Abstract
Context: MEG3 is an imprinted gene encoding a novel noncoding RNA that suppresses tumor cell growth. Although highly expressed in the normal human pituitary, it is unknown which of the normal pituitary cell types and pituitary tumors express MEG3.
Objectives: Our objectives were 1) to investigate cell-type- and tumor-type-specific expression of MEG3 in the human pituitary and 2) to investigate whether methylation in the intergenic differentially methylated region (IG-DMR) at the DLK1/MEG3 locus is involved in the loss of MEG3 expression in tumors.
Design and Methods: RT-PCR, quantitative RT-PCR, Northern blot, and a combination of in situ hybridization and immunofluorescence were used to determine the cell-type- and tumor-type-specific MEG3 expression. Bisulfite treatment and PCR sequencing of genomic DNA were used to measure the CpG methylation status in the normal and tumor tissues. Five normal human pituitaries and 17 clinically nonfunctioning, 11 GH-secreting, seven prolactin-secreting, and six ACTH-secreting pituitary adenomas were used.
Results: All normal human pituitary cell types express MEG3. However, loss of MEG3 expression occurs only in nonfunctioning pituitary adenomas of a gonadotroph origin. All other pituitary tumor phenotypes examined express MEG3. Hypermethylation of the IG-DMR at the DLK1/MEG3 locus is present in nonfunctioning pituitary adenomas.
Conclusions: MEG3 is the first human gene identified expressed in multiple normal human pituitary cell types with loss of expression specifically restricted to clinically nonfunctioning pituitary adenomas. The IG-DMR hypermethylation may be an additional mechanism for MEG3 gene silencing in such tumors.
Loss of expression of the maternally imprinted gene MEG3 in clinically non-functioning pituitary adenomas may play a tumorigenic role.
Pituitary adenomas comprise approximately 7% of all intracranial tumors, and clinically nonfunctioning adenomas are among the most common phenotype (1). Although clinically nonfunctioning tumors typically grow slowly, many are locally invasive, causing hypopituitarism and neurological deficits, and recurrences can occur (2,3). Most importantly, there is no medical therapy available for such tumors. Although it has been known for many years that these tumors are primarily of a gonadotroph cell origin, the underlying pathogenetic factors responsible for their development remain unknown. Clinically nonfunctioning pituitary adenomas are known to represent monoclonal cell proliferations (4,5); however, target oncogenes and tumor suppressor genes, such as ras, c-myc, MEN-1, Rb, p53, nm23, and gsp, have not been shown to play an important role in their pathogenesis (3,6,7). Epigenetic alterations in clinically nonfunctioning tumors, such as hypermethylation of the p16/CDKN2A/MTS1 gene, have been reported. However, this alteration is not specific to these tumors because it has also been described with high frequency in lactotroph-derived tumors and, rarely, in somatotroph- and corticotroph-derived adenomas (8). Therefore, molecular mechanisms specifically underlying the pathogenesis of clinically nonfunctioning pituitary adenomas remain unknown.
MEG3 is an imprinted gene expressed from the maternal allele (9). Using representational difference analysis, we identified this gene as strongly expressed in the normal human pituitary but not expressed in clinically nonfunctioning tumors derived from gonadotroph cells (10). The link between MEG3 expression and tumorigenesis has been supported by the finding that expression of MEG3 cDNA suppresses cell proliferation in a number of human tumor cell lines (10). Of importance, we have recently shown that MEG3 cDNA is able to stimulate the transactivation function of p53 (11). MEG3 is the human homolog of Gtl2 (12), first mapped on mouse distal chromosome 12. Gtl2/MEG3 gives rise to multiple spliced cDNA isoforms, and each of them has similar functions in suppressing cancer cell growth in vitro and stimulating p53-mediated transactivation (11). Interestingly, no strong Kozak consensus sequence for translation has been detected in any of the ATG codons in the potential open reading frames (ORFs) (12,13), suggesting that this gene might function as a noncoding RNA. We have recently shown that the integrity of each potential ORF in MEG3 is not required for its ability to suppress tumor cell growth and to activate the tumor suppressor p53, indicating that MEG3 function is translation-independent. Therefore, the MEG3 gene encodes a noncoding RNA with growth suppression function (11). We also found no genomic abnormalities in the MEG3 locus in most clinically nonfunctioning pituitary adenomas (14). However, two 5′-flanking regions, containing a promoter and an enhancer, respectively, were hypermethylated in human tumors lacking MEG3 expression, and treatment of human cancer cell lines with a methylation inhibitor resulted in MEG3 expression (14,15). These data indicate that hypermethylation is a mechanism involved in tumor suppression by MEG3.
The expression of MEG3 in normal gonadotrophs and loss of MEG3 expression in pituitary adenomas of gonadotroph origin have been reported in our previous studies (10,14). However, an important unanswered question is whether loss of MEG3 expression is specific for gonadotroph-derived tumors or also occurs in other pituitary tumor types. It is also unknown whether MEG3 is expressed in other normal human pituitary cell types. We therefore investigated the specificity of MEG3 expression in normal human pituitary cell types and examined MEG3 expression in different human pituitary tumor phenotypes. We also determined whether hypermethylation of the imprinting control region for the MEG3 locus serves as another mechanism for the loss of MEG3 expression in gonadotroph-derived pituitary tumors.
