Abstract
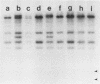
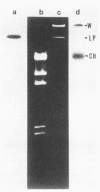
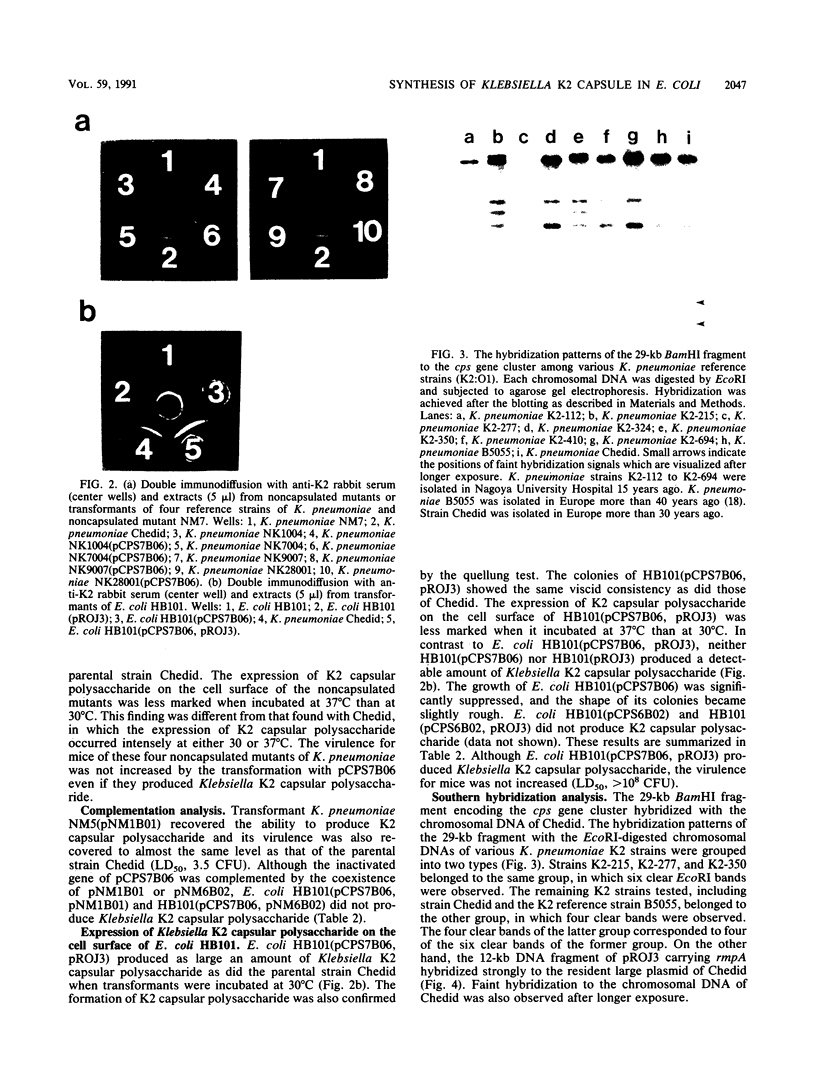
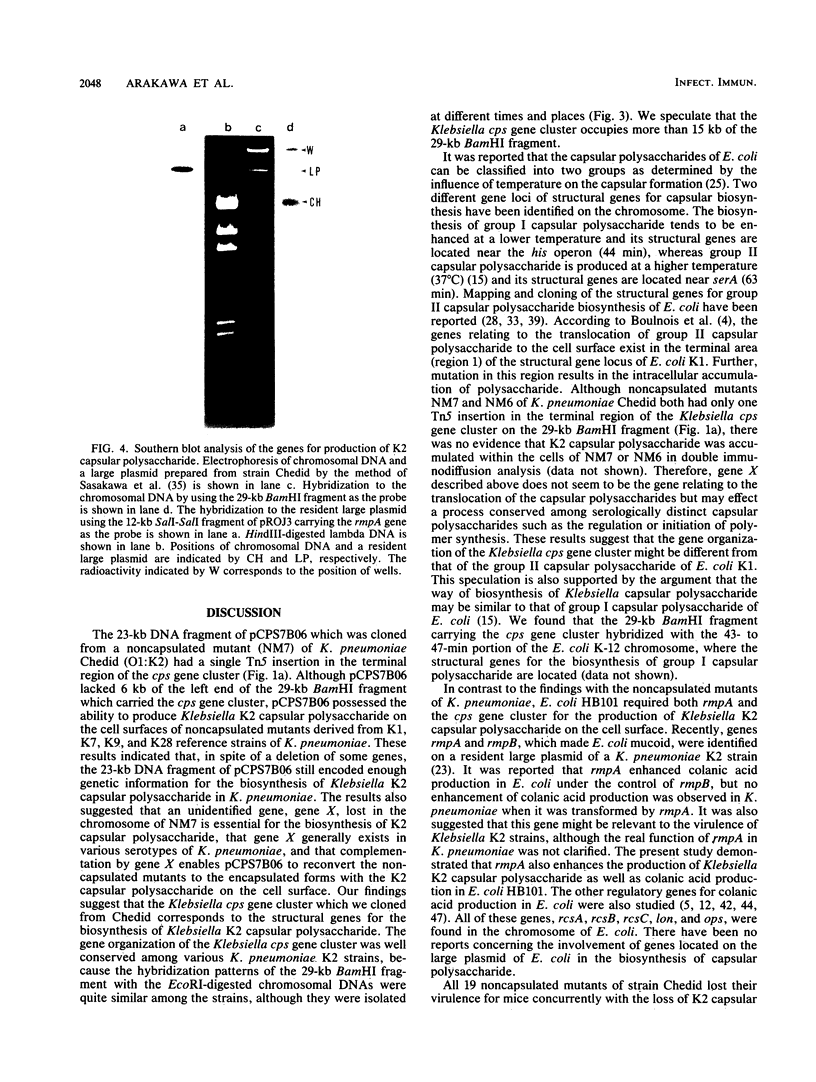
The genes determining the biosynthesis of type 2 (K2) capsular polysaccharide [3----beta Glc1,4----beta Man(1,3----beta GlcUA) 1,4----alpha Glc1----] of Klebsiella pneumoniae Chedid (O1:K2), which is highly virulent for mice, were cloned and introduced into Escherichia coli HB101 and into four noncapsulated mutants derived from K. pneumoniae reference strains of K1, K7, K9, and K28. The recombinant plasmid pCPS7B06 carried 23 kb of a chromosomal DNA fragment of strain Chedid and encoded a part of the Klebsiella cps gene cluster. However, pCPS7B06 encoded enough genetic information for the production of Klebsiella K2 capsular polysaccharide on the cell surfaces of four noncapsulated mutants of K. pneumoniae. On the other hand, both pCPS7B06 and pROJ3 carrying the rmpA gene locus derived from a resident large plasmid of Chedid were required for the biosynthesis of Klebsiella K2 capsular polysaccharide on the cell surface of E. coli HB101. The insertion inactivation analysis using Tn5 revealed that the cps gene cluster occupied more than 15 kb of the chromosome of Chedid. We conclude that rmpA, which has been known to enhance the biosynthesis of colanic acid in E. coli, is also involved in the biosynthesis of Klebsiella capsular polysaccharide in E. coli HB101.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa Y., Ohta M., Kido N., Mori M., Ito H., Komatsu T., Fujii Y., Kato N. Chromosomal beta-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1989 Jan;33(1):63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATSHON B. A., BAER H., SHAFFER M. F. Immunologic paralysis produced in mice by Klebsiella pneumoniae type 2 polysaccharide. J Immunol. 1963 Jan;90:121–126. [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S., Hodge R., Hardy K. R., Jann K. B., Timmis K. N. Analysis of the K1 capsule biosynthesis genes of Escherichia coli: definition of three functional regions for capsule production. Mol Gen Genet. 1987 Jun;208(1-2):242–246. doi: 10.1007/BF00330449. [DOI] [PubMed] [Google Scholar]
- Brill J. A., Quinlan-Walshe C., Gottesman S. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli K-12. J Bacteriol. 1988 Jun;170(6):2599–2611. doi: 10.1128/jb.170.6.2599-2611.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. S., Gemski P., Sadoff J. C., Orskov F., Orskov I. The importance of the K1 capsule in invasive infections caused by Escherichia coli. J Infect Dis. 1984 Feb;149(2):184–193. doi: 10.1093/infdis/149.2.184. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Mortimer P. M., Mansfield V., Germanier R. Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. J Clin Microbiol. 1986 Apr;23(4):687–690. doi: 10.1128/jcm.23.4.687-690.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenico P., Diedrich D. L., Straus D. C. Extracellular polysaccharide production by Klebsiella pneumoniae and its relationship to virulence. Can J Microbiol. 1985 May;31(5):472–478. doi: 10.1139/m85-088. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Trisler P., Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985 Jun;162(3):1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Jann B., Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- Kaijser B., Hanson L. A., Jodal U., Lidin-Janson G., Robbins J. B. Frequency of E. coli K antigens in urinary-tract infections in children. Lancet. 1977 Mar 26;1(8013):663–666. doi: 10.1016/s0140-6736(77)92111-0. [DOI] [PubMed] [Google Scholar]
- Kiseleva B. S., Krasnogolovets V. N. Rol' Klebsiella pneumoniae v étiologii bakterial'nogo sepsisa. Zh Mikrobiol Epidemiol Immunobiol. 1983 Feb;(2):20–25. [PubMed] [Google Scholar]
- Mizuta K., Ohta M., Mori M., Hasegawa T., Nakashima I., Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 1983 Apr;40(1):56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Ohta M., Agata N., Kido N., Arakawa Y., Ito H., Komatsu T., Kato N. Identification of species and capsular types of Klebsiella clinical isolates, with special reference to Klebsiella planticola. Microbiol Immunol. 1989;33(11):887–895. doi: 10.1111/j.1348-0421.1989.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Nassif X., Honoré N., Vasselon T., Cole S. T., Sansonetti P. J. Positive control of colanic acid synthesis in Escherichia coli by rmpA and rmpB, two virulence-plasmid genes of Klebsiella pneumoniae. Mol Microbiol. 1989 Oct;3(10):1349–1359. doi: 10.1111/j.1365-2958.1989.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Sharma V., Orskov I. Influence of growth temperature on the development of Escherichia coli polysaccharide K antigens. J Gen Microbiol. 1984 Oct;130(10):2681–2684. doi: 10.1099/00221287-130-10-2681. [DOI] [PubMed] [Google Scholar]
- Orskov I., Sharma V., Orskov F. Genetic mapping of the K1 and K4 antigens (L) of Escherichia coli. Non-allelism of K(L) antigens with K antigens of O8:K27(A), O8:K8(L) and O9:K57(B). Acta Pathol Microbiol Scand B. 1976 Jun;84(3):125–131. [PubMed] [Google Scholar]
