Abstract
Context/Objective: The aim was to characterize rates of change in serum estradiol (E2) levels across the menopausal transition and into early postmenopause.
Setting/Participants: We studied the Michigan Bone Health and Metabolism Study cohort of 629 women with median age of 38 yr (interquartile range, 7) at the 1992–1993 baseline with annual assessment of E2 levels over the subsequent 15-yr period.
Design/Main Outcome Measures: The purpose was to describe patterns of acceleration/deceleration in logE2 rates of change before and after the final menstrual period (FMP) using nonparametric and piecewise regression modeling.
Results: Between −10 to −2 yr to the FMP, mean fitted serum E2 population values were relatively stable. The 95% confidence bands around the slight increase in E2 rate of change 5 yr prior to the FMP included the value of no change. The fitted population mean E2 value declined 67% from 64.5 pg/ml (se = 3.6) to 21 pg/ml (se = 1.2) in the 4 yr between −2 < FMP < +2. A second significant mean E2 rate of change was identified from 6–8 yr after FMP. Fitted population mean E2 values declined 18% from 18.1 pg/ml (se = 1.3) at FMP = 6 to 14.8 pg/ml (se = 1.3) at FMP = 8. In nonobese women, the mean E2 percent decline was 42% from FMP = 6 to FMP = 8, whereas in obese women, the mean E2 percent decline over this time was 31%.
Conclusions: Population mean serum E2 levels were sustained until approximately 2 yr prior to the FMP. In the ensuing 4-yr period, E2 levels declined 67%. A secondary E2 decline, commencing about 6 yr after the FMP, was observed in nonobese but not obese women.
Patterns in estradiol (E2) levels and rates-of-change occurring 10 years before and after the final menstrual periodare identified in longitudinal data from 629 women. A precipitous decline (↓67%) in E2 levels began 2 years before the final menstrual period FMP to a plateau of 21 pg/ml at 2 years after. A second significant E2 decline at 6 years after the final menstrual period occurred only in non-obese women.
During reproductive life and into the early menopausal transition, levels of circulating estradiol (E2) change minimally when measured in the early follicular phase in cross-sectional (1,2,3,4,5,6) or longitudinal studies (7,8,9). Furthermore, Burger et al. (8,10) reported that there was not a gradual decline in E2 across the menopause transition, as long believed; rather a relatively rapid decline in E2 occurs shortly before the final menstrual period (FMP). In a small cohort of 11 women, Landgren et al. (11) demonstrated that E2 was the last hormone to change before the FMP compared with FSH, LH, progesterone, and the inhibins (11). Why E2 levels are maintained months after other ovarian markers show evidence of follicular senescence is unclear. However, Welt et al. (12) hypothesized that E2 levels persist because of increased ovarian aromatase function in the late menopause transition (12).
Questions remain about the pattern and timing of changes in E2, including whether there is an increased level of E2 (rather than no change) early in the menopausal transition. Mechanism(s) for sustained levels of E2 rather than a gradual decline before the FMP remain to be elucidated, and patterns of E2 levels after the FMP are yet to be described. This report addresses the natural history of E2 change through the mid and late reproductive years and into the menopause using data from a population-based cohort of women, aged 24–44 yr at their initial annual examination, who were followed from 1992–1993 through 2006–2007. We characterized acceleration and deceleration of E2 rates of change in relation to the FMP. We further considered whether smoking behavior, parity, body mass index (BMI), and age at menarche altered these patterns.
Subjects and Methods
Study population and sample size
The Michigan Bone Health and Metabolism Study (MBHMS) is a population-based longitudinal natural history study of folliculogenesis and how changing sex steroid hormones relate to the initiation and development of musculoskeletal and metabolic diseases (13,14). MBHMS is conducted in a cohort of Caucasian women who have been evaluated through their young and midadulthood years. Age-eligible women (n = 664) were identified from two sampling frames: 1) the family records of the Tecumseh (Michigan) Community Health Study developed from a community census from 1959 to 1985; and 2) a 1992 Tecumseh community listing of names, addresses, and ages. More than 80% of the female Tecumseh offspring, aged 24–44 yr, were recruited from the family records listing (n = 543). Furthermore, 121 women, aged 24–44 yr (91% of eligible listed in Kohl’s Directory), were recruited.