Materials and Methods
Tissue and tumor samples
Normal human pituitary glands were obtained 2–16 h postmortem from autopsies performed at the Massachusetts General Hospital. Fresh samples from 17 clinically nonfunctioning, 11 GH-, seven prolactin (PRL)-, and six ACTH-secreting adenomas with immunohistochemical confirmation were obtained from surgical specimens at the Massachusetts General Hospital and the National Institutes of Child Health and Human Development (Bethesda, MD). The median age was 43 (range, 12–83 yr old). The patients included 16 females and 25 males. All pituitary tumors were macroadenomas except the six ACTH-secreting adenomas, which were microadenomas. The study was approved by the Institutional Review Boards of Partners HealthCare and The National Institute of Child Health and Human Development/National Institutes of Health.
In situ hybridization
Digoxigenin-labeled RNA probes (sense and antisense) of human MEG3a were generated by in vitro transcription from the cloned cDNA in pBlueScript-SK vector using the DIG-RNA labeling kit (Roche, Indianapolis, IN) according to the manufacturer’s instructions. This probe recognizes all MEG3 RNA isoforms. Normal pituitaries and tumor samples were fixed with 4% paraformaldehyde for 3–4 h, rinsed with PBS, and washed with 30% sucrose. Four-micrometer frozen sections were obtained using a cryostat and stored at −80 C. In situ hybridization was performed as previously described (10). The in situ hybridization slides with tumor tissue were stained with nuclear fast red (Sigma Chemical Co., St. Louis, MO).
Immunofluorescence
After in situ hybridization, immunofluorescence was performed on the same slides using primary antibodies against GH, FSHβ, PRL, ACTH, and TSHβ (obtained from Dr A. F. Parlow, National Institutes of Health National Hormone and Pituitary Program, Torrance, CA). The sections were rinsed briefly with PBS, incubated with 1% BSA in PBS at room temperature for 30 min, and then incubated overnight at 4 C with primary antibodies diluted in 1% BSA (1:400 dilution). After washes with PBS, the sections were incubated with a secondary immunofluorescent antibody in PBS (antirabbit goat IgG conjugated with rhodamine; Jackson ImmunoResearch, West Grove, PA) at room temperature for 2 h, washed with PBS, and mounted with Vectashield (mounting medium with 4′,6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA).
Colocalization of MEG3 and pituitary hormones
The images for the in situ hybridization and immunostaining signals were photographed using a Nikon dual-field microscope (bright field for the in situ hybridization and fluorescence for the immunostaining) and analyzed with Adobe Photoshop software (Adobe Systems Inc., San Jose, CA). The in situ hybridization images were converted to dark field with a green color and superimposed on the immunofluorescence images.
RT-PCR and Northern blot
Total RNA from the normal anterior pituitaries and the pituitary adenomas was extracted using TRIzol reagent (Invitrogen Life Technologies, Inc., Carlsbad, CA) and reverse transcribed using the RT System from Promega Corp. (Madison, WI) according to the manufacturer’s protocol. PCR was performed under the following condition for both MEG3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 94 C for 2 min, 94 C for 30 sec, 60 C for 30 sec, and 72 C for 1 min for 30 cycles and 72 C for 10 min. The PCR primer sequences used were as follows: MEG3 forward, 5′-ATCATCCGTCCACCTCCTTGTCTTC-3′, and MEG3 reverse, 5′-GTATGAGCATAGCAAAGGTCAGGGC-3′; GAPDH forward, 5′-AATGCCTCCTGCACCACCAAC-3′, and GAPDH reverse, 5′-AAGGCCATGCCAGTG-AGCTTC-3′. The MEG3 primers used here amplify all MEG3 cDNA isoforms. Positive controls with three different normal pituitaries and negative controls without cDNA template were used in each reaction. Northern blot was performed as previously described (10).
Quantitative (real-time) RT-PCR (qRT-PCR)
qRT-PCR for MEG3 expression was performed in a subset of the pituitary adenomas, including 11 clinically nonfunctioning, seven GH-secreting, four PRL-secreting, and six ACTH-secreting tumors. Four normal human pituitaries were used for control. The PCR was performed using a 20-μl working mix containing 1.0 μl of the cDNA template in 1× TaqMan universal Master Mix (Applied Biosystems, Foster City, CA) and 200 nm final concentration of the primers and the probe for MEG3 (Hs01087966_m1, FAM labeled; Applied Biosystems). The TaqMan primers and the probe for MEG3 amplify all MEG3 cDNA isoforms. As an endogenous control, GAPDH (Hs99999905_m1, FAM labeled; Applied Biosystems) was amplified in parallel and used to normalize the results to allow relative quantitative analysis of MEG3 expression. The reaction was run in an Applied Biosystems 7500 Fast Real-Time PCR Sequence Detection System using the following parameters: 50 C for 2 min, 95 C for 10 min, and 40 cycles of 95 C (denature) for 15 sec, with 60 C for 1 min (annealing and extension). Each qRT-PCR was performed in duplicate experiments. Data were expressed as CT values (the cycle number at which logarithmic PCR plots cross a calculated threshold line). Relative RNA expression was given by the formula 2-ΔΔCT, where ΔΔCT = CT(MEG3 tumor − GAPDH tumor) − CT.(MEG3 normal pituitary gland − GAPDH normal pituitary gland)
Genomic DNA extraction
Genomic DNA from normal human pituitaries and from pituitary tumors was extracted using a DNeasy Tissue Kit (QIAGEN, Valencia, CA) according to the manufacturer’s protocol.
