied seems to have had an exceptionally poor outcome compared with that in other studies; we would normally expect to see at least some survivors from the placebo group. Although the authors include a power calculation, this assumes an increase in survival from 1% in the placebo group to 10.3% in the t‐PA group. As this has not been achieved by any previous study, this assumption may have led to the study being underpowered. The authors' stated aim in restricting the study to patients with PEA was to include PTE; however, only one pulmonary embolism was found in the 42 necropsies carried out. Similarly, patients whose initial rhythm was asystole and who then went on to have PEA were included; such patients are known to have poor prognosis. Thus, the study design and inclusion criteria may have obscured any positive effects of treatment and certainly may limit the generalisability of the study to a setting out‐with a clinical trial.
Janata et al16 report a retrospective cohort study of patients with thrombolysis in cardiac arrest due to massive PTE. Although the principal area of interest in the study was bleeding risk after CPR, data relating to survival were also presented. In all, 36 patients treated with recombinant tissue‐type plasminogen activator during cardiac arrest were compared with 30 controls. The diagnosis of PTE was confirmed by a combination of risk factors, presenting symptoms, clinical investigation (echocardiogram or computed tomography of the pulmonary artery) and, in fatal cases, necropsies. ROSC was achieved in 24 (67%) patients v 13 (43%) controls (p = 0.06). 24‐h survival was 53% in the treatment group and 23% in controls (p = 0.01). Hospital discharge was recorded in 7 (19%) patients from the thrombolysis group and in 2 (7%) controls (p = 0.15). Non‐randomised selection and small numbers limit this study, making it little more than a case series. The groups identified have marked differences at baseline and, again, selection of patients was entirely at the discretion of the treating doctor.
Fatovich et al18 conducted a randomised controlled trial comparing outcomes of ROSC, survival to discharge from the emergency department and survival to discharge from hospital for 35 patients treated with 50 mg of tenecteplase or placebo during cardiac arrest. In the prehospital phase, all patients received advanced life support. Intravenous treatment with drugs was withheld until arrival at hospital. The patients were enrolled into the study on arrival at the emergency department. Patients were excluded if cardiac arrest was thought to be due to a non‐cardiac cause. Randomisation was by an opaque sealed envelope, and blinding was rigorously adhered to. The main findings were a marked improvement in ROSC in the treatment group veruss controls (8 (42%) v 1 (6%), 95% CI 11% to 61%). However, only three patients survived to discharge from the emergency department—two from the treatment group and one from the control group. The one patient who survived from the control group was the only patient to achieve hospital discharge. No bleeding complications were observed during the study. The main limitation of this study is the small sample size. Although the authors used a power calculation to estimate that 58 patients would be required in each treatment arm, in the event they recruited a total of only 35 patients. This is said to be owing to funding difficulties. Differences between the groups at baseline may be due to the small sample size. Patients in the treatment group were significantly younger (63 v 72 years, p = 0.04) and were more likely to have had a ventricular fibrillation arrest (63% v 19%, 95% CI 15% to 73%). They had non‐statistically significant shorter response times. All these factors might improve outcome of patients treated for cardiac arrest.
Conclusion
Case reports and animal studies have shown favourable results, and have proposed plausible mechanisms to explain the role of thrombolytic agents in cardiac arrest. All studies to date have been too small to draw any firm conclusion, and each had its limitations as described earlier. Even studies which present the most encouraging data fail to show a considerable difference in survival to hospital discharge. Abu‐Laban et al15 does not replicate the encouraging results of Böttiger et al,13 Lederer et al14 and Fatovich et al.18 Although Abu‐Laban's work is the largest and best‐designed study to date, confounding factors, in particular inclusion of only patients with PEA, might have precluded the demonstration of any positive effect and limited its generalisability to a wider patient group.
To conclusively investigate the efficacy of thrombolysis during cardiac arrest, a much larger study on the early use of thrombolytics in patients with a relatively good prognosis will be required. Under way in five European countries, the Thrombolysis in Cardiac Arrest Trial aims to recruit 1000 patients across 60 international study centres. The aim of this study is to consider the problems with randomisation and blinding encountered with earlier work. In addition to the primary end point of 30 day survival, secondary end points will include neurological performance. Importantly, the trial will include all presenting rhythms and intervention will be at an earlier stage, with delivery after the first cycle of advanced life support. Until the results of this and other larger trials are available, doubts prevail about the efficacy of this treatment modality.
Acknowledgements
We thank Mr Shobhan Thakore for checking the initial search and reading an initial draft.
Abbreviations
CPR - cardiopulmonary resuscitation
PEA - pulseless electrical activity
PTE - pulmonary thromboembolism
ROSC - return of spontaneous circulation
t‐PA - tissue‐type plasminogen activator
Footnotes
Competing interests: None.