This report includes data collected during the 15-yr period, excluding the 18- and 14-month lapses in funding in 1997 and 2003, respectively. During those months, neither data nor specimens were collected. For this report, 629 women contributed one or more sex steroid data points to the longitudinal data analyses. Sixty-four percent of women had data at every time point; 16% of women had 9–11 data points; 13% of women had 4–9 data points; and 7% of women had 1–3 data points. Blood was not drawn (precluding hormone analyses) when participants were pregnant or breastfeeding at the time of the annual visit. Data were censored at time of death for those 14 (2%) participants who have died since the cohort inception.
This study was approved by the University of Michigan Institutional Review Board, and informed consent was obtained from all participants.
Measures of menopausal transition status
Annually, women were asked about the number and regularity of menstrual bleeding events. A woman was classified as premenopausal with no increase in menstrual irregularity in the previous year and at least nine menstrual cycles in a 12-month period. Perimenopause was defined as having menstrual irregularity with nine or fewer menstrual cycles in a 12-month time period. Postmenopause was designated after 12 consecutive months of amenorrhea associated with no other medical cause. FMP was defined retrospectively to the closest month following 12 months of amenorrhea with no alternative physiologically normal explanation.
Menopause associated with chemotherapy and surgery was identified as such. Hysterectomy and oophorectomy were verified by medical record abstraction. Hormone therapy (HT) and oral contraceptive use was assessed at each visit. Information was recorded about preparation components and duration of use and subsequently coded according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification (15). For purposes of these analyses, data from women with hysterectomy/oophorectomy were censored at the time of surgery; data during HT use were censored for the relevant annual data point(s).
Sex steroid hormones
Blood and urine specimens were collected fasting during d 2–7 of the follicular phase of the menstrual cycle (blood was drawn four times more frequently in d 2–5 than in d 6–7 of the d 2–7 window). If a woman was late perimenopausal or postmenopausal so that phlebotomy could not be linked to menses, specimens were collected on the anniversary of her study enrollment ± 15 d. From 1992 to 2000, blood was drawn in the d 2–7 window 72–95% of the time; from 2000 to the present, among menstruating women, blood was drawn in the d 2–7 window 54–64% of the time, reflecting the increasing likelihood of nonregular menses. Specimens were aliquoted and stored at −80 C without thawing until specimens were assayed.
Serum E2 concentrations were measured in duplicate with a modified, off-line ACS-180 (E2–6) immunoassay. Inter- and intraassay coefficients of variation averaged 10.6 and 6.4%, respectively, over the assay range, and the lower limit of detection was 1 pg/ml. Specific antibody cross-reactivities were 0.75% for estrone (E1), 0.28% for estriol, and 0.00% for norethindrone (16). E2 values from 1992–1993 were excluded from analyses because a different antibody was used that year.
Other measures
Height (in centimeters) and weight (in kilograms) were measured with a stadiometer and balance-beam scale, respectively. BMI was calculated by dividing the weight (in kilograms) by height (in meters) squared. Based on data from interviews, participants were classified as never, past, or current smokers. Parity was described based on the number of live births over 28 wk of age. Age at menarche was self-reported.
Data analysis
Variable distributions were examined, and transformations were applied as necessary to satisfy statistical modeling assumptions including normality and constant variance. E2 values were log transformed (natural) for analysis but back-transformed when presenting results to facilitate ease of communication.
To best model the process of change in E2 values over time, we first used nonparametric stochastic mixed modeling estimating a cubic spline function because relationships between the E2 rates of change and time to FMP could not be appropriately modeled by using quadratic or cubic terms (17). E2 instantaneous rates of change and acceleration were estimated as the first- and second-order derivatives of the cubic spline function for logE2. The E2 data were organized into epochs defined by the inflection points in the E2 slope when E2 was regressed on time to FMP. Piecewise linear mixed models, with inflection points defined by differentiating the cubic spline smoothing functions, were used to describe the change of E2 slope (18). Statistical comparisons of slopes from two consecutive intervals around an inflection point were tested to ascertain whether two adjacent slopes differed. Information on the rate of change and acceleration in the rate of change provides additional information about the pattern of change in E2 levels (i.e. how fast do E2 levels decline and is change best modeled as a sudden shift in level or as a continuous decline in production). Information about acceleration or deceleration can be used to identify where the rate of change either increased or decreased (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). We used bootstrapping of 100 samples to build the 95% bootstrap confidence bands for rates of change and acceleration (19,20).