Sodium bisulfite treatment of genomic DNA
A total of 1.5 μg of genomic DNA from the 11 nonfunctioning pituitary adenomas, three GH-secreting adenomas, three PRL-secreting adenomas, and four ACTH-secreting adenomas as well as from the five normal human pituitaries were treated with sodium bisulfite using the MethylDetector Bisulfite Modification Kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. A semi-nested PCR was performed to amplify the intergenic differentially methylated region (IG-DMR) at the MEG3 locus (position 51021–51180; GenBank accession no. AL117190) (15). For the first round, the primer sequences were as follows: IG forward, 5′-TTTTGAGGAGATTGATATTTTTAG-TTTTATT-3′, and IG reverse, 5′-ATAAACTACACTACTAAAAACTACATTTAAA-3′. The second round was performed using the same IG reverse primer and a new primer IG-Fnes (nested forward primer), 5′-TTAGGTTGGAATTGTTAAGAGTTTGTGGATT-3′. PCR was performed using Hotstart Plus DNA polymerase (QIAGEN) under the following conditions: 95 C for 5 min, 94C for 30 sec, 53 C for 30 sec, and 72 C for 1 min for 35 cycles and 72 C for 10 min. PCR products were purified using a QIAquick purification kit (QIAGEN) according to the manufacturer’s protocol. Purified PCR products were subcloned into a TOPO TA cloning vector (Invitrogen). Twenty clones from each PCR product were examined by sequence analysis. All data are expressed as the mean ± sd for descriptive statistics and ± sem for comparing groups. Repeated measures of ANOVA were used to analyze data where appropriate. P < 0.05 was considered significant.
Results
Expression of MEG3 in the normal pituitary
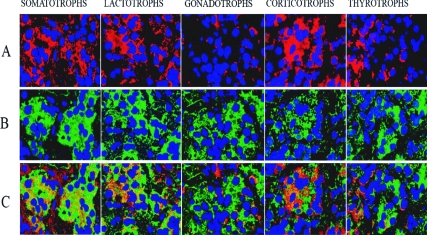
In situ hybridization detected strong positive MEG3 RNA expression in most pituitary cells with stronger expression in the lateral wings of anterior pituitary (data not shown). Using a combination of in situ hybridization and immunofluorescence, MEG3 RNA expression was detected in most of the pituitary cell types (90–95%), including gonadotrophs, somatotrophs, lactotrophs, thyrotrophs, and corticotrophs (Fig. 1). The GH-producing cells had the strongest and most diffuse reaction for MEG3.
Figure 1.
A, Immunofluorescence to detect each pituitary hormone (GH, PRL, FSH, ACTH, and TSH) as indicated, thus identifying different types of pituitary cells; B, in situ hybridization with an antisense probe for MEG3 RNA; C, overlapping of A and B. The areas with yellow represent the cells with coexpression of MEG3 RNA (green) and the pituitary hormone (red), thus showing the expression of MEG3 in each type of pituitary cell.
Selective loss of MEG3 expression in clinically nonfunctioning pituitary adenomas
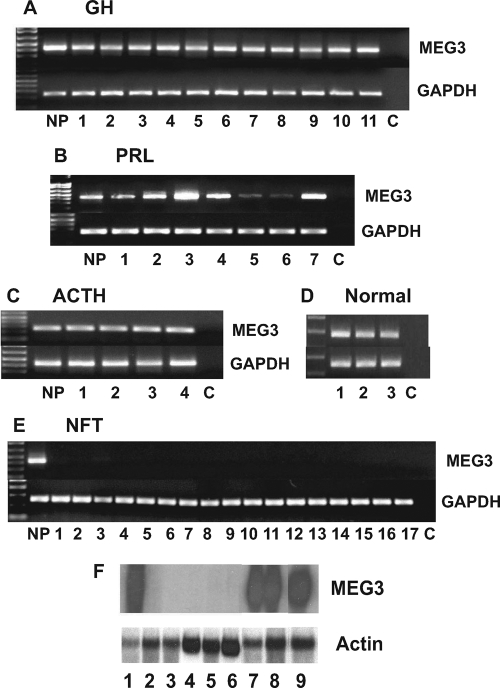
RT-PCR showed MEG3 RNA expression in all 11 GH-secreting, all seven PRL-secreting, and all four ACTH-secreting tumors (Fig. 2, A–C). RT-PCR also detected MEG3 RNA in three normal human pituitaries (Fig. 2D). MEG3 expression in the PRL-secreting tumors was more variable than that in the other functioning tumors. Interestingly, the two prolactinomas with lower MEG3 expression (Fig. 2B, lanes 5 and 6) came from patients with poorly functioning prolactinomas with sparse PRL immunostaining and PRL levels of 68 and 46 ng/ml, respectively, much lower than that in other patients (ranging from 289-8150 ng/ml). In contrast, none of the 17 clinically nonfunctioning tumors expressed MEG3 RNA as determined by RT-PCR (Fig. 2E). Northern blot also showed that MEG3 RNA is detectable in one normal human pituitary, two GH-secreting tumors and one PRL-secreting tumor, but undetectable in five clinically nonfunctioning tumors (Fig. 2F).
