Abstract
Context: Dietary factors may trigger or exacerbate the autoimmune disease process.
Objective: Our objective was to examine dietary glycemic index (GI) and glycemic load (GL) for association with islet autoimmunity (IA) development, and progression from IA to type 1 diabetes.
Design: The Diabetes Autoimmunity Study in the Young follows children at increased genetic type 1 diabetes risk. Diet is collected prospectively via a parent-reported food frequency questionnaire.
Setting: This was an observational study of children in the Denver area.
Patients: A total of 1776 Diabetes Autoimmunity Study in the Young children younger than 11.5 yr was included in the study.
Interventions: There were no interventions.
Main Outcome Measures: IA, defined as the presence of autoantibodies to insulin, glutamic acid decarboxylase, or protein tyrosine phosphatase at two consecutive visits, or the presence of autoantibodies at one visit and diabetic on the next consecutive visit was determined. Type 1 diabetes was diagnosed by a physician. A total of 89 subjects developed IA, and 17 subsequently developed type 1 diabetes during follow-up. Our hypothesis was formulated after data collection.
Results: GI and GL were not associated with IA development. More rapid progression to type 1 diabetes in children with IA was associated with higher dietary GI (hazard ratio: 2.20; 95% confidence interval: 1.17–4.15) and marginally associated with GL (hazard ratio: 1.59; 95% confidence interval: 0.96–2.64) at the first IA-positive visit.
Conclusions: Higher dietary GI and GL are not associated with IA development, but higher GI is associated with more rapid progression to type 1 diabetes in children with IA, perhaps due to increased demand on the β-cell to release insulin. Further study is needed to confirm this finding and identify the underlying biological mechanism.
In 1,776 children at increased risk for type 1 diabetes, higher dietary glycemic index is not associated with development of islet autoimmunity, but with more rapid progression from islet autoimmunity to type 1 diabetes, perhaps due to increased demand on the β-cell to release insulin.
Environmental factors may be responsible for the increased incidence and earlier age of type 1 diabetes onset observed worldwide (1,2,3,4,5). Type 1 diabetes results from the autoimmune destruction of pancreatic β-cells. Although islet autoimmunity (IA) precedes, and is strongly predictive of, type 1 diabetes development (6), little is known about the factors that influence IA development or subsequent progression to type 1 diabetes.
The overload hypothesis proposes a mechanism describing a role for β-cell stress in progression to type 1 diabetes, suggesting that overfeeding and sedentary lifestyles of today’s youth place a high insulin demand on the β-cell (7). Increased insulin production may cause increased β-cell apoptosis (8) and make the β-cell more vulnerable to autoimmune attack (9).
We explored the quality of carbohydrates in the diets of children at increased genetic risk for type 1 diabetes. Consumption of high glycemic index (GI) foods results in large postprandial spikes in blood glucose and a subsequent larger production of insulin. We hypothesized that diets high in GI and/or glycemic load (GL) would stress β-cells, thus increasing β-cell apoptosis and β-cell vulnerability to the immune system. Therefore, such diets may be associated with IA development and more rapid progression to type 1 diabetes.
Subjects and Methods
The Diabetes Autoimmunity Study in the Young (DAISY) is a prospective study of three groups of young children at increased risk for developing type 1 diabetes. One group consists of unaffected first-degree relatives of patients with type 1A diabetes, identified and recruited between birth and age 8 yr through the Barbara Davis Center for Childhood Diabetes in Denver, CO, other diabetes care clinics, and the Colorado IDDM Registry. The second group consists of babies born at St. Josephs Hospital in Denver, CO, and screened by umbilical cord blood samples for diabetes-susceptibility alleles in the human leukocyte antigen (HLA) region. This group is representative of the general population of the Denver metropolitan area. Cord blood is sent to Roche Molecular Systems, Inc. (Alameda, CA) for PCR-based HLA class II typing. The third group consists of siblings of DAISY subjects that have the high-risk HLA genotype. These siblings are enrolled in the DAISY and followed according to DAISY protocol. Siblings of DAISY subjects that test positive for autoantibodies at the family-screening visit are enrolled in the DAISY, and data collected from these children are included in the analyses of the development of type 1 diabetes in autoimmune children. The details of the newborn screening (10) and follow-up (11) have been published elsewhere. The DAISY has enrolled approximately 2500 children at increased risk for type 1 diabetes from 1993–2004.
Prospective follow-up of DAISY subjects included clinic visits at 9, 15, and 24 months (if child enrolled at birth) or at enrolment (if child enrolled later in childhood), and then annually thereafter up to age 15 (10). At every visit, blood was drawn and tested for autoantibodies to insulin, protein tyrosine phosphatase, and glutamic acid decarboxylase using RIAs, as described previously (12,13,14). IA was defined as testing positive for one or more islet autoantibody on two consecutive visits, or autoantibody positive on one visit and diabetic on the next consecutive visit within 1 yr of first autoantibody positive visit. Children who tested positive for an autoantibody were examined every 3–6 months, and glycosylated hemoglobin (HbA1c) and random glucose were also measured. A child was referred to a physician for diagnosis if he/she had a random glucose more than 200 mg/dl and/or a HbA1c more than 6.2%. Criteria used for type 1 diabetes diagnosis included typical symptoms of polyuria and/or polydipsia, and a random glucose more than 200 mg/dl or an oral glucose tolerance test with the 2-h glucose more than 200 mg/dl. Details of intensive monitoring and diagnosis protocol were described previously (15).