Analyses were implemented in Matlab7.0 (The MathWorks, Inc., Natick, MA), SAS version 9.1, SAS macro language, and SAS/IML (SAS Institute, Cary, NC).
Results
The cohort median age was 38 yr (IQR = 7) at baseline and 52 yr (IQR = 7) 15 yr later, as shown in Table 1. The median BMI for the cohort at baseline was 25.3 kg/m2 (IQR = 7.4) and 29.4 kg/m2 (IQR = 8.4) 15 yr later. The median age at menarche was 13 yr (IQR = 1), and the median age at FMP was 51 yr. Over the time period, the number of women who were premenopausal (and not using exogenous hormones) declined from 71% in 1992–1993 to 24% at the 2006–2007 visit; surgical menopause frequency was 20% at the 2006–2007 visit. Almost 15% of the cohort remained nulliparous at the 2006–2007 visit. Fifty-three percent of the cohort never smoked cigarettes.
Table 1.
Description of MBHMS cohort at the 1992–1993 baseline and at the follow-up in 2006–2007
| Baseline | 2006–2007 | |
|---|---|---|
| Age (yr) | 38 (7) | 52 (7) |
| BMI (kg/m2) | 25.3 (7.4) | 29.4 (8.4) |
| E2 (pg/ml) | 63.2 (43.2) | 21.1 (40.7) |
| Age at menarche (yr) | ||
| <12 | 100 (17%) | |
| 12–13 | 348 (61%) | |
| >13 | 124 (22%) | |
| Parity | ||
| Nulliparous | 100 (17%) | 58 (15%) |
| Parity = 1–2 | 288 (50%) | 208 (53%) |
| Parity >2 | 184 (32%) | 128 (33%) |
| Smoking status | ||
| Never | 331 (58%) | 263 (53%) |
| Former | 115 (20%) | 155 (31%) |
| Current | 127 (22%) | 75 (15%) |
| Menopausal status | ||
| Premenopause | 408 (71%) | 117 (24%) |
| Perimenopause | 9 (2%) | 41 (8%) |
| Postmenopause | 149 (30%) | |
| Surgical menopause | 25 (4%) | 99 (20%) |
| Oral contraceptive pill or HT use | 134 (23%) | 87 (18%) |
Data represent median (IQR) or number (%).
E2 and rates of change in the early menopause transition
In the time period between −10 to −2 yr and the FMP, fitted curves (from both piecewise linear or nonparametric curvilinear models) indicated that the logE2 population mean values were relatively stable (Fig. 1). Although there appeared to be a slight increase in the rate of logE2 change about 5 yr before the FMP, the 95% confidence bands included the null value of no difference in rate of change (Fig. 2).
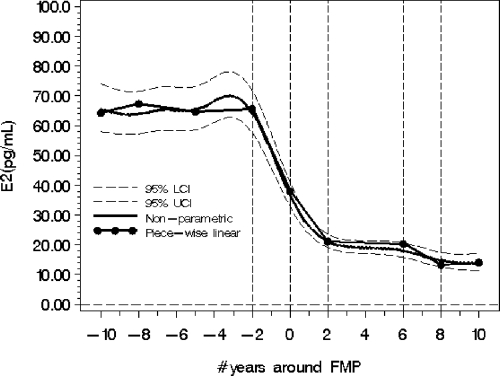
Figure 1.
Population mean backtransformed logE2 levels (pg/ml), both nonparametric and piece-wise fitted models, and upper and lower 95% confidence bands around the nonparametric fitted line for the 20-yr period around the FMP. The vertical reference lines (−2 to +2 and 6 to 8) identify the two time periods in which there are significant declines according to rate of change. LCI, Lower confidence interval; UCI, upper confidence interval.