Figure 2.
A–D, RT-PCR showing MEG3 RNA expression in all cases of functioning pituitary adenomas (A, GH-secreting tumors; B, PRL-secreting tumors; and C, ACTH-secreting tumors) and normal human pituitaries (D). Some prolactinomas have a weaker expression (in two cases) (B). E, No MEG3 RNA is detected in any of the 17 clinically nonfunctioning tumors (NFT). C, Negative control without cDNA template; NP, positive control with normal pituitary cDNA. F, Northern blot showing MEG3 RNA expression in a normal human pituitary (lane 1), 2 GH-secreting tumors (lanes 7 and 8), and a PRL-secreting tumor (lane 9), but not in five clinically nonfunctioning tumors.
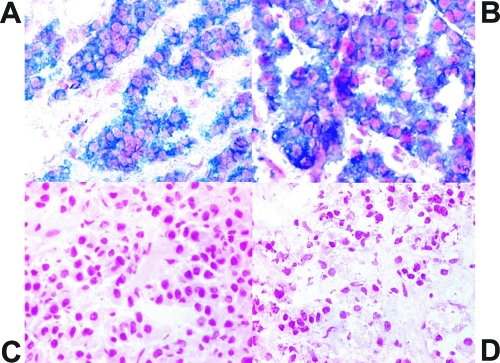
Furthermore, qRT-PCR demonstrated detectable and variable levels of MEG3 RNA in GH-secreting, PRL-secreting, and ACTH-secreting adenomas (Table 1). GH-secreting adenomas had a 38-fold (38.0 ± 31.0, mean ± sd) increase and PRL-secreting adenomas had a 12-fold increase (12.0 ± 15.0) in the levels of MEG3 RNA compared with that seen in the normal pituitaries (1.00 ± 0.0). Most ACTH-secreting adenomas also had increased MEG3 RNA expression (2.85 ± 2.72) compared with that in the normal pituitaries. In contrast, the clinically nonfunctioning pituitary adenomas had a −100-fold (0.01 ± 0.01) decrease (P < 0.001) in the levels of MEG3 RNA compared with that in normal pituitaries (Table 1). Tissue slides from four GH-secreting tumors (tumors number 1–4, as shown in Fig. 2) and four clinically nonfunctioning tumors (tumors number 1–4, as shown in Fig. 2) were used for in situ hybridization. Strong expression of MEG3 RNA was observed in all cells of the GH-secreting tumors, but no MEG3 RNA expression was detected in the clinically nonfunctioning tumors, consistent with the RT-PCR results (Fig. 3).
Table 1.
MEG3 RNA levels in human pituitary tumor samples
| Nonfunctioning adenomas | GH-secreting adenomas | PRL-secreting adenomas | ACTH-secreting adenomas |
|---|---|---|---|
| 0.001 | 90.0 | 0.50 | 1.10 |
| 0.022 | 32.0 | 0.16 | 3.10 |
| 0.001 | 64.0 | 15.0 | 0.62 |
| 0.002 | 5.00 | 32.0 | 1.30 |
| 0.004 | 4.00 | 3.00 | |
| 0.021 | 42.0 | 8.00 | |
| 0.010 | 32.0 | ||
| 0.013 | |||
| 0.000 | |||
| 0.033 | |||
| 0.003 |
Each number indicates the relative MEG3 RNA level in each tumor compared with the average MEG3 RNA level in the normal human pituitaries (n = 4), which is designated as 1, as determined by qRT-PCR.
Figure 3.
Representative results of in situ hybridization for MEG3 RNA in frozen tissues. A, Normal human pituitary gland; B, GH-secreting pituitary tumor; C, clinically nonfunctioning pituitary tumor; D, negative control, the normal pituitary section hybridized with a MEG3 sense probe. The blue indicates MEG3 RNA-positive signal.
