Abstract
Objective
To describe the triage of patients operated for non‐ruptured and ruptured abdominal aortic aneurysms (AAAs) before the endovascular era.
Design
Retrospective single‐centre cohort study.
Methods
All patients treated for an acute AAA between 1998 and 2001 and admitted to our hospital were evaluated in the emergency department for urgent AAA surgery. All time intervals, from the telephone call from the patient to the ambulance department, to the arrival of the patient in the operating theatre, were analysed. Intraoperative, hospital and 1‐year survival were determined.
Results
160 patients with an acute AAA were transported to our hospital. Mean (SD) age was 71 (8) years, and 138 (86%) were men. 34 (21%) of these patients had symptomatic, non‐ruptured AAA (sAAA) and 126 patients had ruptured AAA (rAAA). All patients with sAAA and 98% of patients with rAAA were operated upon. For the patients with rAAA, median time from telephone call to arrival at the hospital was 43 min (interquartile range 33–53 min) and median time from arrival at the hospital to arrival at the operating room was 25 min (interquartile range 11–50 min). Intraoperative mortality was 0% for sAAA and 11% for rAAA (p = 0.042), and hospital mortality was 12% and 33%, respectively (p = 0.014).
Conclusions
A multidisciplinary unified strategy resulted in a rapid throughput of patients with acute AAA. Rapid transport, diagnosis and surgery resulted in favourable hospital mortality. Despite the fact that nearly all the patients were operated upon, survival was favourable compared with published data.
As untreated ruptured abdominal aortic aneurysm (AAA) has an almost 100% mortality, rapid diagnosis and treatment are essential goals. Nevertheless, several recent series report a hospital mortality >50% for patients with acute AAAs.1,2,3 These figures include only patients who arrive alive at the hospital. Therefore, total mortality for acute AAA may approximate 80–90%.4,5,6 Delay in treatment might significantly influence mortality; therefore, measures to reduce time to surgery can be valuable in decreasing mortality. Very few studies describe the delay to surgery.7,8,9,10 AbuRahma et al8 describe a mortality of 73% in patients operated after a delay of >2 h, whereas patients who were operated within 2 h had a mortality of 48% (p<0.05). Both transportation by ambulance and intrahospital management protocol have an influence on delays to surgery.
This study aims to present the results of a strategy of direct ambulance transport to our tertiary referral centre when acute AAA was suspected, followed by minimal diagnostics and emergency surgery for nearly all patients.
Materials and methods
Patients
This is a retrospective cohort study of consecutive patients undergoing emergency surgery for acute AAA at a university centre. All patients admitted alive to our hospital with acute AAAs between 1998 and 2001 were included in the study. Patients were identified from the ambulance database, hospital and medical records using the International Classification of Diseases—Ninth Revision codes and the search terms aneurysm, aorta and aortic surgery.
A rural and stable population provides the referral base for our hospital. Information on survival was retrieved from hospital databases.
Definitions
Acute AAA was defined as any AAA requiring treatment within 24 h. Patients were classified as having an acute symptomatic, non‐ruptured AAA (sAAA) or ruptured AAA (rAAA), according to the absence or presence of discontinuation of the integrity of the aortic wall with blood extravasation as shown on computerised tomography or as found during surgery. Haemorrhagic shock was defined as a systolic blood pressure ⩽100 mm Hg before surgery. Hospital mortality was calculated for all patients who reached the operating theatre alive. Patients treated with emergency endovascular repair during the same period were excluded.
Transport time was defined as the time from telephone call to the ambulance service to arrival in the emergency room. Intrahospital delay was defined as time from arrival of the patient in the emergency department to arrival in the operating theatre. Total delay to surgery was defined as the sum of transport time and intrahospital delay. Distance of transport was defined as the distance from the home address of the patient to our hospital.