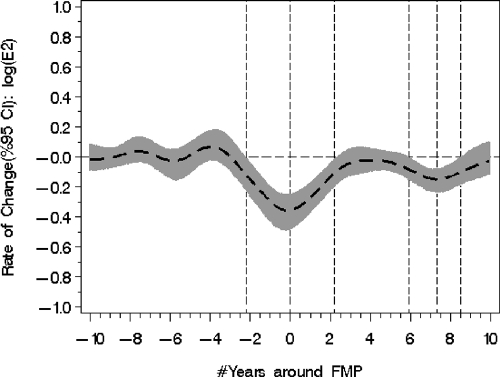
Figure 2.
Significant declines in the instantaneous rates of change occur in logE2 levels at −2, 2, 6, and 8 yr before and after the FMP as identified, with broken vertical lines indicating where the 95% confidence intervals (95% CI) in gray shaded bands exclude the null value of no change.
E2 and rates of change around the FMP
Figure 1 shows the pronounced decline in E2 levels that began, on average, 2 yr before the FMP. Figure 2 shows the marked rates of E2 change referenced to time around the FMP. There were significant differences in the logE2 rates of change identifiable at approximately −2 and +2 yr in relation to the FMP with cut points identified using the highest values of acceleration and deceleration.
Commencing at 2 yr before the FMP, the fitted population mean E2 value declined from 64.5 pg/ml (se = 3.6) to 21 pg/ml (se = 1.2), a decline of 67% in the 4-yr time period around the FMP. These values were determined by back-transforming E2 population means from 4.165 (se = 0.056) at FMP = −2 and 3.054 (se = 0.055) at FMP = +2.
The mean instantaneous rate of change was −0.29 (P < 0.0001) from 2 yr before the FMP. At the time of the FMP, the logE2 instantaneous rates of change began slowing to a value of no change at FMP = +2 yr.
E2 and rates of change in the postmenopause
In the time between 2 and 6 yr after the FMP, the mean logE2 rate of change was −0.009 (P = 0.77), indicating that the rate of change was not significantly different than zero. However, a second significant alteration in the mean logE2 rate of change (−0.21, P = 0.008) was identified in the postmenopause period at 6 yr < FMP < 8 yr, with the maximal instantaneous declining rate occurring around 7.3 yr after FMP (Fig. 2). The back-transformed fitted population mean E2 value declined from 18.1 pg/ml (se = 1.26) at FMP ≈ 6 to 14.8 pg/ml (se = 1.25) at FMP ≈ 8, or an average 18% decline. Eight years after the FMP, the mean rate of change had declined to 0.029 (P = 0.57), a change not significantly different than zero.
Importance of BMI
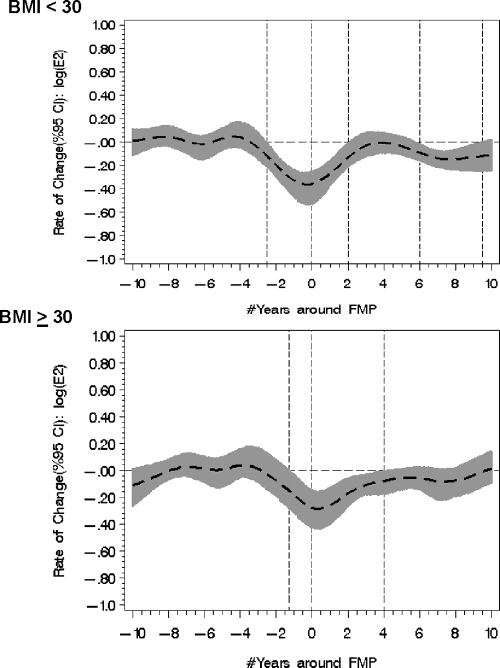
BMI was significantly associated with logE2 measures, but the effect was not constant and varied in relation to the FMP (Fig. 3). The effect was most prominent at yr 6 < FMP < 8 at the second significant rate of change; the decline in logE2 levels was observed in women less than 30 kg/m2 with the mean rate of change 0.27 (se = 0.11, P = 0.01) but was not observed in women with a BMI greater than 30 kg/m2 with the mean rate of change 0.20 (se = 0.18, P = 0.26). The mean E2 percent decline among the nonobese women at 6 > FMP > 8 was from 20.8 pg/ml (se = 2.6) at FMP ≈6 to 12.2 pg/ml (se = 2.3) at FMP ≈8, or an average 42% decline, whereas the mean in obese women was from 20.7 pg/ml (se = 3.1) at FMP ≈6 to 16.4 pg/ml (se = 4.4) at FMP ≈8. Current smoking behavior, parity, and age at menarche were not associated with important differences in logE2 levels or rates of logE2 change (data not shown).
