Abstract
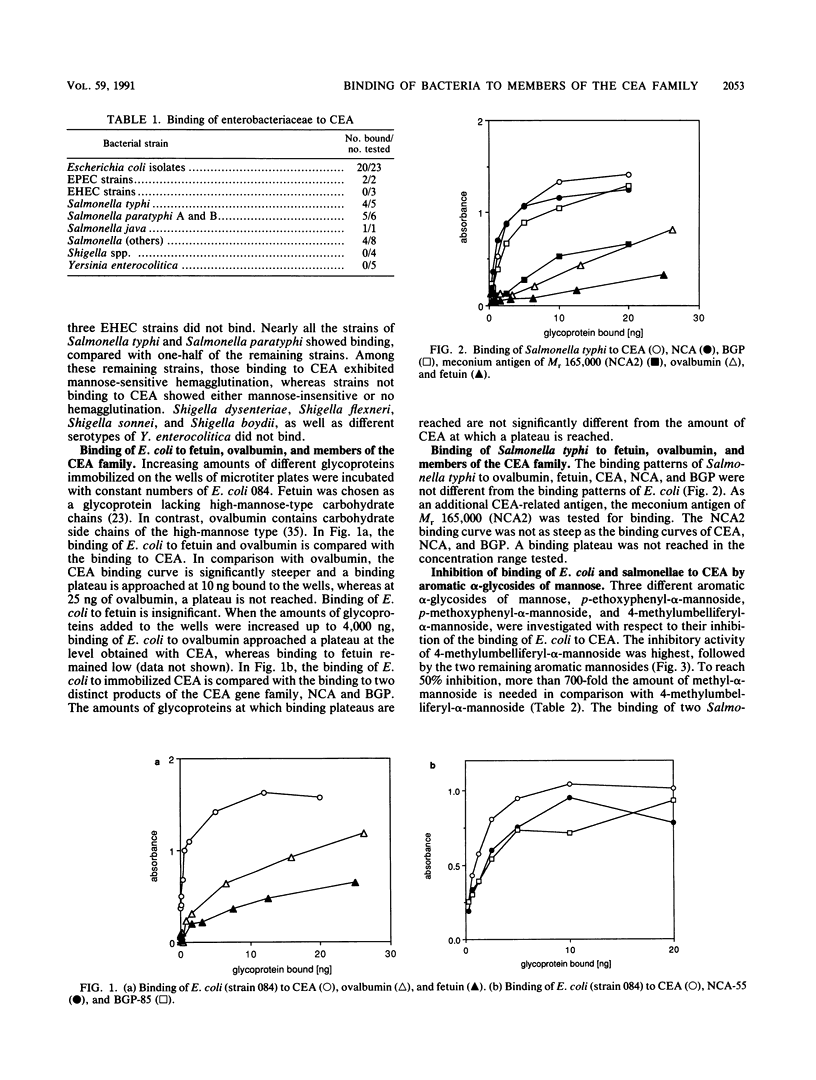
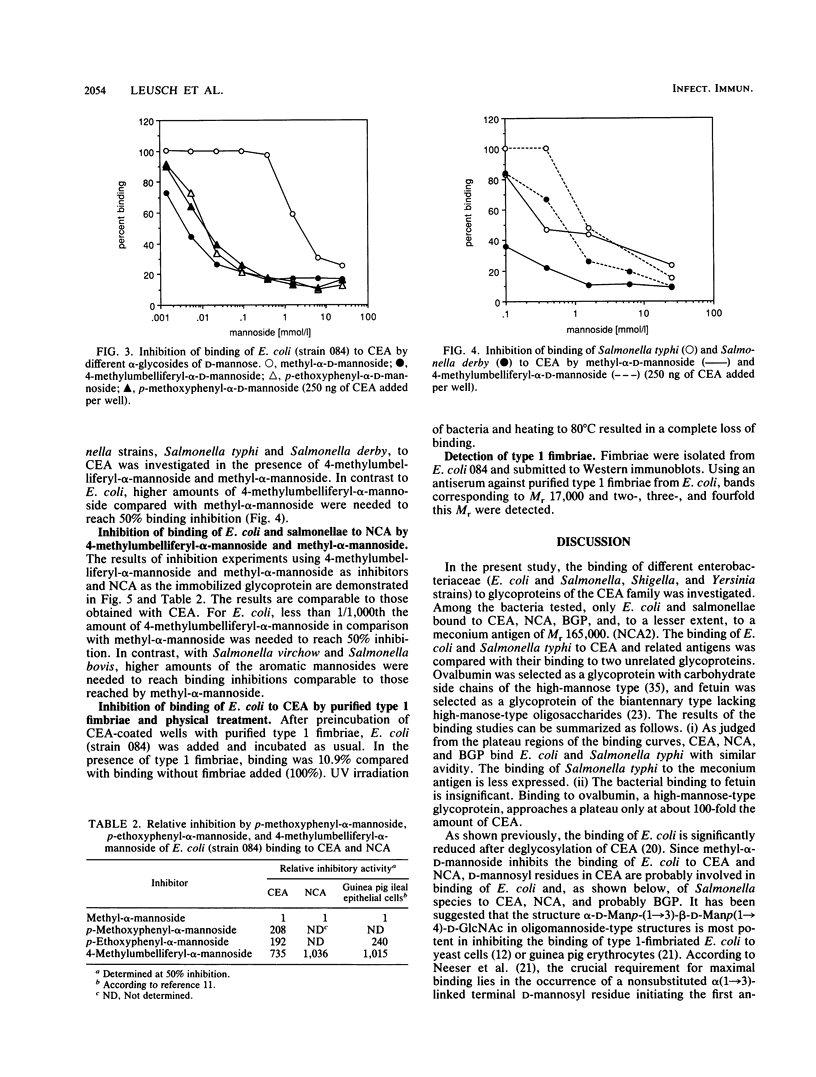
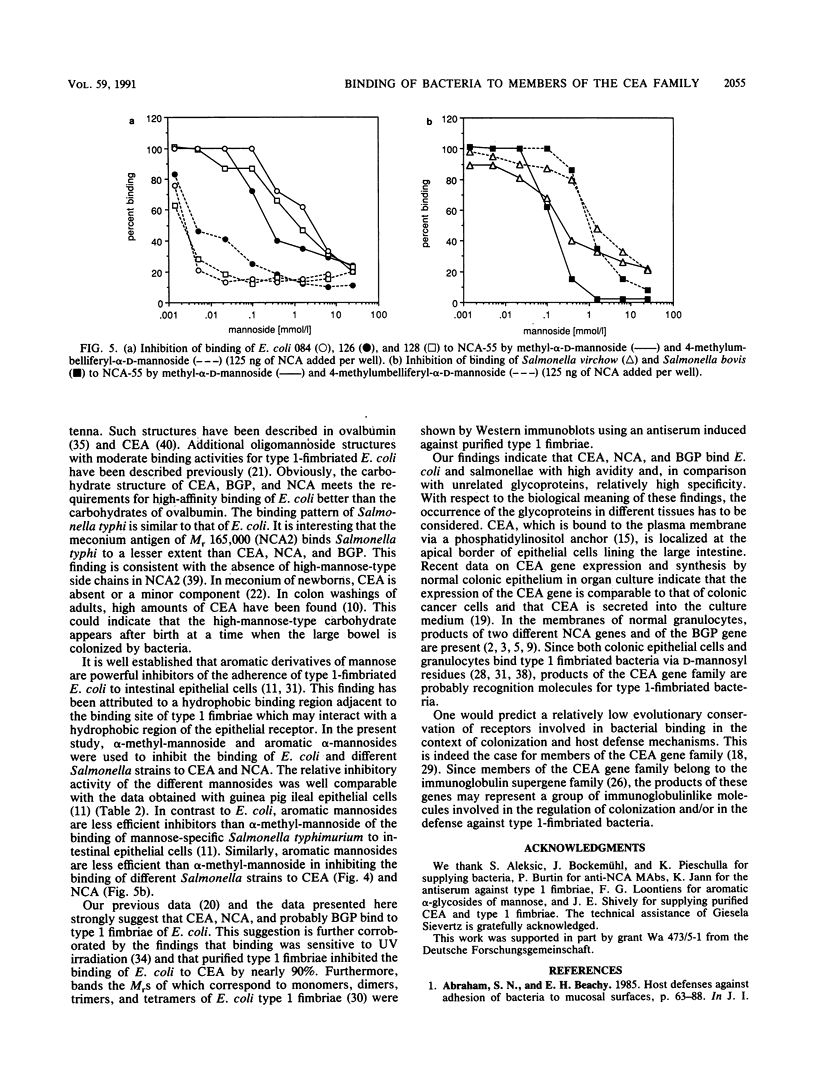
Various Escherichia coli and Salmonella strains bound to glycoproteins of the family of carcinoembryonic antigens (CEA). As judged from plateau regions of the binding curves, CEA, nonspecific cross-reacting antigen of Mr 55,000 (NCA-55), and biliary glycoprotein of Mr 85,000 (BGP-85) showed similar binding activities. The binding to ovalbumin was significantly lower and the binding to fetuin was insignificant under identical experimental conditions. The binding of E. coli and S. typhi to the different glycoproteins was similar as judged from the binding curves. In comparison with alpha-methyl-D-mannopyranoside, aromatic alpha-glycosides of mannose were more potent binding inhibitors of E. coli but not of salmonellae to CEA and NCA-55. These results are similar to those previously obtained with intestinal epithelial cells and yeast cells (N. Firon, S. Ashkenazi, D. Mirelman, I. Ofek, and N. Sharon, Infect. Immun. 55:472-476, 1987). The binding of E. coli to CEA was inhibited by purified type 1 fimbriae. On the basis of the distribution of CEA-like glycoproteins in tissues and body fluids, the results indicate that glycoproteins of the CEA family may be involved in the recognition of bacteria and the regulation of bacterial colonization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa F., Kuroki M., Misumi Y., Oikawa S., Nakazato H., Matsuoka Y. Characterization of a cDNA clone encoding a new species of the nonspecific cross-reacting antigen (NCA), a member of the CEA gene family. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1063–1071. doi: 10.1016/0006-291x(90)90975-s. [DOI] [PubMed] [Google Scholar]
- Audette M., Buchegger F., Schreyer M., Mach J. P. Monoclonal antibody against carcinoembryonic antigen (CEA) identifies two new forms of crossreacting antigens of molecular weight 90,000 and 160,000 in normal granulocytes. Mol Immunol. 1987 Nov;24(11):1177–1186. doi: 10.1016/0161-5890(87)90164-7. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Berling B., Kolbinger F., Grunert F., Thompson J. A., Brombacher F., Buchegger F., von Kleist S., Zimmermann W. Cloning of a carcinoembryonic antigen gene family member expressed in leukocytes of chronic myeloid leukemia patients and bone marrow. Cancer Res. 1990 Oct 15;50(20):6534–6539. [PubMed] [Google Scholar]