Management strategy
When ambulance personnel suspected that the patient had a ruptured aneurysm, our hospital was alerted, so that an experienced vascular surgeon and a radiologist were present when the patient arrived. At the same time, the operating room was alerted. The ambulance personnel inserted two intravenous lines and initiated fluid resuscitation only in the case of shock (ie, systolic blood pressure ⩽100 mm Hg) with altered mental state. On arrival, the diagnosis was confirmed by physical examination, ultrasound and, more recently, computerised tomography to evaluate emergency endovascular repair as a treatment option. For a patient in haemodynamic shock with a palpable aneurysm, no ultrasound was carried out and the patient was immediately transported to the operating theatre. Until aortic clamping was carried out in the operating theatre, we accepted a systolic blood pressure of ⩽100 mm Hg, to reduce retroperitoneal blood losses or to prevent free rupture in the peritoneal cavity. Patients who had a cardiac arrest during transport or in the emergency room but were successfully resuscitated were also offered surgery. Exclusion criteria for surgery were prolonged cardiac arrest despite resuscitation, advanced Alzheimer's disease and a poor Karnofsky performance score11 (⩽40; ie, the patient is disabled and requires special care and help), or severe cardiovascular disease associated with a New York Health Association‐IV performance score. If the clinical picture was unclear or unknown, the patient was still offered surgery.
Surgery was performed with at least one experienced vascular surgeon present. In the operating theatre, a rapid sequence induction of anaesthesia and intubation was carried out after prepping and draping the abdomen. After surgery, all patients remained intubated and mechanically ventilated and were transferred to a tertiary intensive care unit (ICU). This ICU is staffed with nurses in a 1.5:1 patient–nurse ratio, with onsite doctors dedicated solely to the ICU for 24 h a day and supervised by certified intensivists.
Statistics
Data are reported as mean and standard deviation (SD), or as median with interquartile ranges for skewed data. The Student's t test was used to compare normally distributed continuous variables, and the Mann–Whitney U test was used for continuous variables with a skewed distribution. Differences between categorical variables were tested by χ2 analysis. Cumulative survival was calculated using Kaplan–Meier analysis. The log rank test was used to compare survival curves between patients with sAAA and those with rAAA. p Values <0.05 were regarded as significant.
Results
Patient population
During the study period, 160 patients with acute aneurysms were operated upon: 34 with sAAA and 126 with rAAA. An additional 15 patients who received emergency endovascular treatment for their acute AAA were excluded. Table 1 describes the demographics of this population. Age and sex did not differ between patients with sAAA and rAAA; nor did comorbidity profiles between these two groups.
Table 1 Demographic data of patients operated for symptomatic aneurysms.
| All patients n = 160 | sAAA n = 34 | rAAA n = 126 | p Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years), mean (SD) | 71 (8) | 71 (7) | 71 (8) | NS |
| Male sex, n (%) | 138 (86) | 29 (85) | 109 (87) | NS |
| Cerebrovascular disease, n (%) | 18 (11) | 2 (6) | 16 (12) | NS |
| Chronic obstructive pulmonary disease, n (%) | 37 (23) | 7 (21) | 30 (24) | NS |
| Chronic renal failure, n (%) | 12 (8) | 3 (9) | 9 (7) | NS |
| Congestive heart failure, n (%) | 17 (11) | 4 (12) | 13 (10) | NS |
| Coronary artery disease, n (%) | 62 (39) | 15 (44) | 47 (37) | NS |
| Diabetes mellitus, n (%) | 15 (9) | 3 (9) | 12 (10) | NS |
| Hypertension, n (%) | 50 (31) | 11 (32) | 39 (31) | NS |
NS, not significant; rAAA, ruptured AAA; sAAA, symptomatic, non‐ruptured AAA.
All 34 patients presenting to the emergency department with an sAAA were operated upon. In total, 126 patients with rAAA were evaluated for surgery, of which only three (2.4%) patients did not undergo surgery, because of severe cardiovascular disease with a New York Health Association‐IV performance score.
Transport time and intrahospital delay
Median transport time for the 34 patients with sAAA was 43 min (interquartile range 33–52 min). The median distance of transport (between patient pick‐up and hospital) was 22 km (16–31 km). Median intrahospital delay was 160 min (53–452 min).
Median transport time of the 126 operated patients with rAAA was 43 min (33–53 min). The median distance of transport was 22 km (4–30 km). Median intrahospital delay was 25 min (11–50 min). Median total delay to surgery was 67 min (52–102 min; table 2). Of these patients, 66% were referred by a general practitioner or directly by the ambulance services, and 34% were referred from another hospital.