Figure 3.
The association of BMI was inconsistent across the menopause transition, with logE2 rate of change declining among nonobese women (<30 kg/m2) in 6 < FMP < 9.5 in contrast to no decline in obese women (≥30 kg/m2). CI, Confidence intervals.
Discussion
It is hypothesized that in the late reproductive period and early menopausal transition, altered menstrual functioning and the accompanying hormone alterations reflect the failure of follicular recruitment (21) or the recruitment of poor quality antral follicles. This is, in turn, thought to contribute to reduced granulosa cell number or poor responsiveness of the granulosa cell to stimulation by gonadotropins. This sequence of events is associated with lower inhibin levels and higher FSH (10). Ultimately, this results in lower E2 levels. It is widely believed that rates of change in E2 may be important explanatory factors in the frequency and presentation of conditions such as disturbed sleep (22) or hot flash frequency (23).
There are at least three areas of uncertainty related to E2 levels as women progress through the menopause transition and into the postmenopause. First, there is uncertainty about the presence of a transient increase in the E2 rate of change in older, but ovulatory women compared with younger ovulatory women before the FMP. Second, there is controversy as to why E2 levels remain relatively conserved into the later perimenopause period when other indicators of folliculogenesis such as changing FSH, inhibins, anti-Mullerian hormone, and antral follicle count all indicate advancing reproductive aging. Third, there is controversy as to the likelihood that E2-driven biological activity being maintained in the early postmenopausal period can influence bone and cancer cells.
Whether there is a transient increase in E2 in older, but ovulatory women, compared with younger ovulatory women remains a point of uncertainty, with some studies reporting similar (2,24,25), decreased (1), or increased levels (26). Our models suggested a slight increase in the population mean logE2 in women at approximately 5 yr before the FMP, which was associated with a modest increase in the logE2 rate of change. However, the 95% confidence bands included the null value for rate of change, suggesting that a population increase is unlikely. There are several possible explanations for an apparent increase in serum E2 in the perimenopause period. First, in studies such as MBHMS with protocols for specimen collection windows in d 2–7 after the onset of menstrual bleeding, the increasingly shorter follicular phase as women age, as described by Klein et al. (27), may cause the specimen collection window to encompass not only the lower E2 values of the early follicular phase but also the higher values of the midfollicular phase. Notably, in MBHMS and as described in Subjects and Methods, more than 80% of the specimens assayed and reported collected were in the d 2–5 window among those women still menstruating. A second explanation may rest with the increased frequency of contribution from secondary follicles (28) or with the increasing likelihood of anovulatory events (2). After extended exposure to elevated gonadotropins, one or more follicles may become responsive, and E2 levels may rise. It has also been proposed that there is a difference in FSH bioactivity with aging that alters the FSH requirement to sustain follicular development, although actual measurement of bioactive FSH is not consistent with this hypothesis (27). Our data, anchored to the FMP, suggest that the magnitude of any increase that might be present 5 yr before the FMP is insufficient to suggest a major shift in population levels of circulating E2.
We confirmed previous work whose models indicated that the major mean E2 population values decline at approximately 2 yr before the FMP (8,10). Furthermore, we identified an accelerated rate of E2 change until the time of the FMP. At the FMP, a major deceleration in this rate of change occurred, lasting until approximately 2 yr after the FMP. We have previously reported that 2 yr before the FMP, there was a final and acute increase in the logFSH rate of change (29). We propose that this accelerated change in E2 2 yr before the FMP should be evaluated as a signal that is permissive for increasingly greater bone loss, more rapid change in lipids, and potentially greater hot flash frequency among those with hot flashes.
