Abstract
Objectives
To investigate the diagnostic accuracy of presentation ischaemia‐modified albumin (IMA), in addition to cardiac troponin I (TnI), as a strategy to rapidly ascribe low risk to patients with chest pain attending an emergency department, and to determine whether IMA has the potential to reduce transit time in emergency departments.
Methods
A prospective observational study was carried out in two emergency departments (belonging to the John Radcliffe Hospital, Oxford, UK; and the Frenchay Hospital, Bristol, UK) of similar size. Consecutive adult patients presenting with features of possible ischaemic cardiac chest pain and a normal electrocardiogram were eligible. The index test (measurement of IMA and TnI at presentation) and reference standard (delayed TnI measurement, taken at least 8 h after pain onset) were applied to all recruited patients. All clinicians were blinded to the results of the index test. Assays were carried out in a single laboratory using standard techniques.
Results
399 patients were recruited; 277 patients had a result for both the index test and reference standard. The sensitivity was 97.6% (95% confidence interval (CI) 87.4 to 99.9), negative predictive value 97% (95% CI 84.2 to 99.9) and specificity 13.6% (95% CI 9.5 to 18.7). Sensitivity analysis showed similar findings in three alternative scenarios. Receiver operating characteristic analysis indicated that a different “cut‐off” value for IMA would not improve the properties of the test. The median potential time saved (n = 268) was 6 h and 10 min.
Conclusion
The diagnostic accuracy of presentation IMA in this study does not support its use as an effective risk stratification tool for patients with chest pain in the emergency department. The sensitivity is insufficiently high, with a small number of false negatives undermining the safety of the test. Frequent false positives produce a low specificity that limits the practical value of the test.
Chest pain accounts for 2–4% of all new presentations at emergency departments in the UK.1 It is estimated that 30% of patients presenting with chest pain will be diagnosed with an acute coronary syndrome.2 “Rule‐out” pathways allow risk stratification of some groups of patients presenting with chest pain.3 This process facilitates the safe and early discharge of patients, many of whom will undergo further investigation (eg, stress testing) as outpatients during the next few weeks. This approach avoids inconvenient and costly hospital admission.4
The current gold standard for risk stratification of chest pain is the delayed measurement of cardiac troponin. Its prognostic sensitivity is highest from 8 h after the onset of pain5; hence patients often need to wait before blood can be analysed usefully. Emergency departments in the UK are under increasing pressure to process patients in less time.6 Therefore, a test that stratifies the risk of patients with chest pain more rapidly would be valuable, facilitating earlier discharge.
The measurement of ischaemia‐modified albumin (IMA), also referred to as albumin cobalt binding, could be such a test. It is a biochemical assay based on the observation that human albumin has the capacity to bind transition metals. In the presence of ischaemia (affecting the myocardium or elsewhere), the N terminus of albumin is modified and affects transition metal binding. This change is quantifiable.7
The aims of this study were
To investigate the diagnostic accuracy of presentation IMA, in addition to cardiac troponin, as a strategy to ascribe low risk to patients with chest pain in the emergency department.
To determine whether IMA can reduce transit time in emergency departments.
Methods
Design and setting
This prospective observational two‐centre study recruited patients from two emergency departments (belonging to the John Radcliffe Hospital, Oxford, UK; and the Frenchay Hospital, Bristol, UK). Each of them see about 80 000 new patients annually.
Study population
Consecutive adult patients presenting with features of possible ischaemic cardiac chest pain were eligible at the discretion of the consenting physician. Included patients were required to have a normal electrocardiogram (ECG), which was defined as the absence of all of the following:
ST segment elevation or depression ⩾0.5 mm.
T‐wave inversion ⩾1 mm (in leads other than III, aVR and V1).
Left bundle branch block.8
Exclusion criteria
The following patients were excluded:
Patients who had been in pain for >8 h on admission, because existing protocols specify immediate troponin analysis in this group.
Patients whose pain had ceased >2 h previously, because IMA levels fall rapidly once an ischaemic event has ended.
Asymptomatic patients, and those unable to relate the time that their symptoms began or ended (if the pain was not persisting).
