Abstract
Aims
To determine the prevalence of overweight and obesity among patients with type 1 and type 2 diabetes mellitus attending a secondary care diabetes clinic in the United Kingdom, and to assess the impact of overweight and obesity on glycaemic control and cardiovascular risk factors in patients with type 2 diabetes.
Methods
3637 patients with diabetes were identified from the hospital electronic diabetes register, 916 with type 1 diabetes (mean (SD) age 40.4 (15.1) years, 496 male) and 2721 with type 2 diabetes (mean (SD) age 62.5 (11.8) years, 1436 male). Data on body mass index (BMI), glycaemic control, lipid profiles, and blood pressure were extracted.
Results
Of patients with type 1 diabetes, 55.3% were overweight (BMI ⩾25 kg/m2), 16.6% were obese (BMI ⩾30 kg/m2), and 0.4% had morbid obesity (BMI ⩾40 kg/m2). In contrast, 86% of patients with type 2 diabetes were overweight or obese, 52% were obese, and 8.1% had morbid obesity. Obese patients with type 2 diabetes were younger, had poorer glycaemic control, higher blood pressures, worse lipid profiles, and were more likely to be receiving antihypertensive and lipid lowering drugs compared with patients with BMI <30 kg/m2.
Conclusions
Obesity is the rule among patients attending this hospital diabetes clinic, with 86% of those with type 2 diabetes overweight or obese. Obesity is associated with significantly worse cardiovascular risk factors in this patient group, suggesting that more active interventions to control weight gain would be appropriate.
Keywords: cardiovascular disease, diabetes mellitus, obesity, secondary care
Obesity is the major potentially modifiable risk factor for type 2 diabetes.1 Intervention studies in which modest weight loss has been achieved by lifestyle interventions in overweight subjects with impaired glucose tolerance,2,3,4 including in combination with antiobesity drug treatment,5 show that weight loss is associated with improved insulin‐glucose homoeostasis and a reduced risk of developing diabetes. It is already well established that those developing type 2 diabetes have a higher body weight than control populations,6 reflecting the strong epidemiological association between obesity and the development of diabetes.
In contrast, much less attention has been paid to the significance of obesity in clinic populations with diabetes. This is of important interest because obesity is an independent risk factor for cardiovascular disease,7,8 an effect likely to be mediated, at least in part through its known associations with the metabolic syndrome. Increased incidence and deaths from cardiovascular disease are the major challenge in both type 29 and type 1 diabetes.10 Modest weight loss leads to considerable improvements in all aspects of cardiovascular disease risk.11 In type 2 diabetes, weight loss has a beneficial effect on indices of glycaemic control and treatment requirements, and those who achieve pronounced weight loss experience normalisation of insulin sensitivity and blood glucose concentrationss.12 In clinical practice this is best exemplified by bariatric surgery in obese type 2 diabetic patients, which often leads to enduring remission of the disease.13
At present, the prevalence and impact of obesity on clinical workload and services provided for people with diabetes has not attracted much attention, and comparatively few obese patients with diabetes currently are offered the option of structured weight management as an integral part of their treatment. This analysis was undertaken to determine the prevalence of overweight and obesity, together with their association with biochemical and clinical end points in patients with type 2 diabetes attending a large hospital diabetic clinic in the United Kingdom.
Patients and methods
The study population comprised 3637 patients identified from the hospital electronic diabetes register. This register includes all routinely collected demographic and clinical data of adult patients who attend the diabetes clinic of a large secondary care hospital for their annual review. The clinic serves an urban population of 380 000 in the north of Liverpool, United Kingdom, which is predominantly white from all socioeconomic groups. The information extracted was that from visits to the clinic in 2002. Extracted data were anonymised before this analysis. The study was approved by the audit department of the institution, and was conducted in accordance with the Declaration of Helsinki of the World Medical Association.