It is unclear why sustained E2 levels are maintained months after other ovarian markers show evidence of follicular senescence. The positive correlations between follicle size or maturity and estrogen and progesterone concentrations in dominant follicle aspirates (30) led us to speculate that the identified acute accelerations in the FSH rate of change and E2 rate of change at 2 yr before the FMP reflect the frequent failure to establish the corpus luteum and deterioration in the reciprocal relationship of FSH with the inhibins (31). Welt et al. (12) hypothesized that E2 levels are maintained because of increased ovarian aromatase function in the late menopause transition where ovarian aromatase function was approximated using a ratio of E1 and androstenedione levels (12).
Notably, we also identified a significant additional alteration in the logE2 rate of change between 6 and 8 yr after the FMP, but the direction and magnitude of that rate of change varied according to BMI. Women with a greater BMI (≥30 kg/m2) did not develop the second decline in logE2 levels. We speculate that this second decline in E2 rates of change represents exhaustion of theca cells. The relative absence of this second decline in obese women may be related to supplementation of the E2 pool by androstenedione and E1 from extragonadal sources, including adipose tissue. Androstenedione is converted to E1 by aromatase, and then E1 can be converted to E2 (32) by type 1 17-β-hydroxysteroid dehydrogenase, which catalyzes the reversible reaction between E1 and the more biologically active E2. Typically, the gradient in this bidirectional enzyme action is thought to favor the conversion of E2 to E1; however, the greater E1 levels in obese women relative to the low E2 levels in all women during the postmenopause may reverse the gradient to favor the formation of a small but detectable amount of E2 from E1. Moreover, the inverse relationship of BMI and E2 levels observed in the premenopause and early perimenopause (6) reverses in the postmenopause (9) and supports this potential mechanism.
It remains to be determined whether the higher levels of E2 at 6 to 8 yr after the FMP in obese postmenopausal women compared with nonobese postmenopausal women has important health implications. Some studies have reported that postmenopausal sex hormone concentrations are risk factors for several major chronic diseases of women, including osteoporosis, breast cancer, and endometrial cancer, and, with less certainty, coronary heart disease and osteoarthritis. Several studies have found that very low concentrations of either total or bioavailable E2 and high concentrations of SHBG (which binds estrogen and testosterone, making these hormones less available to target receptors) are associated with increased risk for hip fracture (33,34,35), low bone mineral density (36,37), and more rapid bone mineral density loss (38). A meta-analysis from the Endogenous Hormones and Breast Cancer Collaborative Group (39) found a greater risk for postmenopausal breast cancer with increasing concentrations of all sex hormones examined, including total E2, free E2, non-SHBG-bound E2, E1, E1 sulfate, and androstenedione. Endometrial cancer risk has also been associated with higher concentrations of E2, E1, and testosterone and lower concentrations of SHBG (40).
The strengths of this study include having a large cohort of women representative of a general population group rather than a selected clinical subsample. Cohort participation has been excellent over a 15-yr period that encompassed the late reproductive period, the menopause transition, and the early postmenopause. E2 was measured in duplicate with an assay that, over time, employed the same antibody that does not cross-react with other sex steroids. Across most of the study period, specimens were collected annually in the early follicular phase of the menstrual cycle (primarily in d 2 to 5 after initiation of menstrual bleeding) to allow for the comparison of values in a standardized time frame. However, these data reflect total E2 rather than free E2, and this study does not include measures of androstenedione or E1 to confirm our interpretation of events occurring in the E2 rate of change of the early postmenopause. Finally, findings in Caucasian women may not be generalizable to other race/ethnic groups because we have reported somewhat lower E2 in Chinese and Japanese women in the Study of Women’s Health Across the Nation (9).