A semiquantitative food frequency questionnaire (FFQ) (Nutrition Questionnaire Service Center, Boston, MA) was used to collect the child’s diet via the parental report. This FFQ has been validated using multiple 24-h recalls and biomarkers in the DAISY population (16,17). Starting when the child is 2 yr old and annually thereafter, parents completed the FFQ for their child’s diet in the previous year. Parents classify their child’s intake of foods into nine response categories ranging from “never or less than once per month” to “6+ times per day.” The average daily intake of nutrients was calculated by multiplying each item’s frequency of consumption by its nutrient content and summing the nutrient contributions of all foods. FFQs were checked for feasibility and completeness before analysis. All cases of IA and type 1 diabetes that developed before age 1 yr were not included in these analyses due to the lack of FFQ collection before age 1 yr.
Nutrient values (daily average) for energy, carbohydrates, fiber, GI, and GL were calculated from reported intakes. The GI and GL were calculated as described in Ref. 18, using glucose as the reference. In brief, GI is defined as the glycemic response (i.e. the incremental area under the glucose response curve) measured for 2 h after consumption of a test food, relative to the glycemic response to a reference food containing the same amount of carbohydrates. The average daily GI is calculated by summing the products of the carbohydrate content per serving for each food consumed times the average number of servings of that food consumed per day, times each food’s GI, all divided by the total daily carbohydrate intake. GL was calculated by multiplying the carbohydrate content of each food by its GI value, then multiplying this value by the frequency of consumption and summing over all food items. The U.S. Department of Agriculture Nutrient Database for Standard Reference (SR) Release 10 was used to calculate nutrient values for the diet surveys collected from January 1996 to May 1999, SR Release 11 from June 1999 to February 2003, and SR Release 14 from March 2003 to present. Reported dietary intakes were adjusted for total energy intake using the residual method (19).
Dietary data were linked to an autoantibody measurement if the 1-yr time period of the questionnaire encompassed the time directly preceding the clinic visit at which the autoantibody was measured. For the analysis of the association between the diet and IA development, we analyzed multiple diet records per subject up until the age of first autoantibody positive visit (in those that developed IA) or age at last follow-up.
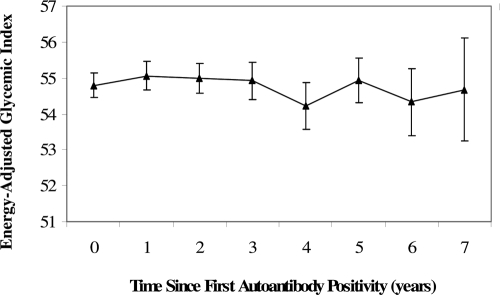
For the analysis of diet and the progression to type 1 diabetes in IA-positive children, we examined a single dietary measure corresponding to the age at first positive visit, for the following reason. When a child was diagnosed with type 1 diabetes outside of a scheduled DAISY visit, we did not contact the family after diagnosis to collect an FFQ because the intense dietary advice that a family receives at diagnosis may alter their recollection of the child’s pre-diagnosis diet. Thus, we do not have diet information corresponding to diagnosis on a number of children who developed type 1 diabetes in the analysis cohort. Because dietary GI is relatively stable in the autoimmune period (Fig. 1), and due to our concerns that these missing data would reduce our sample size, and the differential amounts of missing data may introduce bias, we defined our dietary exposure as a fixed variable at the age at first autoantibody positive visit.
Figure 1.
Means and ses of energy adjusted GI intake in the autoimmune period.
The following demographical and cohort selection variables were collected at the enrolment interview: gender, race/ethnicity, maternal education, annual household income, and type 1 diabetes status of first-degree relatives. Weight and height were measured at every clinic visit. Body mass index (BMI) was calculated as weight (kg)/height (m)2 for all clinic visits at age 2 yr or older. BMI z scores were calculated using Centers for Disease Control and Prevention growth charts (http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm).
Because puberty is a time of hormonal changes that result in rapid growth and increased insulin resistance, we only analyzed records collected between ages 1 and 11.0 yr for girls, and between ages 1 and 11.5 yr for boys. Records collected after these ages were censored. These age cutoffs were obtained by collecting self-Tanner staging questionnaires (20) on a subset of DAISY children (n = 604). The age cutoffs represent the median ages at which children reported being at Tanner stage II. The Colorado Multiple Institutional Review Board approved all study protocols. Informed consent was obtained from the parents/legal guardians of all children.