Table 2 Blood pressure at arrival at the emergency department, delay to surgery, diagnostic approach, level of aortic clamping and length of stay of patients operated for symptomatic aneurysms.
| All patients n = 160 | sAAA n = 34 | rAAA n = 126 | p Value | |
|---|---|---|---|---|
| Systolic blood pressure at arrival | ||||
| Cardiac arrest | 8 (5%) | 0 | 8 (6%) | NS |
| RR 20–60 mm Hg | 31 (19%) | 0 | 31 (25%) | <0.01 |
| RR 60–100 mm Hg | 65 (41%) | 7 (21%) | 58 (46%) | NS |
| RR >100 mm Hg | 56 (35%) | 27 (79%) | 29 (23%) | NS |
| Delay to surgery (min) | ||||
| Transport time | 42 (33–49) | 43 (33–52) | 43 (33–53) | NS |
| Intrahospital delay | 29 (12–61) | 160 (53–452) | 25 (11–50) | <0.001 |
| Diagnostic investigation | ||||
| Physical examination only | 70 (43%) | 8 (24%) | 62 (49%) | 0.007 |
| Ultrasound | 40 (25%) | 8 (24%) | 32 (25%) | NS |
| Computerised tomography | 65 (41%) | 23 (68%) | 42 (33%) | <0.001 |
| Intraoperative data | ||||
| Suprarenal clamping (yes) | 8 (5%) | 0 | 8 (6%) | NS |
| Length of stay (days), median (IQR) | ||||
| Intensive care | 3 (1–8) | 2 (1–3) | 4 (2–10) | 0.001 |
| Hospital | 12 (8–18) | 9 (7–11) | 14 (9–21) | 0.001 |
IQR, interquartile range; NS, not significant; rAAA, ruptured AAA; sAAA, symptomatic, non‐ruptured AAA.
The diagnostic investigation was different in patients with sAAA and rAAA: an ultrasound or computed tomography scan was used in 76% and 51%, respectively (p = 0.007).
Outcome
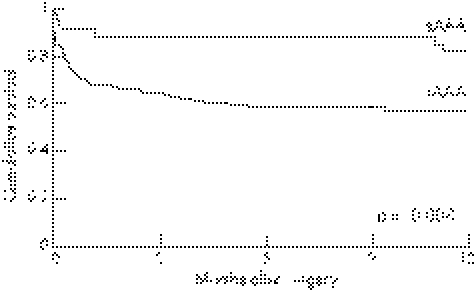
Overall intraoperative mortality was 0 for patients with sAAA and 14 (11%) for patients with rAAA (p = 0.042). Thirty‐day and hospital mortality was 3 (9%) and 4 (12%), respectively, for sAAA. Thirty‐day and hospital mortality for patients with rAAA was 38 (30%) and 42 (33%), respectively (p = 0.011 and 0.014). Haemorrhagic shock was observed in 7 (21%) patients with sAAA versus 97 (77%) patients with rAAA. None of the patients with sAAA and eight with rAAA had a cardiac arrest during ambulance transport or in the emergency department. Four of these patients died intraoperatively, two died in the ICU and another two patients survived until discharge from hospital. Of these two patients, one died after 4 months; the other is still alive after >5 years of follow‐up. One‐year mortality was 6 (18%) for sAAA and 54 (43%) for rAAA, see figure 1 (p = 0.008; log rank). Follow‐up was complete in all patients.
Figure 1 Kaplan–Meier curve of the 1‐year survival in patients with symptomatic, non‐ruptured abdominal aortic aneurysm (sAAA) and ruptured abdominal aortic aneurysm (rAAA).
Discussion
This study shows that a unified strategy for the treatment of patients with acute AAAs is associated with a hospital mortality of 12% and 35%, for sAAA and rAAA, respectively, despite offering surgery to >95% of all patients. We infer that the combined transport time and intrahospital delay of only 67 min has contributed to this relatively favourable outcome.
The survival curves for sAAA and rAAA suggest that longer‐term survival is similar, which reflects the similar effect of associated comorbidities.
In the literature, hospital mortality for patients operated for rAAA ranges from 20% to 70%.1,2,12,13,14 This wide range is partly explained by selection bias, with some centres having restrictive and others liberal indications for surgery. This, however, is not the only explanation, as in some series12,13,15,16,17 in which surgery was offered to an average of 73% (95% confidence interval (CI) 72% to 74%) of the patients with ruptured aneurysms, the cumulative hospital mortality was 40% (95% CI 38% to 41%).