Significant rates of E2 change were not observed until approximately 2 yr before the FMP; this pattern did not differ according to smoking status, parity, BMI, or age at menarche. From −2 yr to the FMP, there was a precipitous decline in E2 levels, reflected in a marked rate of change. This rate of change led to a new plateau level of 21 pg/ml at 2 yr after the FMP. There was a second significant decline in E2 at 6 yr after the FMP among the nonobese, accompanied by increased rate of change, but this was not observed in obese women. Future studies are needed to identify those mechanisms associated with the sustained E2 levels long after other markers of folliculogenesis show significant change. Additional studies are also needed to replicate our observed second E2 decline in the early postmenopause among nonobese women (compared with obese women) and to determine whether these E2 levels are important for subsequent disease risk.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants AR051384, AR040888, and AR20557 (M. Sowers, Principal Inves-tigator).
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 22, 2008
Abbreviations: BMI, Body mass index; E1, estrone; E2, estradiol; FMP, final menstrual period; HT, hormone therapy; IQR, interquartile range; MBHMS, Michigan Bone Health and Metabolism Study.
References
- Sherman BM, West JH, Korenman SG 1976 The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab 42:629–636 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lenton EA, Sexton L, Cooke ID 1988 The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Hum Reprod 3:851–855 [DOI] [PubMed] [Google Scholar]
- Sherman BM, Korenman SG 1975 Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest 55:699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf MG, Donald RA, Livesey JH 1981 Pituitary-ovarian function in normal women during the menopausal transition. Clin Endocrinol (Oxf) 14:245–255 [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Hopper JL, Shelley JM, Green A, Smith A, Dennerstein L, Morse C 1995 The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab 80:3537–3545 [DOI] [PubMed] [Google Scholar]
- Randolph Jr JF, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL 2003 Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 88:1516–1522 [DOI] [PubMed] [Google Scholar]
- Rannevik G, Carlstrom K, Jeppsson S, Bjerre B, Svanberg L 1986 A prospective long-term study in women from pre-menopause to post-menopause: changing profiles of gonadotrophins, oestrogens and androgens. Maturitas 8:297–307 [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L 1999 Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84:4025–4030 [DOI] [PubMed] [Google Scholar]
- Randolph Jr JF, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ 2004 Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 89:1555–1561 [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Robertson DM, Dennerstein L 2002 Hormonal changes in the menopause transition. Recent Prog Horm Res 57:257–275 [DOI] [PubMed] [Google Scholar]
- Landgren BM, Collins A, Csemiczky G, Burger HG, Baksheev L, Robertson DM 2004 Menopause transition: annual changes in serum hormonal patterns over the menstrual cycle in women during a nine-year period prior to menopause. J Clin Endocrinol Metab 89:2763–2769 [DOI] [PubMed] [Google Scholar]
- Welt CK, Jimenez Y, Sluss PM, Smith PC, Hall JE 2006 Control of estradiol secretion in reproductive ageing. Hum Reprod 21:2189–2193 [DOI] [PubMed] [Google Scholar]
- Sowers MF, Kshirsagar A, Crutchfield MM, Updike S 1992 Joint influence of fat and lean body composition compartments on femoral bone mineral density in premenopausal women. Am J Epidemiol 136:257–265 [DOI] [PubMed] [Google Scholar]
- Sowers M, Willing M, Burns T, Deschenes S, Hollis B, Crutchfield M, Jannausch M 1999 Genetic markers, bone mineral density, and serum osteocalcin levels. J Bone Miner Res 14:1411–1419 [DOI] [PubMed] [Google Scholar]
- 2007 Guidelines for ATC classification. Oslo, Norway: World Health Organization Collaborating Centre for Drug Statistics Methodology. Accessed online at http://www.whocc.no/atcddd [Google Scholar]
- England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR 2002 Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clin Chem 48:1584–1586 [PubMed] [Google Scholar]
- Zhang D, Lin X, Sowers M 1998 Semiparametric stochastic mixed models for longitudinal data. J Am Stat Assoc 93:710–719 [Google Scholar]
- Neter J, Wasserman W, Kutner M 1985 Applied linear statistical models. 2nd ed. Homewood, IL: Irwin [Google Scholar]