Pregnant patients.
Patients on renal replacement therapy and those clinically diagnosed to have jaundice, as these conditions are known to influence IMA.
Study procedures
The index test (measurement of IMA and troponin I (TnI) at presentation) and reference standard (delayed TnI measurement, taken at least 8 h after pain onset) were applied to all recruited patients. However, the study had no effect on patient management, as standard and established protocols were followed and all clinicians were blinded to the results of the index test. After obtaining consent from patients, blood was taken according to usual practice, with an additional venous sample for measurement of IMA and TnI on presentation. A second blood test, to measure TnI, was taken 8 h after the onset of pain. This was made available to the treating clinician according to usual practice.
A copy of the admission ECG was retained for subsequent review by an expert in emergency medicine, who was independent of the study and unaware of the clinical presentation and blood results.
Laboratory methods
IMA measurements were made using a Beckman LX 20 analyser on serum samples frozen at −20°C. In a subset of the first 68 samples collected at Bristol, analysis was also carried out in real time, as would be the case if IMA were to be adopted into routine practice. All samples were handled according to the manufacturer's recommendations, with analysis taking place with a time lapse of no more than 2.5 h in the unfrozen state from collection to analysis. A value of ⩾86 was taken as positive, as advised by the manufacturer for this assay platform, with a corresponding coefficient of variation (CV) of 3.5%.
As troponin assays are carried out on different analysers, the assay and cut‐off values for TnI differ between Oxford and Bristol. Therefore, the reference TnI samples for the study were all analysed at Bristol, on a Beckman Access with a value of ⩾0.06 μg/l taken as positive. This value imparts the same 30‐day risk of death or myocardial infarction as the value of ⩾0.04 μg/l, which is the value that exceeds the 99th centile of a reference group.9
Follow‐up
No further follow‐up was arranged, because TnI measured 8 h after onset of pain is known to be an excellent risk stratification tool, predicting 30‐day outcome.5 This study was not powered to detect differences in clinical outcome such as mortality or myocardial infarction.
Outcome measurements
The primary outcome analysis compared TnI measured 8 h after onset of pain (the reference standard) with IMA and TnI measured at presentation to the emergency department (the index test).
The secondary outcome analysis compared the time the initial IMA and TnI sample was taken with the time the delayed TnI sample was taken. This indicated the potential time saving as a result of using IMA.
Sample size
This study sought to estimate the diagnostic properties of IMA with suitably narrow confidence intervals (CIs). On the basis of previous research, we assumed that the sensitivity of the new test would be approximately 95%.10 If 50 patients have a positive reference standard, then the 95% CIs around this estimate are +/− 6%. A previous study of troponin in the early risk stratification of chest pain enrolled consecutive patients in the emergency department and found that 15% had a raised troponin level.5 We assumed that the prevalence of raised delayed troponin would be similar in our population, which meant that a sample size of 330 patients would provide 50 patients with a positive reference standard. To allow for incomplete data, 400 patients were recruited.
Data analysis
If one or both of the presentation TnI or IMA measurements were raised, this was taken as a positive index test. This was compared with the reference standard of the delayed TnI result. From these data, sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio and negative likelihood ratio were calculated.
Receiver operating characteristic (ROC) curves were plotted for the frozen and fresh IMA samples, to determine whether a different “cut‐off” value for IMA would improve the properties of the test.
The median time interval between the initial and delayed drawing of blood (and hence the potential time saving from adopting IMA) was analysed using the Wilcoxon signed rank test for matched pairs.
Results
Recruitment started in October 2003 and finished in February 2005. Table 1 shows the patient characteristics.