The patients attending the diabetes clinic include nearly all those with type 1 diabetes who reside within the catchment area of the hospital. The type 2 patients seen tend to have problems such as poor glycaemic control and multiple microvascular and macrovascular diabetic complications who are referred from primary care for specialist input and monitoring by a diabetologist.
A standard set of information is collected on all patients attending the diabetes clinic. These include known microvascular and macrovascular complications and current drugs for diabetes and other conditions. Blood pressure, height, and weight are measured, and body mass index (BMI) is calculated (BMI = w/h2; w = weight in kg, h = height in m). Overweight and obesity were defined using the current World Health Organisation definitions: underweight: BMI <18.5 kg/m2, normal weight: BMI 18.5–24.9 kg/m2, overweight (preobese): BMI 25–29.9 kg/m2, class 1 obese: BMI 30–34.9 kg/m2, class 2 obese: BMI 35–39.9 kg/m2, class 3 (morbid) obese: BMI > 40 kg/m2.14 Biochemistry investigations routinely undertaken include glycosylated haemoglobin (HBA1c) (DCCT‐aligned), non‐fasting total cholesterol, triglycerides, HDL‐cholesterol, and creatinine. An early morning midstream urine specimen is tested for clinical proteinuria, and if this is negative the urinary microalbumin‐creatinine ratio is determined as the screening test for microalbuminuria.
Statistical methods
The differences between the obese and non‐obese patients were examined with the use of the t test for normally distributed continuous data and when a normal distribution could not be achieved despite logarithmic transformation of data, the Mann‐Whitney test was used. Continuous data examined included age, BMI, HBA1c, blood pressure, total cholesterol, triglycerides, HDL‐cholesterol, and creatinine. Differences in terms of categorical variables (for example, presence or absence of treated hypertension and dyslipidaemia) were tested using the χ2 test, with continuity correction for 2 by 2 tables. The one way analysis of variance (Bonferroni test) was used to test for trends across the BMI groups for normally distributed data such as HBA1c, creatinine, and blood pressure. For non‐normally distributed data (cholesterol, triglycerides, and HDL‐cholesterol) the Kruskall‐Wallis test was used. Significance was defined as p<0.05 (two tailed). Results were analysed using SPSS version 10.0 for Windows (SPSS, Chicago, IL).
Results
Table 1 shows the demographic and clinical characteristics of the clinic population and as expected, patients with type 2 diabetes were older and more obese.
Table 1 Demographic and clinical characteristics of the patients with type 1 and type 2 diabetes.
| Characteristic | Type 1 diabetes | Type 2 diabetes | p Value |
|---|---|---|---|
| Sex (% male) | 54.2 | 53.5 | 0.8 |
| Age (years) | 40.4 (15.1) | 62.5 (11.8) | <0.0001 |
| BMI (kg/m2) | 26 (4.5) | 31.1 (6.3) | <0.0001 |
| HBA1c (%) | 8.9 (1.7) | 8.3(1.7) | <0.0001 |
| Treatment (%) | |||
| Insulin | 94.6 | 17.9 | <0.0001 |
| OHA | 0 | 60.7 | <0.0001 |
| OHA + insulin | 5.4 | 13.1 | <0.0001 |
| Diet alone | 0 | 8.3 | <0.0001 |
| Blood pressure (mm Hg) | |||
| Systolic | 127.4 (23.3) | 144.9 (23.7) | <0.0001 |
| Diastolic | 68.9 (11.8) | 74.1 (12.6) | <0.0001 |
| Total cholesterol (mmol/l) | 5.6 (3.1) | 5.2 (1.3) | 0.001 |
| Triglycerides (mmol/l) | 1.5 (1.0) | 2.3 (1.3) | 0.0001 |
| HDL (mmol/l) | 1.65 (0.4) | 1.3 (0.4) | <0.0001 |
| Creatinine (μmol/l) | 106.2 (56.7) | 108.9 (46.0) | 0.2 |
OHA, oral hypoglycaemic agents. Values are expressed as mean (SD).