Methylation status of the IG-DMR in clinically nonfunctioning pituitary tumors
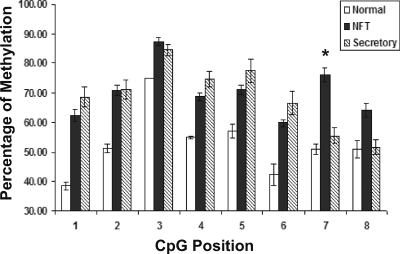
Genomic DNA from 11 nonfunctioning, three GH-secreting, three PRL-secreting, and four ACTH-secreting pituitary adenomas as well as five normal pituitaries was examined for methylation in CpG dinucleotides in DLK1/MEG3 IG-DMR. There are a total of eight CpG dinucleotides in this region. As shown in Table 2, there is an approximately 50% methylation in these CpGs in the normal pituitaries, consistent with the fact that MEG3 is an imprinted gene; therefore, the paternal allele is hypermethylated for the silencing of gene expression from this allele. In contrast, the percentages of CpG methylation in clinically nonfunctioning tumors are significantly higher at each CpG position (P < 0.05), indicating that this region is hypermethylated in such tumors. In the pituitary tumors with hormone secretion, we observed an increased percentage of methylation at the CpG positions 1–6. However, in secretory tumors, the percentages of methylation at CpG position 7 and 8 are similar to those in the normal pituitaries, much lower than the percentages of methylation at these two positions observed in the clinically nonfunctioning tumors (Table 2). As shown in Fig. 4, there is a uniform increase of methylation in each individual CpG in the clinically nonfunctioning tumors compared with the normal pituitaries, and in particular, the percentage of methylation at the CpG positions 7 and 8 in the clinically nonfunctioning tumors is much higher that that in the hormone-secreting tumors (76 vs. 56% for CpG 7, P = 0.031; 64 vs. 52% for CpG 8, P = 0.189).
Table 2.
Percentages of CpG methylation within the IG-DMR in normal pituitaries and pituitary tumors
| CpG1 | CpG2 | CpG3 | CpG4 | CpG5 | CpG6 | CpG7 | CpG8 | |
|---|---|---|---|---|---|---|---|---|
| Secretory tumors | ||||||||
| GH-secreting | ||||||||
| 1 | 65 | 95 | 85 | 60 | 60 | 90 | 45 | 70 |
| 2 | 65 | 55 | 70 | 75 | 90 | 70 | 85 | 70 |
| 3 | 35 | 85 | 90 | 55 | 35 | 75 | 65 | 50 |
| PRL-secreting | ||||||||
| 1 | 100 | 45 | 100 | 100 | 100 | 40 | 35 | 35 |
| 2 | 95 | 50 | 100 | 100 | 100 | 100 | 45 | 45 |
| 3 | 40 | 60 | 65 | 55 | 40 | 60 | 55 | 45 |
| ACTH-secreting | ||||||||
| 1 | 60 | 70 | 70 | 70 | 80 | 25 | 60 | 30 |
| 2 | 80 | 100 | 95 | 85 | 95 | 90 | 55 | 55 |
| 3 | 75 | 60 | 75 | 70 | 90 | 45 | 40 | 35 |
| 4 | 70 | 90 | 95 | 75 | 85 | 70 | 70 | 80 |
| Mean | 69% | 71% | 85% | 75% | 78% | 67% | 56% | 52% |
| NFT | ||||||||
| 1 | 50 | 100 | 100 | 66 | 66 | 66 | 75 | 50 |
| 2 | 40 | 50 | 86 | 95 | 60 | 66 | 95 | 95 |
| 3 | 57 | 87 | 74 | 57 | 87 | 57 | 61 | 74 |
| 4 | 33 | 86 | 95 | 57 | 48 | 81 | 33 | 24 |
| 5 | 33 | 50 | 83 | 67 | 50 | 58 | 100 | 83 |
| 6 | 100 | 68 | 95 | 77 | 59 | 65 | 100 | 100 |
| 7 | 57 | 48 | 57 | 57 | 95 | 43 | 100 | 48 |
| 8 | 90 | 60 | 100 | 40 | 90 | 40 | 40 | 40 |
| 9 | 60 | 90 | 70 | 70 | 70 | 70 | 80 | 50 |
| 10 | 94 | 65 | 100 | 82 | 76 | 76 | 65 | 59 |
| 11 | 70 | 76 | 100 | 88 | 82 | 47 | 88 | 82 |
| Mean | 62% | 71% | 87% | 69% | 71% | 61% | 76% | 64% |
| Normal | ||||||||
| 1 | 43 | 56 | 75 | 50 | 56 | 68 | 55 | 50 |
| 2 | 44 | 44 | 75 | 56 | 37 | 44 | 37.5 | 62.5 |
| 3 | 31 | 56 | 75 | 56 | 62 | 25 | 60 | 37 |
| 4 | 44 | 44 | 75 | 56 | 62.5 | 50 | 62.5 | 68 |
| 5 | 31 | 56 | 75 | 56 | 68 | 25 | 50 | 37.5 |
| Mean | 39% | 51% | 75% | 55% | 57% | 42% | 53% | 51% |
Each number indicates the percentage of methylation at each CpG dinucleotide position with the IG-DMR among 20 clones analyzed. Normal indicates normal human pituitaries. NFT, Clinically nonfunctioning pituitary adenomas.
Figure 4.
Comparison of methylation at each CpG position (from position 1 to position 8) within the IG-DMR in the normal human pituitaries (white bars), clinically nonfunctioning pituitary adenomas (NFT, black bars), and the secretory pituitary adenomas (striped bars). The methylation status of each CpG in this region is quantified by the percentage of methylated CpGs among all PCR products analyzed. Values are the mean ± sem. Difference with statistical significance is observed at each CpG position between the normal human pituitaries and the clinically nonfunctioning pituitary adenomas. Between the clinically nonfunctioning adenomas and the hormone-secreting adenomas, the percentage of methylation at the CpG positions 7 and 8 in the clinically nonfunctioning tumors is much higher that that in the hormone-secreting tumors (76 vs. 56% for CpG 7, P = 0.031; 64 vs. 52% for CpG 8, P = 0.189).