Table 1 Patient characteristics.
| Oxford | Bristol | |
|---|---|---|
| No of patients | 238 | 161 |
| Median age, range (years) | 63, 27–98 | 59, 25–94 |
| Women | 87 | 61 |
| Men | 151 | 100 |
| Disease prevalence, 95% CI (%) | 12.2, 8.6 to 17.0 | 17.4, 8.6 to 17.0 |
Summary of patients receiving the index test and reference standard
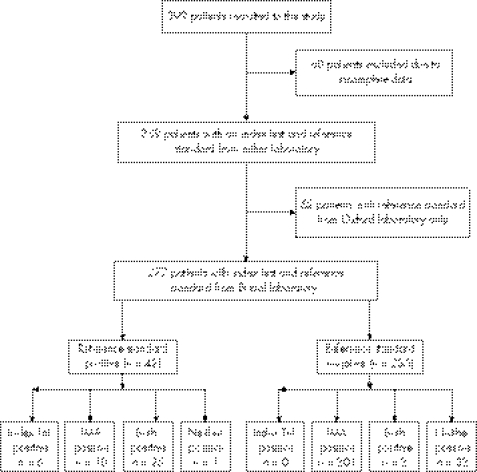
Figure 1 shows the availability of study data in the recruited patients. A total of 399 patients were recruited, but in 60 of these index or reference test data were missing, making analysis impossible. An additional 62 patients had no reference standard from the Bristol laboratory, but did have a reference standard (delayed TnI result) from the Oxford laboratory. Thus, there were 277 patients with per‐protocol data, and a further 62 with a valid index test result and a reference standard from the Oxford laboratory.
Figure 1 Flow diagram showing recruited patients and the availability of trial data. IMA, ischaemia‐modified albumin; TnI, troponin I.
Primary outcome measure
Table 2 shows the results of the primary analysis, including the 277 patients with a complete set of per‐protocol data.
Table 2 Primary analysis, comparing the results of the index test with the reference standard in all patients with a complete per‐protocol dataset.
| Index test | Delayed troponin | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 41 | 203 | 244 |
| Negative | 1 | 32 | 33 |
| Total | 42 | 235 | 277 |
Sensitivity analyses
In addition to the primary analysis, three sensitivity analyses (A–C) were carried out to further examine the robustness of the data. Sensitivity analysis A included all 339 patients with an index test and reference standard from either laboratory. This increased the number of patients than that specified in the original sample size calculation, and the results are shown in table 3. Table 3 is similar to table 2, except that the number of false negatives is increased from 1 to 4.
Table 3 Sensitivity analysis A, comparing the results of the index test with the reference standard from either laboratory.
| Index test | Delayed troponin | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 40 | 250 | 290 |
| Negative | 4 | 45 | 49 |
| Total | 44 | 295 | 339 |
Sensitivity analysis B used only fresh IMA data: this was not frozen but analysed in real time. This analysis would best reflect actual clinical practice if the test were introduced into routine care, but it contains smaller numbers because the fresh analysis was stopped midway through the study when recruitment fell below expected levels, leading to wastage of analytical materials. The numbers are much smaller (table 4), but similar to the primary analysis.
Table 4 Sensitivity analysis B, comparing the results of the index test with the reference standard in all patients with a fresh ischaemia‐modified albumin analysis.
| Index test | Delayed troponin | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 8 | 38 | 46 |
| Negative | 1 | 21 | 22 |
| Total | 9 | 59 | 68 |
Sensitivity analysis C excluded patients who were subsequently found, on ECG review by an independent expert in emergency medicine, to have an abnormal ECG, and thus to have been inappropriately recruited to the study. Of the 399 patients recruited, 294 had their ECGs reviewed by an independent expert; 234 (80%) of these were judged to be normal. Of these 234 patients, 201 had a complete per‐protocol dataset. The results are shown in table 5, and again resemble the primary analysis.
Table 5 Sensitivity analysis C, comparing the results of the index test with the reference standard in patients with a normal electrocardiogram.
| Index test | Delayed troponin | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 11 | 155 | 166 |
| Negative | 3 | 32 | 35 |
| Total | 14 | 187 | 201 |
Table 6 shows the calculated test properties of the primary analysis and all three sensitivity analyses, with 95% CIs.