The distributions of BMI were analysed separately for patients with type 1 and type 2 diabetes. Table 2 presents the results. Comparison of the percentages showed that the prevalence of overweight and obesity was far higher among patients with type 2 diabetes.
Table 2 Distributions of body mass index (BMI) in patients with type 1 and type 2 diabetes.
| BMI category (kg/m2) | Type 1 diabetes mellitus (n = 916) (%) | Type 2 diabetes mellitus (n = 2721) (%) |
|---|---|---|
| <18.5 | 12 (1.47) | 9 (0.3) |
| 18.5–24.9 | 353 (43.2) | 335 (13.3) |
| 25–29.9 | 316 (38.7) | 837 (34) |
| ⩾30 | 136 (16.6) | 1283 (52) |
| ⩾40 | 3 (0.4) | 199 (8.1) |
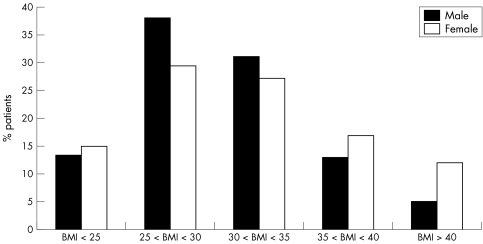
In view of the known sex difference in the prevalence of obesity particularly class III obesity in the United Kingdom,15 the distributions of BMI were determined separately for men and women with type 2 diabetes. Figure 1 shows these data. There were some significant sex differences in the BMI distributions in those with type 2 diabetes. Whereas there was a male preponderance in the overweight group (BMI 25–29.9) (60.5% male v 39.5% female, p<0.0001), and the class 1 obese group (BMI 30–34.9) (57.4% male v 42.6% female, p<0.0001), most class 3 obese patients with type 2 diabetes were women (67.3% female v 32.7% male, p<0.0001).
Figure 1 Distribution of body mass index (BMI) in patients with type 2 diabetes mellitus.
Table 3 shows the effects of obesity (BMI dichotomised around 30 kg/m2) on glycaemic control, blood pressure, and lipid profiles for obese and non‐obese patients with type 2 diabetes. Compared with the non‐obese patients, the obese patients had worse glycaemic control and lipid profiles and higher blood pressures. These differences were found despite the younger age of the obese patients.
Table 3 Contrasting clinical features of obese and non‐obese patients with type 2 diabetes mellitus.
| Type 2 diabetes and BMI <30 | Type 2 diabetes and BMI ⩾30 | p Value | ||||
|---|---|---|---|---|---|---|
| Female (n = 516 ) | Male (n = 706 ) | Female (n = 612) | Male (n = 626) | Female (BMI <30 v BMI ⩾30) | Male (BMI <30 v BMI⩾30) | |
| Age (y) | 66.2 (11.6) | 64.1 (10.7) | 61.0 (12.1) | 58.6 (11.0) | < 0.0001 | < 0.0001 |
| HBA1c (%) | 8.3 (1.8) | 8.0 (1.6) | 8.3 (1.6) | 8.3 (1.6) | 0.6 | < 0.0001 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 143.1 (26.4) | 141.5 (23.3) | 148.3 (23.4) | 146.8 (21.2) | 0.001 | < 0.0001 |
| Diastolic | 71.8 (13.2) | 73.2 (12.4) | 74.1 (12.2) | 77.5 (11.7) | 0.003 | < 0.0001 |
| Total cholesterol (mmol/l) | 5.4 (1.1) | 5.0 (1.1) | 5.5 (1.1) | 5.2 (1.1) | 0.2 | 0.008 |
| Triglycerides (mmol/l) | 2.1 (1.1) | 2.0 (1.3) | 2.3 (1.1) | 2.6 (1.4) | 0.03 | < 0.0001 |
| HDL (mmol/l) | 1.5 (0.5) | 1.3 (0.3) | 1.4 (0.5) | 1.2 (0.3) | < 0.0001 | < 0.0001 |
| Creatinine (μmol/l) | 101.8 (36.7) | 115.9 (57.7) | 100.3 (43.4) | 113.5 (39.9) | 0.6 | 0.4 |
Values are expressed as mean (SD).