- Buchegger F., Schreyer M., Carrel S., Mach J. P. Monoclonal antibodies identify a CEA crossreacting antigen of 95 kD (NCA-95) distinct in antigenicity and tissue distribution from the previously described NCA of 55 kD. Int J Cancer. 1984 May 15;33(5):643–649. doi: 10.1002/ijc.2910330515. [DOI] [PubMed] [Google Scholar]
- Burtin P., Chavanel G., Hendrick J. C., Frenoy N. Antigenic variants of the nonspecific cross-reacting antigen (NCA). J Immunol. 1986 Aug 1;137(3):839–845. [PubMed] [Google Scholar]
- Drzeniek Z., Lamerz R., Fenger U., Wagener C., Haubeck H. D. Identification of membrane antigens in granulocytes and colonic carcinoma cells by a monoclonal antibody specific for biliary glycoprotein, a member of the carcinoembryonic antigen family. Cancer Lett. 1991 Feb;56(2):173–179. doi: 10.1016/0304-3835(91)90093-w. [DOI] [PubMed] [Google Scholar]
- Egan M. L., Pritchard D. G., Todd C. W., Go V. L. Isolation and immunochemical and chemical characterization of carcinoembryonic antigen-like substances in colon lavages of healthy individuals. Cancer Res. 1977 Aug;37(8 Pt 1):2638–2643. [PubMed] [Google Scholar]
- Firon N., Ashkenazi S., Mirelman D., Ofek I., Sharon N. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect Immun. 1987 Feb;55(2):472–476. doi: 10.1128/iai.55.2.472-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983 Aug 16;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- Fritsche R., Mach J. P. Isolation and characterization of carcinoembryonic antigen (CEA) extracted from normal human colon mucosa. Immunochemistry. 1977 Feb;14(2):119–127. doi: 10.1016/0019-2791(77)90290-7. [DOI] [PubMed] [Google Scholar]
- Giampapa C. S., Abraham S. N., Chiang T. M., Beachey E. H. Isolation and characterization of a receptor for type 1 fimbriae of Escherichia coli from guinea pig erythrocytes. J Biol Chem. 1988 Apr 15;263(11):5362–5367. [PubMed] [Google Scholar]
- Hefta S. A., Hefta L. J., Lee T. D., Paxton R. J., Shively J. E. Carcinoembryonic antigen is anchored to membranes by covalent attachment to a glycosylphosphatidylinositol moiety: identification of the ethanolamine linkage site. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4648–4652. doi: 10.1073/pnas.85.13.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoda Y., Neumaier M., Hefta S. A., Drzeniek Z., Wagener C., Shively L., Hefta L. J., Shively J. E., Paxton R. J. Molecular cloning of a cDNA coding biliary glycoprotein I: primary structure of a glycoprotein immunologically crossreactive with carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6959–6963. doi: 10.1073/pnas.85.