Table 6 Properties of the index test in the primary analysis, and in all three sensitivity analyses.
| Property | Primary analysis (n = 277) | Sensitivity analysis A: any reference standard (n = 339) | Sensitivity analysis B: fresh IMA analysis (n = 68) | Sensitivity analysis C: normal ECG (n = 201) |
|---|---|---|---|---|
| Sensitivity (%) | 97.6 (87.4 to 99.9) | 90.9 (78.3 to 97.5) | 88.9 (56.5 to 99.4) | 78.6 (49.2 to 95.3) |
| Specificity (%) | 13.6 (9.5 to 18.7) | 15.3 (11.7 to 19.8) | 35.6 (24.6 to 48.3) | 17.1 (12.4 to 23.2) |
| Positive predictive value (%) | 16.8 (12.3 to 22.1) | 13.8 (10.1 to 18.4) | 17.4 (9.1 to 30.7) | 6.7 (3.4 to 11.7) |
| Negative predictive value (%) | 97.0 (84.2 to 99.9) | 92.0 (80.8 to 97.8) | 95.5 (78.2 to 99.8) | 91.4 (77.6 to 97) |
| Positive likelihood ratio | 1.1 (1.05 to 1.2) | 1.1 (1.0 to 1.2) | 1.4 (1.0 to 1.7) | 1.0 (0.7 to 1.3) |
| Negative likelihood ratio | 0.2 (0.02 to 1.2) | 0.6 (0.2 to 1.5) | 0.3 (0.05 to 2.0) | 1.2 (0.41 to 3.36) |
| Prevalence (%) | 15.2 (11.2 to 19.9) | 13.0 (9.6 to 17.0) | 13.2 (5.6 to 21.3) | 7.0 (3.9 to 11.4) |
ECG, electrocardiogram; IMA, ischaemia‐modified albumin.
95% CIs are shown in brackets.
ROC curves for frozen IMA results
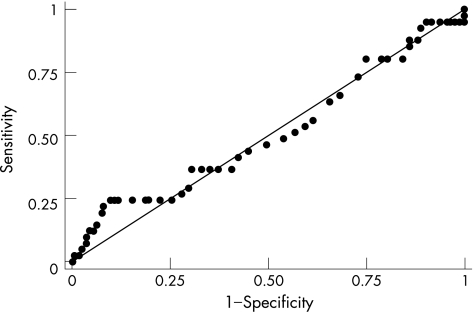
An ROC curve was plotted for the frozen IMA results (fig 2). The diagonal line indicates that the probability of a positive result given that the patient has the disease is equal to the probability of a positive result, given that the patient does not have the disease (ie, no better than guessing). The area under the curve is 0.512, with all points lying fairly close to the diagonal line.
Figure 2 Receiver operating characteristic curve for the frozen ischaemia‐modified albumin results.
Secondary outcome measure
The median interval time between the initial and delayed drawing of blood (n = 268) was 6 h and 10 min. This time saving was highly significant (p<0.001).
Discussion
The diagnostic accuracy of IMA in this study does not support its use as an effective risk stratification tool for patients in the emergency department with chest pain of possible ischaemic cardiac origin. The reported sensitivities are insufficiently high, and the large number of false positives generates a low specificity. The ROC curves for both the fresh and frozen IMA results show that if a higher cut‐off were used to improve specificity, this would have an unacceptable effect on the test's sensitivity. Therefore, there is no IMA cut‐off that will give high values for both sensitivity and specificity.
Emergency department chest pain pathways seek to facilitate safe and early discharge by accurately identifying patients at low risk of subsequent cardiac events. Delayed troponin has become established as an excellent test for this purpose, with the exception that it cannot often be measured usefully at the time of presentation. The index test of IMA and troponin on presentation achieved a negative predictive value of 97% and a sensitivity of 97.6% in the primary analysis, and lower values in all three sensitivity analyses, although with wide CIs. This suggests that patients discharged on the basis of a negative IMA and troponin on presentation would have a ⩾2% risk of subsequently developing raised troponin, and the associated cardiac risks. We believe that this would be unacceptable to most emergency clinicians, particularly when the wide CIs are taken into account.
The low specificity of the test is also a limitation, as in practice the number of patients with chest pain discharged on the basis of an initial IMA and TnI result would be very low (12% in our primary analysis). We showed a clear potential to save time and shorten stay in the emergency department, as expected. This seems to be the main potential benefit of IMA in the emergency department, but needs to be supported by adequate test performance to be clinically useful.