Patients were then categorised into four groups based on their BMI: group 1 with BMI <25 kg/m2 group 2 with BMI 25–29.9 kg/m2; group 3, BMI 30–34.9 kg/m2; group 4 with BMI >35 kg/m2. We examined the differences in HBA1c, creatinine, total cholesterol, triglycerides, HDL‐cholesterol, systolic and diastolic blood pressure across these four groups. Table 4 shows the analysis according to the WHO categories. This analysis confirms, for men and women separately, that increasing BMI is associated with higher blood pressure, higher levels of triglycerides, and lower levels of HDL cholesterol. A trend towards poorer glycaemic control with increasing BMI was statistically significant in men but not in women.
Table 4 Glycaemic control, creatinine, blood pressure, and lipid profiles across the four different bands of BMI in female and male patients with type 2 diabetes mellitus.
| Group 1 | Group 2 | Group 3 | Group 4 | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI < 25 | 25 ⩽ BMI <30 | 30 ⩽ BMI <35 | BMI ⩾35 | |||||||
| Female (n = 167) | Male (n = 177) | Female (n = 331) | Male (n = 506) | Female (n = 306) | Male (n = 412) | Female (n = 324) | Male (n = 237) | Female (n = 1128) | Male (n = 1332) | |
| HBA1c (%) | 8.1 (1.8) | 8.2 (1.6) | 8.4 (1.8) | 7.9 (1.6) | 8.4 (1.7) | 8.2 (1.5) | 8.3 (1.6) | 8.5 (1.7) | NS | * |
| Blood pressure | ||||||||||
| Systolic (mm Hg) | 142.9 (27.3) | 138.1 (23.7) | 143.9 (26.0) | 142.6 (23.0) | 147.7 (24.2) | 145.9 (21.2) | 148.0 (23.0) | 148.0 (21.5) | NS | † |
| Diastolic (mm Hg) | 71.3 (12.4) | 72.1 (12.4) | 72.4 (13.3) | 73.3 (12.4) | 73.0 (13.0) | 76.8 (11.9) | 74.6 (11.7) | 79.0 (11.4) | ‡ | § |
| Creatinine (μmol/l) | 100.0 (24.2) | 111.6 (34.9) | 103.0 (42.4) | 115.8 (48.0) | 99.2 (27.8) | 118.5 (57.6) | 101.5 (53.1) | 108.8 (34.2) | NS | NS |
| Total cholesterol (mmol/l) | 5.2 (1.2) | 4.8 (1.2) | 5.3 (1.6) | 4.9 (1.4) | 5.3 (1.4) | 5.1 (1.5) | 5.3 (1.2) | 4.7 (1.4) | 0.8 | 0.2 |
| Triglycerides (mmol/l) | 1.4 (1.2) | 1.3 (1.2) | 2.0 (1.3) | 1.7 (1.4) | 2.2 (1.3) | 2.2 (1.4) | 2.1 (1.3) | 2.3 (1.7) | <0.0001 | <0.0001 |
| HDL‐cholesterol (mmol/l) | 1.5 (0.5) | 1.3 (0.5) | 1.4 (0.6) | 1.2 (0.4) | 1.3 (0.4) | 1.1 (0.3) | 1.3 (0.4) | 1.1 (0.3) | <0.0001 | <0.0001 |
Values of HBA1c, blood pressure, and creatinine (normally distributed) are expressed as mean (SD). Values of cholesterol, triglycerides, and HDL‐cholesterol (non‐normally distributed) are expressed as median (interquartile range). *A significant difference existed between groups 2 and 4 (p<0.0001); †a significant difference exists between groups 1 and 3 (p = 0.001), 1 and 4 (p<0.0001), and 2 and 4 (p = 0.015); ‡a significant difference was found between groups 1 and 4 (p = 0.036); §a significant difference exists between groups 1 and 3 (p<0.0001), 1 and 4 (p<0.0001), 2 and 3 (p<0.0001) and 2 and 4 (p<0.0001). NS, non‐significant.