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodelja V., Lucas K., Barnert S., von Kleist S., Thompson J. A., Zimmermann W. Identification of a carcinoembryonic antigen gene family in the rat. Analysis of the N-terminal domains reveals immunoglobulin-like, hypervariable regions. J Biol Chem. 1989 Apr 25;264(12):6906–6912. [PubMed] [Google Scholar]
- Kuroki M., Arakawa F., Yamamoto H., Shimura H., Ikehara Y., Matsuoka Y. Active production and membrane anchoring of carcinoembryonic antigen observed in normal colon mucosa. Cancer Lett. 1988 Dec 1;43(1-2):151–157. doi: 10.1016/0304-3835(88)90228-5. [DOI] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Winberg J. In vitro adhesion of uropathogenic Escherichia coli to human periurethral cells. Infect Immun. 1980 Jun;28(3):972–980. doi: 10.1128/iai.28.3.972-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leusch H. G., Hefta S. A., Drzeniek Z., Hummel K., Markos-Pusztai Z., Wagener C. Escherichia coli of human origin binds to carcinoembryonic antigen (CEA) and non-specific crossreacting antigen (NCA). FEBS Lett. 1990 Feb 26;261(2):405–409. doi: 10.1016/0014-5793(90)80603-g. [DOI] [PubMed] [Google Scholar]
- Neeser J. R., Koellreutter B., Wuersch P. Oligomannoside-type glycopeptides inhibiting adhesion of Escherichia coli strains mediated by type 1 pili: preparation of potent inhibitors from plant glycoproteins. Infect Immun. 1986 May;52(2):428–436. doi: 10.1128/iai.52.2.428-436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier M., Fenger U., Wagener C. Monoclonal antibodies for carcinoembryonic antigen (CEA) as a model system: identification of two novel CEA-related antigens in meconium and colorectal carcinoma tissue by Western blots and differential immunoaffinity chromatography. J Immunol. 1985 Nov;135(5):3604–3609. [PubMed] [Google Scholar]
- Nilsson B., Nordén N. E., Svensson S. Structural studies on the carbohydrate portion of fetuin. J Biol Chem. 1979 Jun 10;254(11):4545–4553. [PubMed] [Google Scholar]
- Parkkinen J., Virkola R., Korhonen T. K. Identification of factors in human urine that inhibit the binding of Escherichia coli adhesins. Infect Immun. 1988 Oct;56(10):2623–2630. doi: 10.1128/iai.56.10.2623-2630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton R. J., Mooser G., Pande H., Lee T. D., Shively J. E. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):920–924. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard D. G., Todd C. W. Purification of carcinoembryonic antigen by removal of contaminating mucopolysaccharides. Cancer Res. 1976 Dec;36(12):4699–4701. [PubMed] [Google Scholar]