Previously published studies describing the clinical properties of IMA show substantial variation in setting, population, intervention and outcomes. Although most studies report high sensitivities, the specificities vary.10,11,12,13,14 Two groups have published results from the UK.10,15,16 Most studies have recruited all patients presenting with chest pain, rather than those entering a low‐risk “rule‐out” pathway. This increases disease prevalence, and may overestimate the test accuracy.17
We have considered IMA as a risk stratification tool in comparison to troponin, rather than as a quantifiable measure of cardiac ischaemia. Indeed, the meaning of raised IMA is not yet clearly understood. Some authors speculate that the high number of IMA false positives in this context is attributable to the properties of the test, suggesting that IMA is also a marker for reversible cardiac ischaemia.10,15 Although this is possible, the implications are unclear, and difficult to investigate further owing to the absence of any clear reference standard for cardiac ischaemia.18 It would also suggest that in our conventionally “low‐risk” cohort of patients, 86% had chest pain due to acute coronary syndrome or reversible cardiac ischaemia, which seems unlikely. A marker of reversible cardiac ischaemia without immediate prognostic implications would be of lesser interest to emergency doctors, who are usually seeking a means of rapid risk stratification.
Troponin and IMA were combined as the index test, as IMA levels fall rapidly once an ischaemic event has ended.19 Therefore, as IMA level decreases, troponin is likely to increase. However, despite excluding patients who had been pain free for >2 h at the time of presentation, it is interesting to note that of the 41 patients in the primary analysis who had a true positive index test, 6 had raised troponin alone, 25 had raised troponin and IMA, and 10 had raised IMA alone. Thus, not all patients with recent or persisting chest pain and raised troponin also have raised IMA, and the additional benefit of IMA over an admission troponin alone is relatively small. Interestingly, this is similar to the study by Collinson et al, where only 2 of 37 patients had raised IMA alone.16
Our findings are robust. The main results of the primary analysis are replicated throughout the three sensitivity analyses. The disease prevalence across the two centres is similar, although not identical, and at the prespecified level. Differences in disease prevalence may reflect the fact that a slightly different population has been recruited in the two centres, although this would be expected in a real‐world, two‐centre study, and the study demographics are comparable at both sites. This is a pragmatic study, reflected by the inadvertent entry into a “rule‐out” pathway of patients with an abnormal ECG. However, this is a clinical reality in an emergency department setting, and reflects the effectiveness of IMA in clinical practice, rather than its efficacy under optimal conditions. As a result, sensitivity analysis C is the least relevant, as it fails to reflect what would happen if the test were introduced into routine practice. Conversely, sensitivity analysis B best reflects the way that the test would be used in an emergency department, but is undermined by small numbers.
The major weakness of this study is the loss of data in 60 patients, and failure to obtain a Bristol reference standard in a further 62. Unfortunately, accurate record keeping required the cooperation of many clinicians, and some Oxford samples were lost during freezing and transport to Bristol. To reach our prespecified sample size, it was necessary to use an Oxford reference standard in 62 patients (sensitivity analysis A). This is disappointing and reduces the number of patients in the primary analysis; however, the results are consistent throughout.
Conclusion
Evidence to support the use of IMA as a risk stratification tool for possible ischaemic cardiac chest pain in emergency departments in the UK is insufficient from this study. Although the negative predictive value and the sensitivity are high, false negatives do occur and undermine the safety of the test. A low specificity also limits its usefulness. Economic analysis may be helpful to determine the effect of IMA on healthcare resources.
Abbreviations
ECG - electrocardiogram
IMA - ischaemia‐modified albumin
ROC - receiver operating characteristic
TnI - troponin I
Footnotes
Funding: Inverness Medical, the company that owns and markets IMA, met the laboratory and recruitment costs. Additional funding was received from a Boehringer Ingelheim research grant, administered by the College of Emergency Medicine.
Competing interests: None.
Ethical approval: Separate ethical approval was sought and obtained from the local ethics committee at both Oxford Radcliffe Hospital and North Bristol NHS Trust.