Treated hypertension was more prevalent in obese than in non‐obese patients with type 2 diabetes (65% compared with 55.4%, p<0.0001), and treated dyslipidaemia was commoner among the overweight and obese patients (BMI >25 kg/m2) (50.8% compared with 45%, p = 0.04). It was also shown that obese patients with type 2 diabetes require more antihypertensive drugs to control their blood pressure compared with non‐obese (41% of non‐obese patients required monotherapy to achieve blood pressure targets compared with 32.7% of obese patients; p = 0.08, and 9.8% of obese required 4 or more drugs to control their blood pressure compared with 5.4% of non‐obese; p<0.0001).
Discussion
These data show that overweight and obesity are common in a representative population of type 2 diabetic patients attending a hospital diabetes clinic, in keeping with known epidemiological associations. This was in noticeable contrast with patients with type 1 diabetes, in whom obesity was far less common. According to the health survey for England in 2002, about 34.5% of all adult men and 43.5% of adult women in the general population had BMI <25 kg/m2, while 43.4% of men and 33.7% of women were overweight (BMI 25–29.9 kg/m2), 22.1% of men and 22.8% of women had BMI >30 kg/m2, and 0.8% of men and 2.6% of women had BMI >40 kg/m2. The highest prevalence of obesity was in women in the 55–64 age group, of whom 29.1% were obese, while the highest prevalence of class 3 obesity was 4.2% in women aged 45–54 years.15 Thus, this study shows that the prevalence of obesity in type 2 diabetes is considerably higher than in the general population. The female excess of class 3 obesity is consistent with the population data, although the prevalence is still about fourfold greater in women with type 2 diabetes in this study. Interestingly, obesity in type 1 diabetes was much less common than in the background population.
These data highlight several fundamental questions about diabetes. Firstly, to what extent are differences in obesity between those with established type 1 and type 2 diabetes “primary” differences? Obviously, the majority with type 2 diabetes have developed diabetes because they were a selected population who were already overweight, whereas the type 1 population probably included fewer people predisposed to obesity. Secondly, could differences between those with type 1 and type 2 diabetes reflect differences in aspects of management? Thirdly, to what extent might obesity influence long term clinical outcomes?
The differences in BMI between type 1 and type 2 diabetes probably reflect fundamental differences between the two forms of diabetes. Those presenting with new type 2 diabetes are more overweight than non‐diabetic subjects,16 and obesity plays a causal part in the pathogenesis of type 2 diabetes. In contrast, obesity does not predispose to type 1 diabetes, and patients with type 1 diabetes, although younger, were less obese (BMI ⩾30 kg/m2, 16.6% of patients with type 1 diabetes compared with 22.5% in general population). It is plausible that type 1 diabetes offers “protection” against weight gain because periods of poor glycaemic control often lead to weight loss. Gradual weight gain was seen in patients with type 2 diabetes in the UKPDS study.16 Although this may reflect a continuation of the original predisposing factors that caused weight gain and diabetes in the first place, in conjunction with the ageing process and gradual decline in physical activity, current weight is also influenced by modifiable factors such as poor diet, and to some extent use of some drugs, such as sulphonylureas, insulin, and now thiazolidinediones. It is probable that previous dietary advice placed insufficient emphasis on the treatment of obesity in type 2 diabetes. Recent guidelines give greater emphasis to lifestyle changes including the need to increase physical activity.17 In this study, however, we were not able to quantify the contribution to current BMI of potentially avoidable weight gain since the time of the original diagnosis of diabetes. Finally, in the United Kingdom at least, it is clear that there are insufficient numbers of trained dietitians to offer weight reduction programmes to all patients with diabetes who might benefit.18