References
- 1.Bedell S E, Delbanco T L, Cook E F.et al Survival after cardiopulmonary resuscitation in the hospital. N Engl J Med 1983309569–576. [DOI] [PubMed] [Google Scholar]
- 2.Ballew K A, Philbrick J T, Caven D E.et al Predictors of survival follow in hospital cardiopulmonary resuscitation: a moving target. Arch Intern Med 19941542426–2432. [PubMed] [Google Scholar]
- 3.Nichol G, Detsky A S, Stiell I G.et al Effectiveness of emergency medical services for victims of out of hospital cardiac arrest: a metanalysis. Ann Emerg Med 199627700–710. [DOI] [PubMed] [Google Scholar]
- 4.Kudenchuk P J, Cobb L A, Copass M K.et al Amiodarone for resuscitation after out‐of‐hospital cardiac arrest due to ventricular fibrillation. N Engl J Med 1999341871–878. [DOI] [PubMed] [Google Scholar]
- 5.Nolan J P, Morley P T, Vanden Hoek T L.et al Therapeutic hypothermia after cardiac arrest. An advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation 2003108118–121. [DOI] [PubMed] [Google Scholar]
- 6.Nolan J P, De Latorre F J, Steen P A.et al Advanced life support drugs: do they work? Curr Opin Crit Care 20028212–218. [DOI] [PubMed] [Google Scholar]
- 7.Silfvast T. Cause of death in unsuccessful prehospital resuscitation. J Int Med 1991229331–335. [DOI] [PubMed] [Google Scholar]
- 8.Spaulding C M, Joly L M, Rosenberg A.et al Immediate coronary angiography in survivers of out‐of‐hospital cardiac arrest. N Engl J Med 19973361629–1633. [DOI] [PubMed] [Google Scholar]
- 9.Fibrinolytic Therapy Trialists' (FTT) Collaborative Group Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994343311–322. [PubMed] [Google Scholar]
- 10.Arcasoy S M, Kreit J W. Thrombolytic therapy of pulmonary embolism: a comprehensive review of current evidence. Chest 19991151695–1707. [DOI] [PubMed] [Google Scholar]
- 11.Sackett D L, Richardson W S, Rosenberg W.Evidence based medicine: how to practice and teach EBM. Edinburgh: Churchill Livingstone, 1998
- 12.Jadad A R, Moore A, Carroll D.et al Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996171–12. [DOI] [PubMed] [Google Scholar]
- 13.Böttiger B W, Bode C, Kern S.et al Efficacy and safety of thrombolytic therapy after initially unsuccessful cardiopulmonary resuscitation: a prospective clinical trial. Lancet 20013571583–1585. [DOI] [PubMed] [Google Scholar]
- 14.Lederer W, Lichtenberger C, Pechlaner C.et al Recombinant tissue plasminogen activator during cardiopulmonary resuscitation in 108 patients with out‐of‐hospital cardiac arrest. Resuscitation 20015071–76. [DOI] [PubMed] [Google Scholar]
- 15.Abu‐Laban R B, Christenson J M, Innes G D.et al Tissue plasminogen activator in cardiac arrest with pulseless electrical activity. N Engl J Med 20023461522–1528. [DOI] [PubMed] [Google Scholar]
- 16.Janata K, Holzer M, Kurkciyan I. Major bleeding complications in cardiopulmonary resuscitation: the place of thrombolytic therapy in cardiac arrest due to massive pulmonary embolism. Resuscitation 20035749–55. [DOI] [PubMed] [Google Scholar]
- 17.Lederer W, Lichtenberger C, Pechlaner C.et al Long‐term survival and neurological outcome of patients who received recombinant tissue plasminogen activator during out‐of‐hospital cardiac arrest. Resuscitation 200461123–129. [DOI] [PubMed] [Google Scholar]
- 18.Fatovich D M, Dobb G J, Clugston R A. A pilot randomised trial of thrombolysis in cardiac arrest (The TICA Trial). Resuscitation 200461309–313. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber W, Gabriel D, Sterz F.et al Thrombolytic therapy after cardiac arrest and its effect on neurological outcome. Resuscitation 20025263–69. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz‐Bailen M, Aguayo de Hoyos E, Diaz‐Castellanos M A. Role of thrombolysis in cardiac arrest. Intensive Care Med 200127438–441. [DOI] [PubMed] [Google Scholar]
- 21.Voipio V, Kuisma M, Alaspaa A.et al Thrombolytic treatment of acute myocardial infarction after out‐of‐hospital cardiac arrest. Resuscitation 200149251–258. [DOI] [PubMed] [Google Scholar]