Discussion
We found that MEG3 is strongly expressed in all cell types of the normal human pituitary gland as determined by in situ hybridization and immunohistochemistry, consistent with the strong MEG3 expression found in the normal human pituitary using both regular RT-PCR and qRT-PCR. MEG3 RNA expression was observed in tumor cells of all functioning pituitary adenomas examined, including lactotroph, somatotroph and corticotroph tumors. In striking contrast, no expression of MEG3 was found in clinically nonfunctioning tumors. This specific loss of expression in gonadotroph-derived tumors is consistently demonstrated by regular RT-PCR and qRT-PCR as well as in situ hybridization. Therefore, MEG3 represents the first human gene identified whose expression is selectively lost only in clinically nonfunctioning pituitary tumors. These data suggest that MEG3 may play an important role in a specific pathway controlling clinically nonfunctioning pituitary tumor pathogenesis. qRT-PCR revealed the highest MEG3 expression in somatotroph-derived pituitary adenomas. This is likely due to the almost pure population of somatotroph cells in these tumors, because we have observed the highest MEG3 expression in the lateral wings of the anterior pituitary where there are more somatotroph and lactotroph cells. We would like to point out that because MEG3 expression levels in different cell types in the normal pituitary are different, we cannot conclude that MEG3 is overexpressed in the functioning tumors.
It had been hypothesized that the MEG3 gene may be unable to generate protein products based on the lack of Kozak sequences near the potential ORFs within its RNA (12). We recently reported that MEG3 is capable of stimulating p53-mediated transcription activation, thus providing a strong functional link between MEG3 and p53, one of the most important tumor suppressors. MEG3 expression leads to cellular accumulation of p53 protein and selective activation of p53 downstream target genes. Using p53-mediated transactivation as a functional assay, we have provided experimental evidence demonstrating that MEG3 functions as a noncoding RNA (11). Furthermore, we have reported that suppression of tumor cell growth by MEG3 is retinoblastoma (Rb) dependent (11). The tumor suppressor Rb is well known for its involvement in preventing pituitary tumorigenesis. Loss of expression of Rb or its activator p16Ink4a in human pituitary tumors has been reported by several groups (8,16,17,18,19). Inactivation of Rb by simian virus 40 T antigen in mice results in the development of pituitary tumors derived from gonadotrophs (20), which resemble human clinically nonfunctioning pituitary tumors. Therefore, MEG3 may play an important tumor suppressor role in Rb-related pathways to prevent the development of clinically nonfunctioning pituitary tumors.
We previously reported that MEG3 expression was stimulated by cAMP (21). cAMP is one of the most important second messengers. It is involved in mediating functions or regulating production of all major pituitary hormones (22,23). We have now observed that MEG3 is expressed in all cell types of the normal human pituitary. Interestingly, loss of MEG3 expression is restricted only to gonadotroph-derived clinically nonfunctioning tumors, which coincidently lack significant hormone secretion. In addition, the two prolactinomas with low levels of MEG3 expression (nos. 5 and 6; see Fig. 2B) are poorly functioning PRL-secreting tumors. Taken together, these data may also suggest that MEG3 is physiologically involved in the control of pituitary hormone production and function.
We have previously shown that the mechanism for the loss of MEG3 expression in human pituitary tumors is not related to genomic DNA abnormalities such as gene deletion or mutation. Rather, MEG3 gene promoter and enhancer hypermethylation has been demonstrated as an epigenetic mechanism for the loss of MEG3 expression in nonfunctioning pituitary adenomas (14). The expression of MEG3 in human cancer cell lines can be induced by a demethylation reagent (14). In this study, we observed additional hypermethylation in the IG-DMR in 14q32, between DLK1 and the MEG3 gene and known to control imprinting status, in clinically nonfunctioning tumors. In mice, the IG-DMR of the Dlk1/Gtl2 domain is a control element for the whole imprinting gene cluster in chromosome 14q. Paulsen et al. (24) first identified this CpG-rich tandem repeat in the intergenic region of Dlk1/Gtl2 that was conserved between mouse, sheep, and humans. Takada et al. (25) identified this CpG island located 15 kb upstream of the Gtl2 and 70 kb downstream of the Dlk1 promoter. Deletion in the IG-DMR from the maternal chromosome causes loss of imprinting of all imprinting genes in the maternal allele. However, deletion of the paternal allele does not affect the imprinted gene expression, indicating that it is the IG-DMR in the maternal allele that controls genetic imprinting (26,27). The presence of hypermethylation in this IG-DMR in clinically nonfunctioning pituitary tumors compared with the normal pituitaries reported here may be an additional mechanism and likely works together with the promoter and enhancer hypermethylation to contribute to the loss of MEG3 expression in human pituitary tumors. Hypermethylation has been found in relation to the loss of expression of various genes in pituitary adenomas such as GADD45γ, p16, Rb, and death-associated protein kinase genes (28,29). Considering that pituitary adenomas are slowly growing benign tumors, it is likely that the epigenetic mechanisms play a more important role in the pathogenesis of these tumors than genomic defects, which often lead to more malignant features.