- Pelkonen S., Häyrinen J., Finne J. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J Bacteriol. 1988 Jun;170(6):2646–2653. doi: 10.1128/jb.170.6.2646-2653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Quie P. G. Bacterial surface components and the pathogenesis of infectious diseases. Annu Rev Med. 1981;32:29–43. doi: 10.1146/annurev.me.32.020181.000333. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Roberts I., Mountford R., High N., Bitter-Suermann D., Jann K., Timmis K., Boulnois G. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Murayama S. Y., Makino S., Yoshikawa M. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect Immun. 1986 Feb;51(2):470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Yoshikawa M. A series of Tn5 variants with various drug-resistance markers and suicide vector for transposon mutagenesis. Gene. 1987;56(2-3):283–288. doi: 10.1016/0378-1119(87)90145-4. [DOI] [PubMed] [Google Scholar]
- Schiffer M. S., Oliveira E., Glode M. P., McCracken G. H., Jr, Sarff L. M., Robbins J. B. A review: relation between invasiveness and the K1 capsular polysaccharide of Escherichia coli. Pediatr Res. 1976 Feb;10(2):82–87. doi: 10.1203/00006450-197602000-00002. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Vann W. F., Aaronson W. Genetic and molecular analyses of Escherichia coli K1 antigen genes. J Bacteriol. 1984 Feb;157(2):568–575. doi: 10.1128/jb.157.2.568-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoons-Smit A. M., Verweij-van Vught A. M., MacLaren D. M. The role of K antigens as virulence factors in Klebsiella. J Med Microbiol. 1986 Mar;21(2):133–137. doi: 10.1099/00222615-21-2-133. [DOI] [PubMed] [Google Scholar]
- Stauffer G. V., Plamann M. D., Stauffer L. T. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene. 1981 Jun-Jul;14(1-2):63–72. doi: 10.1016/0378-1119(81)90148-7. [DOI] [PubMed] [Google Scholar]
- Stout V., Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990 Feb;172(2):659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Kido N., Arakawa Y., Mori M., Naito S., Ohta M., Kato N. Rapid small-scale preparation method of cell surface polysaccharides. Microbiol Immunol. 1990;34(7):635–641. doi: 10.1111/j.1348-0421.1990.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Trisler P., Gottesman S. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1984 Oct;160(1):184–191. doi: 10.1128/jb.160.1.184-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij-Van Vught A. M., Namavar F., Peerbooms P. G., Sparrius M., Maclaren D. M. The role of different K antigens of Escherichia coli in phagocytosis by polymorphonuclear leukocytes. J Med Microbiol. 1984 Apr;17(2):141–150. doi: 10.1099/00222615-17-2-141. [DOI] [PubMed] [Google Scholar]
- Zinkewich-Péotti K., Fraser J. M. New locus for exopolysaccharide overproduction in Escherichia coli K-12. J Bacteriol. 1988 Mar;170(3):1405–1407. doi: 10.1128/jb.170.3.1405-1407.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ØRSKOV I. O antigens in the Klebsiella group. Acta Pathol Microbiol Scand. 1954;34(2):145–156. doi: 10.1111/j.1699-0463.1954.tb00811.x. [DOI] [PubMed] [Google Scholar]