The third question we addressed was whether obesity influenced the prevalence of cardiovascular risk factors and glycaemic control. Obesity in type 2 diabetes was associated with poorer glycaemic control, blood pressure, and lipid profiles, and increased use of lipid lowering and antihypertensive drugs. As these data are cross sectional it is not possible, from this study alone, to establish causality. Epidemiological data also show that obesity amplifies the current risk of coronary heart disease in people with diabetes.19 However, obesity is also predictive of future coronary and cerebrovascular disease events in those who go on to develop diabetes.19,20 It is important to consider whether the treatment of obesity, comparatively late in the natural history of cardiovascular disease, would be likely to make any impact. In favour of this proposition, it is known that weight loss in overweight patients with type 2 diabetes rapidly reverses the state of insulin resistance and can restore normal blood glucose concentrations.12 A variety of intervention studies show that patients with type 2 diabetes who succeed in losing weight often enjoy modest improvements in glycaemic control and cardiovascular risk profiles,13 as long as the weight loss is maintained. The clearest example of this is bariatric surgery in morbidly obese patients with type 2 diabetes. This commonly results in major improvements in all biochemical parameters, withdrawal of treatments for diabetes, dyslipidaemia, and hypertension, and long term remission of diabetes often occurs.21 Epidemiological data also show that modest weight loss in patients with diabetes leads to a reduction in mortality.22,23 Therefore, it is plausible, not only that obesity is a risk factor for diabetes, but that obesity is also a continuing risk factor for complications in those with established diabetes.
Potential deficiencies of this study include the usual difficulties in interpreting data gathered principally for clinical purposes. The population with type 2 diabetes in this study is a selected population, as most type 2 patients are managed in the community. Thus, this hospital patient group is more likely to include patients with multiple problems, difficult glycaemic control, and those who have been referred from the community for treatment with insulin. In contrast, patients with type 1 diabetes are nearly all seen at the hospital clinic, such that our data are likely to give a far more representative picture of the overall prevalence of obesity in type 1 diabetes compared with type 2 diabetes. Notwithstanding this criticism, and the possibility that the overall prevalence of overweight and obesity might be somewhat lower in patients with type 2 diabetes in the community, it is clear that obesity is far more of a problem in type 2 diabetes.
In summary, this study shows a high prevalence of obesity, including class 3 morbid obesity, in those with type 2 diabetes, and suggests that this may lead to worse glycaemic control and cardiovascular risk factors. The main therapeutic goals for type 2 diabetes in the USA,24 Europe,25 and the United Kingdom26,27,28 continue to be the attainment of glycaemic and cardiovascular risk reduction targets, whereas obesity itself has received far less attention, time, and still fewer resources. Ultimately, it is not known whether it might be more cost effective to combat obesity rather than to concentrate all resources on palliating diseases like type 2 diabetes with polypharmacy. Our findings highlight the continuing need for long term studies to evaluate and compare different weight reduction interventions in people with established type 2 diabetes.
Footnotes
Funding: none.
Conflicts of interest: none declared.