Interestingly, when we examined the methylation patterns of this IG-DMR in the hormone-secreting pituitary tumors, which all express MEG3, we observed a methylation increase at the CpG positions 1–6. However, the percentages of methylation at CpG positions 7 and 8 in hormone-secreting tumors are similar to those in the normal pituitaries, much lower than the percentages of methylation at these two positions observed in nonsecreting tumors. Recent studies with detailed analysis of particular CpG methylation patterns have shown that methylation at very few specific CpG positions can dramatically affect gene expression. For example, Demura and Bulun (30) reported that in the CYP19 I.3/II promoter fragment of 571 bp, differential methylation at only three CpG dinucleotide positions determined cAMP responsiveness of aromatase expression. Weaver et al. (31,32) showed that methylation of a single CpG was capable of affecting glucocorticoid receptor gene expression. In human adrenocortical adenomas, methylation at one single CpG position in the H19 promoter region is correlated with decreased H19 expression and increased IGF-II expression in adenomas compared with that in the normal adrenal glands (33). Taking together, these data clearly indicated that differences in methylation status even at one or very few CpG sites, such as what we reported here between secretory and nonsecretory pituitary tumors, is sufficient to cause gene expression or silencing.
In summary, we have found specific loss of MEG3 expression in clinically nonfunctioning pituitary adenomas. Thus, MEG3 is the first gene identified whose loss of expression is restricted to clinically nonfunctioning pituitary adenomas, suggesting that it may play a critical role in control of tumor formation from this cell type. The increased CpG methylation within the IG-DMR in the DLK1/MEG3 locus, especially at CpG positions 7 and 8, along with that in MEG3 promoter and enhancer region as we have reported before, demonstrate an overall hypermethylation in the important regulatory regions of MEG3 gene in human clinically nonfunctioning tumors. This increased methylation contributes to loss of expression and antiproliferative function of this noncoding RNA gene specifically in such tumors.
Footnotes
This work was supported by National Institutes of Health Grant R01-DK-40947, The Jarislowsky Foundation, and scholarships from Pontificia Universidad Catolica de Chile and the Chilean Government (R.G.).
Disclosure Statement: B.S. has equity interests in Pfizer, Roche, and Novartis. E.T.H.-W. has equity interests in Becton Dickinson. The other authors have nothing to declare.
First Published Online July 15, 2008
Abbreviations: IG-DMR, Intergenic differentially methylated region; ORF, open reading frame; PRL, prolactin; qRT-PCR, quantitative (real-time) RT-PCR; Rb, retinoblastoma.
References
- Al-Shraim M, Asa SL 2006 The 2004 World Health Organization classification of pituitary tumors: what is new? Acta Neuropathol 111:1–7 [DOI] [PubMed] [Google Scholar]
- Ezzat S, Asa SL 2006 Mechanisms of disease: the pathogenesis of pituitary tumors. Nat Clin Pract Endocrinol Metab 2:220–230 [DOI] [PubMed] [Google Scholar]
- Grossman AB, Korbonits M 2004 Akting and cycling: a tale of the pituitary. Horm Res 62(Suppl 3):117–123 [DOI] [PubMed] [Google Scholar]
- Alexander JM, Biller BM, Bikkal H, Zervas NT, Arnold A, Klibanski A 1990 Clinically nonfunctioning pituitary tumors are monoclonal in origin. J Clin Invest 86:336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman V, Fagin J, Gonsky R, Kovacs K, Melmed S 1990 Clonal origin of pituitary adenomas. J Clin Endocrinol Metab 71:1427–1433 [DOI] [PubMed] [Google Scholar]
- Farrell WE 2006 Pituitary tumours: findings from whole genome analyses. Endocr Relat Cancer 13:707–716 [DOI] [PubMed] [Google Scholar]
- Melmed S 2003 Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest 112:1603–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DJ, Bicknell JE, McNicol AM, Clayton RN, Farrell WE 1999 Hypermethylation of the p16/CDKN2A/MTSI gene and loss of protein expression is associated with nonfunctional pituitary adenomas but not somatotrophinomas. Genes Chromosomes Cancer 24:328–336 [PubMed] [Google Scholar]
- Miyoshi N, Wagatsuma H, Wakana S, Shiroishi T, Nomura M, Aisaka K, Kohda T, Surani MA, Kaneko-Ishino T, Ishino F 2000 Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells 5:211–220 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou Y, Mehta KR, Danila DC, Scolavino S, Johnson SR, Klibanski A 2003 A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. J Clin Endocrinol Metab 88:5119–5126 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhong Y, Wang Y, Zhang X, Batista DL, Gejman R, Ansell PJ, Zhao J, Weng C, Klibanski A 2007 Activation of p53 by MEG3 non-coding RNA. J Biol Chem 282:24731–24742 [DOI] [PubMed] [Google Scholar]