The average time of 43 min between the emergency call and arrival of patients at our emergency department reflects the efficiency of our transport system and the size of our catchment area in the northern Netherlands. In addition, the fact that the paramedics alert the hospital before their arrival and that the vascular surgeon and the radiology staff are in hospital at the time of the patients' arrival reduces intrahospital delay.
Many other factors affect outcome after acute AAA. Another important determinant of outcome is the experience and the specialty of the operating surgeon, with worse survival reported for less experienced surgeons or for general surgeons than for vascular surgeons.18,19,20 Postoperative treatment also affects outcome. Sandison et al21 observed marked differences in mortality between two ICUs that received patients operated by the same surgical team. Thus, the availability of a well‐staffed surgical ICU may also have been beneficial for the prognosis of our patients.
Comparison of results between hospitals and surgeons is confounded by patient selection, modality of patient transport, distances, surgical experience, postoperative care, definition of symptomatic aneurysms and of haemodynamic stability. The retrospective design of our study limits the inferences that may be drawn. However, our extensive search for patients with symptomatic aneurysms (including hospital, doctor and emergency services records), the regional pattern of referral and the completeness of our follow‐up provide an insight to the results that may be obtained with a unified strategy of aggressive management of patients with symptomatic aneurysms.
The clinical implications of our findings may vary depending on the setting in which treatment of acute AAAs is performed. As geographical conditions that determine the distance that patients should travel vary widely, the effectiveness of measures to limit transport delay may also vary. Faster means of transportation and a protocol for the management of these patients are strategies to improve results.
An additional approach may be the implementation of an endovascular programme for the treatment of symptomatic aneurysms. In elective AAA repair, endovascular treatment has shown reduced organ dysfunction and lower 30‐day mortality than in open AAA repair,22,23 although long‐term survival is comparable.24,25 Endovascular treatment for symptomatic AAAs showed promising results; however, suitability was low.26 Furthermore, a potential hazard of this approach is the inevitable delay to surgery caused by the necessary computed tomography. Therefore, the effectiveness of emergency endovascular procedures in the treatment of rAAA needs confirmation in prospective clinical trials.
Further studies on the management of acute AAA should include a description of the protocols used, and report the delays involved as well as the number and type of patients who were denied surgical treatment.
Conclusion
In an environment and an ambulance system that has short delays of transportation, a unified strategy of treatment of acute AAA provides hospital survivals comparable to the best reported in the literature, despite a policy of offering surgery to most patients.
Acknowledgements
We thank C S Cinà for his valuable comments on this article.
Abbreviations
AAA - abdominal aortic aneurysm
ICU - intensive care unit
rAAA - ruptured abdominal aortic aneurysm
sAAA - symptomatic, non‐ruptured abdominal aortic aneurysm
Footnotes
Competing interests: None declared.
References
- 1.Lawrence P F, Gazak C, Bhirangi L.et al The epidemiology of surgically repaired aneurysms in the United States. J Vasc Surg 199930632–640. [DOI] [PubMed] [Google Scholar]
- 2.Bown M J, Sutton A J, Bell P R.et al A meta‐analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg 200289714–730. [DOI] [PubMed] [Google Scholar]
- 3.Dueck A D, Kucey D S, Johnston K W.et al Long‐term survival and temporal trends in patient and surgeon factors after elective and ruptured abdominal aortic aneurysm surgery. J Vasc Surg 2004391261–1267. [DOI] [PubMed] [Google Scholar]
- 4.Kantonen I, Lepantalo M, Brommels M.et al Mortality in ruptured abdominal aortic aneurysms. The Finnvasc Study Group. Eur J Vasc Endovasc Surg 199917208–212. [DOI] [PubMed] [Google Scholar]