References
- 1.Fothergill N, Hunt M, Touquet R. Audit of patients with chest pain presenting to an accident and emergency department over a six‐month period. Arch Emerg Med 199310155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storrow A, Gibler W. Chest pain centers: diagnosis of acute coronary syndromes. Ann Emerg Med 2000449–460. [PubMed]
- 3.ACC/AHA Guidelines for the management of patients with unstable angina and non‐ST elevation myocardial infarction. J Am Coll Cardiol 200036970–1062. [DOI] [PubMed] [Google Scholar]
- 4.Goodacre S, Dixon S. Is a chest pain observation unit likely to be cost effective at my hospital? Extrapolation of data from a randomised controlled trial. Emerg Med J 200522418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamm C, Goldman B, Heeschen M D.et al Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. New Engl J Med 19973371648–1653. [DOI] [PubMed] [Google Scholar]
- 6.Department of Health (DoH) Emergency care. UK: DoH, 2004, http://www.dh.gov.uk/PolicyAndGuidance/OrganisationPolicy/EmergencyCare/EmergencyCareArticle/ (accessed 30 Aug 2005)
- 7.Bar Or D, Lau D, Rao N.et al Reduction in cobalt binding capacity of human albumin with myocardial ischemia. Ann Emerg Med 199934s56 [Google Scholar]
- 8.The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIa Investigators Randomized trial of intravenous heparin versus recombinant hirudin for acute coronary syndromes. Circulation 1994901631–1637. [DOI] [PubMed] [Google Scholar]
- 9.Morrow D A, Rifai N, Sabatine M S.et al Evaluation of the AccuTnI cardiac troponin I assay for risk assessment in acute coronary syndromes. Clin Chem 2003491396–1398. [DOI] [PubMed] [Google Scholar]
- 10.Sinha M, Roy D, Gaze D.et al The role of ischemia modified albumin (IMA), a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J 20042129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu A, Morris D L, Fletcher D R.et al Analysis of the albumin cobalt binding (ACB™) test as an adjunct to cardiac troponin for the early detection of acute myocardial infarction. Cardiovasc Toxicol 20011147–152. [DOI] [PubMed] [Google Scholar]
- 12.Kontos M, Schorer S, Kirk J D.et al Ischemia modified albumin: a new biomarker of myocardial ischemia for early diagnosis of acute coronary syndromes. J Am Coll Cardiol 200341(Suppl 2)340A [Google Scholar]
- 13.Anwaruddin S, Januzzi J L, Lewandrowski K B.et al Ischemia modified albumin improves the sensitivity and negative predictive value of standard cardiac biomarkers for the diagnosis of myocardial ischemia. J Am Coll Cardiol 200443(Suppl)258A [Google Scholar]
- 14.Bhagavan N, Lai E M, Rios P A.et al Evaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem 200349581–585. [DOI] [PubMed] [Google Scholar]
- 15.Roy D, Quiles J, Aldama G.et al Ischaemia modified albumin for the assessment of patients presenting to the emergency department with acute chest pain but normal or non‐diagnostic 12‐lead electrocardiograms and negative cardiac troponin T. Int J Cardiol 200497297–301. [DOI] [PubMed] [Google Scholar]
- 16.Collinson P, Gaze D, Bainbridge K.et al Utility of admission cardiac troponin and “ischemia modified albumin” measurements for rapid evaluation and rule out of suspected acute myocardial infarction in the emergency department. Emerg Med J 200623256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bossuyt P M, Reitsma J B, Bruns J B.et al Standards for reporting of diagnostic accuracy. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration, Clin Chem 2003497–18. [DOI] [PubMed] [Google Scholar]
- 18.Adams J. Markers to define ischemia: are they ready for prime time use in patients with acute coronary syndromes? Curr Cardiol Rep 20046253–258. [DOI] [PubMed] [Google Scholar]
- 19.Bar‐Or D, Winkler J V, VanBenthuysen K.et al Reduced albumin‐cobalt binding with transient myocardial ischaemia after elective percutaneous transluminal coronary angioplasty: a preliminary comparison to creatinine kinase‐MB, myoglobin, and troponin I. Am Heart J 2001141985–991. [DOI] [PubMed] [Google Scholar]