References
- 1.Pinkney J. Prevention and cure of type 2 diabetes. BMJ 2002325232–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindstrom J, Eriksson J G.et al Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 20013441343–1350. [DOI] [PubMed] [Google Scholar]
- 3.Knowler W C, Barrett‐Connor E, Fowler S E.et al Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002346393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan X R, Li G W, Hu Y H.et al Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care 199720537–544. [DOI] [PubMed] [Google Scholar]
- 5.Torgerson J S, Hauptman J, Boldrin M N.et al XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 200427155–161. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study Characteristics of newly presenting type 2 diabetic patients: estimated insulin sensitivity and islet β‐cell function. Multi‐centre study. Diabet Med 19885444–448. [PubMed] [Google Scholar]
- 7.Manson J E, Colditz G A, Stampfer M J.et al A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med 1990322882–889. [DOI] [PubMed] [Google Scholar]
- 8.Rimm E B, Stampfer M J, Giovannucci E.et al Body size and fat distribution as predictors of coronary heart disease among middle‐aged and older US men. Am J Epidemiol 19951411117–1127. [DOI] [PubMed] [Google Scholar]
- 9.Kannel W B, McGee D L. Diabetes and cardiovascular disease. The Framingham study. JAMA 19792412035–2038. [DOI] [PubMed] [Google Scholar]
- 10.Laing S P, Swerdlow A J, Slater S D.et al Mortality from heart disease in a cohort of 23,000 patients with insulin‐treated diabetes. Diabetologia 200346760–765. [DOI] [PubMed] [Google Scholar]
- 11.Jung R T. Obesity as a disease. Br Med Bull 199753307–321. [DOI] [PubMed] [Google Scholar]
- 12.Henry R R, Wiest‐Kent T A, Scheaffer L.et al Metabolic consequences of very‐low‐calorie diet therapy in obese non‐insulin‐dependent diabetic and nondiabetic subjects. Diabetes 198635155–164. [DOI] [PubMed] [Google Scholar]
- 13.Pinkney J H, Wilding J P H. The poorly controlled patient with type 2 diabetes. In: Gill GV, ed. Unstable and brittle diabetes. London: Martin Dunitz, 2004
- 14.World Health Organisation Obesity: preventing and managing the global epidemic. Geneva: World Health Organisation, 2000 [PubMed]
- 15.Department of Health Health survey for England 2002. http://www.doh.gov.uk/public/hse02.htm (accessed 2 Apr 2004)
- 16.UK Prospective Diabetes Study (UKPDS) Group Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998352837–853. [PubMed] [Google Scholar]
- 17.Connor H, Annan F, Bunn E.et al The implementation of nutritional advice for people with diabetes. Diabet Med 200320786–807. [DOI] [PubMed] [Google Scholar]
- 18.Winocour P H, Mearing C, Ainsworth A.et al Association of British Clinical Diabetologists (ABCD): survey of specialist diabetes care services in the UK. 2000. Dietetic services and nutritional issues. Diabet Med 20021939–43. [DOI] [PubMed] [Google Scholar]
- 19.Cho E, Manson J E, Stampfer M J.et al A prospective study of obesity and risk of coronary heart disease among diabetic women. Diabetes Care 2002251142–1148. [DOI] [PubMed] [Google Scholar]
- 20.Hu F B, Stampfer M J, Haffner S M.et al Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care 2002251129–1134. [DOI] [PubMed] [Google Scholar]
- 21.Pinkney J H, Sjostrom C D, Gale E A. Should surgeons treat diabetes in severely obese people? Lancet 20013571357–1359. [DOI] [PubMed] [Google Scholar]
- 22.Williamson D F, Thompson T J, Thun M.et al Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000231499–1504. [DOI] [PubMed] [Google Scholar]
- 23.Lean M E, Powrie J K, Anderson A S. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med 19907228–233. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Clinical practice recommendations. Diabetes Care 200427S1–150. [PubMed] [Google Scholar]
- 25.European Diabetes Policy Group A desktop guide to type 2 diabetes mellitus. European Diabetes Policy Group 1999. Diabet Med 199916716–730. [PubMed] [Google Scholar]
- 26.National Institute for Health and Clinical Excellence Full guidelines for type 2 diabetes: management of blood glucose 2001. htttp, // www.nice.org.uk (accessed 2 Apr 2004)
- 27.National Institute for Health and Clinical Excellence Full guidelines for type 2 diabetes: blood pressure management 2002. http://www.nice.org.uk (accessed 2 Apr 2004)
- 28.National Institute for Health and Clinical Excellence Full guidelines for type 2 diabetes: lipids management 2002. http://www.nice.org.uk/ (accessed 2 Apr 2004)