- Efron B, Tibshirani R 1986 Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Science 1:54–77 [Google Scholar]
- Claeskens G, Van Keilegom I 2003 Bootstrap confidence bands for regression curves and their derivatives. Ann Statistics 31:1852–1884 [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF 1987 Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab 65:1231–1237 [DOI] [PubMed] [Google Scholar]
- Sowers MF, Zheng H, Kravitz HM, Matthews, Bromberger JT, Gold EB, Owens J, Consens F, Hall M2008 Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep, in press [PMC free article] [PubMed] [Google Scholar]
- Freedman RR 2000 Menopausal hot flashes. In: Lobo RA, Kelsey K, Marcus R, eds. Menopause: biology and pathobiology. San Diego: Academic Press; 215–227 [Google Scholar]
- Reyes FI, Winter JS, Faiman C 1977 Pituitary-ovarian relationships preceding the menopause. I. A cross-sectional study of serum follicle-stimulating hormone, luteinizing hormone, prolactin, estradiol, and progesterone levels. Am J Obstet Gynecol 129:557–564 [PubMed] [Google Scholar]
- Fitzgerald CT, Seif MW, Killick SR, Elstein M 1994 Age related changes in the female reproductive cycle. Br J Obstet Gynaecol 101:229–233 [DOI] [PubMed] [Google Scholar]
- Musey VC, Collins DC, Musey PI, Martino-Saltzman D, Preedy JR 1987 Age-related changes in the female hormonal environment during reproductive life. Am J Obstet Gynecol 157:312–317 [DOI] [PubMed] [Google Scholar]
- Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR 1996 Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab 81:1038–1045 [DOI] [PubMed] [Google Scholar]
- Ahmed Ebbiary NA, Lenton EA, Salt C, Ward AM, Cooke ID 1994 The significance of elevated basal follicle stimulating hormone in regularly menstruating infertile women. Hum Reprod 9:245–252 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Zheng H, McConnell D, Nan B, Randolph Jr JF2008 Follicle stimulating hormone (FSH) and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab 93:3958–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dessel HJ, Schipper I, Pache TD, van Geldorp H, de Jong FH, Fauser BC 1996 Normal human follicle development: an evaluation of correlations with oestradiol, androstenedione and progesterone levels in individual follicles. Clin Endocrinol (Oxf) 44:191–198 [DOI] [PubMed] [Google Scholar]
- Sowers MFR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zheng D, Harlow S, Randolph JF2000 Anti-Mullerian hormone (AMH) and inhibin-B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 93:3478–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailly S, Gougeon A, Milgrom E, Bomsel-Helmreich O, Papiernik E 1981 Androgens and progestins in the human ovarian follicle: differences in the evolution of preovulatory, healthy nonovulatory, and atretic follicles. J Clin Endocrinol Metab 53:128–134 [DOI] [PubMed] [Google Scholar]
- Chapurlat RD, Garnero P, Breart G, Meunier PJ, Delmas PD 2000 Serum estradiol and sex hormone-binding globulin and the risk of hip fracture in elderly women: The EPIDOS study. J Bone Miner Res 15:1835–1841 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B 1998 Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med 339:733–738 [DOI] [PubMed] [Google Scholar]
- Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD 2000 Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J Bone Miner Res 15:1526–1536 [DOI] [PubMed] [Google Scholar]
- Ettinger B, Pressman A, Sklarin P, Bauer DC, Cauley JA, Cummings SR 1998 Associations between low levels of serum estradiol, bone density, and fractures among elderly women: The study of osteoporotic fractures. J Clin Endocrinol Metab 83:2239–2243 [DOI] [PubMed] [Google Scholar]
- Heshmati HM, Khosla S, Robins SP, O'Fallon WM, Melton 3rd LJ, Riggs BL 2002 Role of low levels of endogenous estrogen in regulation of bone resorption in late postmenopausal women. J Bone Miner Res 17:172–178 [DOI] [PubMed] [Google Scholar]
- Stone K, Bauer DC, Black DM, Sklarin P, Ensrud KE, Cummings SR 1998 Hormonal predictors of bone loss in elderly women: a prospective study. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 13:1167–1174 [DOI] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson Jr HE, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Key TJ, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR, Longcope C, Endogenous Hormones Breast Cancer Collaborative Group 2003 Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 95:1218–1226 [DOI] [PubMed] [Google Scholar]
- Kaaks R, Lukanova A, Kurzer MS 2002 Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 11:1531–1543 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.