- 5.Bengtsson H, Bergqvist D. Ruptured abdominal aortic aneurysm: a population‐based study. J Vasc Surg 19931874–80. [DOI] [PubMed] [Google Scholar]
- 6.Heikkinen M, Salenius J P, Auvinen O. Ruptured abdominal aortic aneurysm in a well‐defined geographic area. J Vasc Surg 200236291–296. [DOI] [PubMed] [Google Scholar]
- 7.Farooq M M, Freischlag J A, Seabrook G R.et al Effect of the duration of symptoms, transport time, and length of emergency room stay on morbidity and mortality in patients with ruptured abdominal aortic aneurysms. Surgery 19961199–14. [DOI] [PubMed] [Google Scholar]
- 8.AbuRahma A F, Woodruff B A, Lucente F C.et al Factors affecting survival of patients with ruptured abdominal aortic aneurysm in a West Virginia community. Surg Gynecol Obstet 1991172377–382. [PubMed] [Google Scholar]
- 9.Johansen K, Kohler T R, Nicholls S C.et al Ruptured abdominal aortic aneurysm: the Harborview experience. J Vasc Surg 199113240–245. [PubMed] [Google Scholar]
- 10.Adam D J, Mohan I V, Stuart W P.et al Community and hospital outcome from ruptured abdominal aortic aneurysm within the catchment area of a regional vascular surgical service. J Vasc Surg 199930922–928. [DOI] [PubMed] [Google Scholar]
- 11.Karnofsky D A, Abelman W H, Craver L F.et al The use of nitrogen mustards in palliative treatment of carcinoma. Cancer 19481634–656. [Google Scholar]
- 12.Basnyat P S, Biffin A H, Moseley L G.et al Mortality from ruptured abdominal aortic aneurysm in Wales. Br J Surg 199986765–770. [DOI] [PubMed] [Google Scholar]
- 13.Norman P E, Semmens J B, Lawrence‐Brown M M.et al Long term relative survival after surgery for abdominal aortic aneurysm in western Australia: population based study. BMJ 1998317852–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford E S. Ruptured abdominal aortic aneurysm. J Vasc Surg 199113348–350. [DOI] [PubMed] [Google Scholar]
- 15.Evans S M, Adam D J, Bradbury A W. The influence of gender on outcome after ruptured abdominal aortic aneurysm. J Vasc Surg 200032258–262. [DOI] [PubMed] [Google Scholar]
- 16.Bradbury A W, Makhdoomi K R, Adam D J.et al Twelve‐year experience of the management of ruptured abdominal aortic aneurysm. Br J Surg 1997841705–1707. [PubMed] [Google Scholar]
- 17.Dueck A D, Johnston K W, Alter D.et al Predictors of repair and effect of gender on treatment of ruptured abdominal aortic aneurysm. J Vasc Surg 200439784–787. [DOI] [PubMed] [Google Scholar]
- 18.Ouriel K, Geary K, Green R M.et al Factors determining survival after ruptured aortic aneurysm: the hospital, the surgeon, and the patient. J Vasc Surg 199011493–496. [DOI] [PubMed] [Google Scholar]
- 19.Dimick J B, Cowan J A, Jr, Stanley J C.et al Surgeon specialty and provider volumes are related to outcome of intact abdominal aortic aneurysm repair in the United States. J Vasc Surg 200338739–744. [DOI] [PubMed] [Google Scholar]
- 20.Pearce W H, Parker M A, Feinglass J.et al The importance of surgeon volume and training in outcomes for vascular surgical procedures. J Vasc Surg 199929768–776. [DOI] [PubMed] [Google Scholar]
- 21.Sandison A J, Wyncoll D L, Edmondson R C.et al ICU protocol may affect the outcome of non‐elective abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 199816356–361. [DOI] [PubMed] [Google Scholar]
- 22.Greenhalgh R M, Brown L C, Kwong G P.et al Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30‐day operative mortality results: randomised controlled trial. Lancet 2004364843–848. [DOI] [PubMed] [Google Scholar]
- 23.Prinssen M, Verhoeven E L, Buth J.et al A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 20043511607–1618. [DOI] [PubMed] [Google Scholar]
- 24.EVAR trial participants Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 20053652179–2186. [DOI] [PubMed] [Google Scholar]
- 25.Blankensteijn J D, de Jong S E, Prinssen M.et al Two‐year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med 20053522398–2405. [DOI] [PubMed] [Google Scholar]
- 26.Kapma M R, Verhoeven E L, Tielliu I F.et al Endovascular treatment of acute abdominal aortic aneurysm with a bifurcated stentgraft. Eur J Vasc Endovasc Surg 200529510–515. [DOI] [PubMed] [Google Scholar]