- Schuster-Gossler K, Bilinski P, Sado T, Ferguson-Smith A, Gossler A 1998 The mouse Gtl2 gene is differentially expressed during embryonic development, encodes multiple alternatively spliced transcripts, and may act as an RNA. Dev Dyn 212:214–228 [DOI] [PubMed] [Google Scholar]
- Croteau S, Charron MC, Latham KE, Naumova AK 2003 Alternative splicing and imprinting control of the Meg3/Gtl2-Dlk1 locus in mouse embryos. Mamm Genome 14:231–241 [DOI] [PubMed] [Google Scholar]
- Zhao J, Dahle D, Zhou Y, Zhang X, Klibanski A 2005 Hypermethylation of the promoter region is associated with the loss of MEG3 gene expression in human pituitary tumors. J Clin Endocrinol Metab 90:2179–2186 [DOI] [PubMed] [Google Scholar]
- Astuti D, Latif F, Wagner K, Gentle D, Cooper WN, Catchpoole D, Grundy R, Ferguson-Smith AC, Maher ER 2005 Epigenetic alteration at the DLK1-GTL2 imprinted domain in human neoplasia: analysis of neuroblastoma, phaeochromocytoma and Wilms’ tumour. Br J Cancer 92:1574–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryns VL, Alexander JM, Klibanski A, Arnold A 1993 The retinoblastoma gene in human pituitary tumors. J Clin Endocrinol Metab 77:644–646 [DOI] [PubMed] [Google Scholar]
- Woloschak M, Roberts JL, Post KD 1994 Loss of heterozygosity at the retinoblastoma locus in human pituitary tumors. Cancer 74:693–696 [DOI] [PubMed] [Google Scholar]
- Woloschak M, Yu A, Post KD 1997 Frequent inactivation of the p16 gene in human pituitary tumors by gene methylation. Mol Carcinog 19:221–224 [DOI] [PubMed] [Google Scholar]
- Woloschak M, Yu A, Xiao J, Post KD 1996 Frequent loss of the P16INK4a gene product in human pituitary tumors. Cancer Res 56:2493–2496 [PubMed] [Google Scholar]
- Kumar TR, Graham KE, Asa SL, Low MJ 1998 Simian virus 40 T antigen-induced gonadotroph adenomas: a model of human null cell adenomas. Endocrinology 139:3342–3351 [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang X, Zhou Y, Ansell PJ, Klibanski A 2006 Cyclic AMP stimulates MEG3 gene expression in cells through a cAMP-response element (CRE) in the MEG3 proximal promoter region. Int J Biochem Cell Biol 38:1808–1820 [DOI] [PubMed] [Google Scholar]
- De Cesare D, Sassone-Corsi P 2000 Transcriptional regulation by cyclic AMP-responsive factors. Prog Nucleic Acids Res Mol Biol 64:343–369 [DOI] [PubMed] [Google Scholar]
- Richards JS 2001 New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218 [DOI] [PubMed] [Google Scholar]
- Paulsen M, Takada S, Youngson NA, Benchaib M, Charlier C, Segers K, Georges M, Ferguson-Smith AC 2001 Comparative sequence analysis of the imprinted Dlk1-Gtl2 locus in three mammalian species reveals highly conserved genomic elements and refines comparison with the Igf2–H19 region. Genome Res 11:2085–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Paulsen M, Tevendale M, Tsai CE, Kelsey G, Cattanach BM, Ferguson-Smith AC 2002 Epigenetic analysis of the Dlk1-Gtl2 imprinted domain on mouse chromosome 12: implications for imprinting control from comparison with Igf2–H19. Hum Mol Genet 11:77–86 [DOI] [PubMed] [Google Scholar]
- Seitz H, Royo H, Bortolin ML, Lin SP, Ferguson-Smith AC, Cavaille J 2004 A large imprinted microRNA gene cluster at the mouse Dlk1-Gtl2 domain. Genome Res 14:1741–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SP, Youngson N, Takada S, Seitz H, Reik W, Paulsen M, Cavaille J, Ferguson-Smith AC 2003 Asymmetric regulation of imprinting on the maternal and paternal chromosomes at the Dlk1-Gtl2 imprinted cluster on mouse chromosome 12. Nat Genet 35:97–102 [DOI] [PubMed] [Google Scholar]
- Bahar A, Bicknell JE, Simpson DJ, Clayton RN, Farrell WE 2004 Loss of expression of the growth inhibitory gene GADD45γ, in human pituitary adenomas, is associated with CpG island methylation. Oncogene 23:936–944 [DOI] [PubMed] [Google Scholar]
- Farrell WE, Clayton RN 2003 Epigenetic change in pituitary tumorigenesis. Endocr Relat Cancer 10:323–330 [DOI] [PubMed] [Google Scholar]
- Demura M, Bulun SE 2008 CpG dinucleotide methylation of the CYP19 I.3/II promoter modulates cAMP-stimulated aromatase activity. Mol Cell Endocrinol 283:127–132 [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ 2004 Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854 [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M 2005 Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25:11045–11054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZH, Suppola S, Liu J, Heikkila P, Janne J, Voutilainen R 2002 Association of H19 promoter methylation with the expression of H19 and IGF-II genes in adrenocortical tumors. J Clin Endocrinol Metab 87:1170–1176 [DOI] [PubMed] [Google Scholar]