- Rodriguez-Ortega M., Ofek I., Sharon N. Membrane glycoproteins of human polymorphonuclear leukocytes that act as receptors for mannose-specific Escherichia coli. Infect Immun. 1987 Apr;55(4):968–973. doi: 10.1128/iai.55.4.968-973.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudert F., Zimmermann W., Thompson J. A. Intra- and interspecies analyses of the carcinoembryonic antigen (CEA) gene family reveal independent evolution in primates and rodents. J Mol Evol. 1989 Aug;29(2):126–134. doi: 10.1007/BF02100111. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987 Jun 15;217(2):145–157. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- Siepen D., Paxton R. J., Neumaier M., Shively J. E., Wagener C. Carcinoembryonic antigen (CEA) and two crossreacting antigens of 165 kD and 105 kD isolated from meconium exhibit identical amino terminal sequences. Biochem Biophys Res Commun. 1987 Aug 31;147(1):212–218. doi: 10.1016/s0006-291x(87)80108-0. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Wagener C., Yang Y. H., Crawford F. G., Shively J. E. Monoclonal antibodies for carcinoembryonic antigen and related antigens as a model system: a systematic approach for the determination of epitope specificities of monoclonal antibodies. J Immunol. 1983 May;130(5):2308–2315. [PubMed] [Google Scholar]
- Wold A. E., Thorssén M., Hull S., Edén C. S. Attachment of Escherichia coli via mannose- or Gal alpha 1----4Gal beta-containing receptors to human colonic epithelial cells. Infect Immun. 1988 Oct;56(10):2531–2537. doi: 10.1128/iai.56.10.2531-2537.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Totani K., Iwaki Y., Kuroki M., Matsuoka Y., Endo T., Kobata A. Carbohydrate structures of nonspecific cross-reacting antigen-2, a glycoprotein purified from meconium as an antigen cross-reacting with anticarcinoembryonic antigen antibody. Occurrence of complex-type sugar chains with the Gal beta 1----3GlcNAc beta 1----3Gal beta 1----4GlcNAc beta 1----outer chains. J Biol Chem. 1989 Oct 25;264(30):17873–17881. [PubMed] [Google Scholar]
- Yamashita K., Totani K., Kuroki M., Matsuoka Y., Ueda I., Kobata A. Structural studies of the carbohydrate moieties of carcinoembryonic antigens. Cancer Res. 1987 Jul 1;47(13):3451–3459. [PubMed] [Google Scholar]