- 22.Kurkciyan I, Meron G, Sterz F.et al Major bleeding complications after cardiopulmonary resuscitation: impact of thrombolytic treatment. J Intern Med 2003253128–135. [DOI] [PubMed] [Google Scholar]
- 23.Tenaglia A N, Califf R M, Candela R J.et al Thrombolytic therapy in patients requiring cardiopulmonary resuscitation. Am J Cardiol 1991681015–1019. [DOI] [PubMed] [Google Scholar]
- 24.Böttiger B W, Spöhr F. The risk of thrombolysis in association with cardiopulmonary resuscitation—no reason to withhold this causal and effective therapy. J Int Med 200325399–101. [DOI] [PubMed] [Google Scholar]
- 25.Kosits L. Administration of tenecteplase after prolonged cardiopulmonary resuscitation in a 46‐year‐old man with ventricular fibrillation. J Emerg Nurs 200329507–510. [DOI] [PubMed] [Google Scholar]
- 26.Renkes‐Hegendörfer U, Herrmann K. Successful treatment of a case of fulminant massive pulmonary embolism with streptokinase. Anaesthesist 197423500–501. [PubMed] [Google Scholar]
- 27.The TIMI Study Group The Trombolysis in Myocardial Infarction Trial. N Engl J Med 1985312932–936. [DOI] [PubMed] [Google Scholar]
- 28.Newmann D H, Greenwald I, Callaway C W. Cardiac arrest and the role of thrombolytic agents. Ann Emerg Med 200035472–480. [PubMed] [Google Scholar]
- 29.Gando S, Kameue T, Nanzaki S.et al Massive fibrin formation with consecutive impairment of fibrinolysis in patients with out‐of‐hospital cardiac arrest. Thromb Haemost 199777278–282. [PubMed] [Google Scholar]
- 30.Böttiger B W, Motsch J, Böhrer H.et al Activation of blood coagulation after cardiac arrest is not balanced adequately by activation of endogenous fibrinolysis. Circulation 1995922572–2578. [DOI] [PubMed] [Google Scholar]
- 31.Cannon C P, Gibson C M, McCabe C H.et al TNK‐tissue plasminogen activator compared with front‐loaded alteplase in acute myocardial infarction: results of the TIMI 10B trial. Circulation 1998982805–2814. [DOI] [PubMed] [Google Scholar]
- 32.Fischer M, Hossmann K A. No‐reflow after cardiac arrest. Intens Care Med 199521132–141. [DOI] [PubMed] [Google Scholar]
- 33.Fischer M, Böttiger B W, Popov‐Cenic S.et al Thrombolysis using plasminogen activator and heparin reduces cerebral no‐reflow after resuscitation from cardiac arrest: an experimental study in the cat. Intens Care Med 1996221214–1223. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y H, Park J H, Hong S H.et al Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science 1999284647–650. [DOI] [PubMed] [Google Scholar]
- 35.Kilic E, Hermann D M, Hossman K A. Recombinant tissue plasminogen activator reduces infarct size after reversible thread occlusion of middle cerebral artery occlusion in mice. Neuroreport 199910107–111. [DOI] [PubMed] [Google Scholar]
- 36.Newman M F, Kirchner J L, Phillips‐Bute B.et al Longitudinal assessment of neurocognitive function after coronary artery bypass surgery. N Engl J Med 2001344395–402. [DOI] [PubMed] [Google Scholar]
- 37.Murkin J M, Newman S P, Stump D A.et al Statement of consensus on the assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg 1995591289–1295. [DOI] [PubMed] [Google Scholar]
- 38.Van de Werf F, Ardissino D, Betriu A.et al Management of acute myocardial infarction in patients presenting with ST‐segment elevation; the Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 20032428–66. [DOI] [PubMed] [Google Scholar]
- 39.Scholz K H, Tebbe U, Herrmann C.et al Frequency of complications of cardiopulmonary Resuscitation after thrombolysis during acute myocardial infarction. Am J Cardiol 199269724–728. [DOI] [PubMed] [Google Scholar]
- 40.Schiele R, Rustige J, Burcyk U.et al Thrombolysis after resuscitation in acute myocardial infarction. J Am Coll Cardiol 199627(Suppl A)279A [Google Scholar]
- 41.Stewart B F, Weaver W D, Parsons L S.et al Reperfusion therapy after pre‐hospital cardiac arrest and its influence on outcome after acute myocardial infarction. J Am Coll Cardiol 199627(Suppl A)278A [Google Scholar]
- 42.Spöhr F, Böttiger B W. Safety of thrombolysis during cardiopulmonary resuscitation. Drug Saf 200326367–379. [DOI] [PubMed] [Google Scholar]