A total of 89 DAISY children developed IA. Of the 89 IA-positive children, 10 tested antibody positive at their first clinic visit; these 10 left-censored children could not be included in the analysis of the development of IA. Seventeen of these 89 IA-positive children developed type 1 diabetes (described in the supplemental table, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
IA analysis
A Cox proportional hazards model was used to explore associations between GI, GL, and IA development. We analyzed 7560 diet records on 1776 DAISY children, 79 of whom developed IA. GI and GL were included in the model as time-varying covariates and analyzed both as continuous variables and in tertiles. Because children with high GI diets may be more overweight compared with their peers (21), and obesity may be associated with type 1 diabetes development (22,23), we explored the BMI z score as a potential mediating factor between GI, GL, and IA development.
Type 1 diabetes analysis
Using Cox proportional hazards models, we explored whether dietary GI and GL at the first autoantibody positivity were associated with the development of type 1 diabetes in the 89 children that developed IA. The length of follow-up was measured starting with the first autoantibody positive visit and ending with either type 1 diabetes diagnosis (cases) or most recent clinic visit (noncases). Dietary data for GI and GL were analyzed both continuously and in tertiles. Finally, we explored the BMI z score as a potentially mediating factor between GI, GL, and more rapid type 1 diabetes development in autoimmune children.
Results
Development of IA
The mean age at the first autoantibody positive visit was 4.8 yr in the 79 DAISY children in this analysis (Table 1). GI intake throughout childhood in these subjects is described in Table 2. Children that developed IA were more likely to have a high-risk HLA genotype or have a family history of type 1 diabetes than children that did not develop IA. There were no differences in ethnicity, maternal age, maternal education, or income between children that did and did not develop IA.
Table 1.
Descriptive characteristics of the IA analysis cohort and description of the reported childhood diet with time to development of IA
| Dietary component | Developed IA (n = 79) (%) | Did not develop IA (n = 1697) (%) | HR (95% CI) |
|---|---|---|---|
| High-risk genotype HLA-DR3/4,DQ8 | 36.7 | 20.5 | 2.12 (1.33–3.36) |
| Family history of type 1 diabetes | 63.3 | 49.6 | 1.74 (1.10–2.77) |
| Female | 53.2 | 47.6 | 1.20 (0.77–1.87)a |
| Non-Hispanic white ethnicity | 79.8 | 75.8 | 1.07 (0.61–1.88)a |
| Maternal education >12 yr | 87.3 | 79.1 | 1.54 (0.79–3.01)a |
| Annual income ≥$30,000 | 81.6 | 78.0 | 1.16 (0.65–2.09)a |
| Mean age (yr) at first IA-positive visit or most recent visit (sd) | 4.82 (2.49) | 6.20 (2.80) | N/A |
| BMI z score (1581 subjects, 67 cases) | N/Ab | N/Ab | 1.19 (0.94–1.51)a |
| Total calories (kcal/d) | N/Ab | N/Ab | 1.01 (0.80 −1.26)a,c |
| Total carbohydrates (g/d) | N/Ab | N/Ab | 0.91 (0.73–1.15)c,d |
| Total fiber (g/d) | N/Ab | N/Ab | 0.95 (0.75 −1.21)c,d |
| GI | N/Ab | N/Ab | 0.95 (0.76–1.19)c,d |
| GL | N/Ab | N/Ab | 0.91 (0.72 −1.14)c,d |
Adjusted for HLA genotype and family history of type 1 diabetes.
Not applicable (N/A) due to the time-varying nature of the data. See Table 2 for details regarding these variables.
HR and 95% CI represent 1 sd change in the diet variable.
Diet variables were adjusted for energy intake using the residual method (19). Models adjusted for total calories, HLA genotype, and family history of type 1 diabetes.
Table 2.
Description of time-varying diet and childhood size data used in the IA outcome analysis
| IA analysis cohort | Mean at age 3 yr (sd) | Mean at age 5 yr (sd) | Mean at age 8 yr (sd) |
|---|---|---|---|
| Time-varying variable | n = 1002 | n = 801 | n = 519 |
| Total calories (kcal/d) | 2157.44 (734.64) | 2138.91 (738.30) | 2134.41 (693.67) |
| Total carbohydrates (g/d) | 279.44 (100.58) | 271.71 (100.99) | 269.08 (93.67) |
| Total fiber (g/d) | 18.25 (8.03) | 17.55 (7.54) | 17.76 (93.84) |
| GI | 54.97 (3.41) | 55.24 (3.03) | 55.40 (2.80) |
| GL | 152.48 (57.21) | 149.44 (57.46) | 148.89 (53.06) |
| BMI z score | 0.08 (1.07) (n = 927) | 0.24 (0.97) (n = 738) | 0.21 (1.02) (n = 468) |
Adjusting for total energy intake, HLA DR,DQ genotype, and family history of type 1 diabetes, GI and GL were not associated with IA development (Table 1). Adjusting for ω-3 and ω-6 fatty acid intake, dietary factors found to be associated with IA in the DAISY previously (24), did not change these results. In the subanalysis on the 1581 children (67 of which developed IA) for whom at least one BMI z score was available, the BMI z score was not associated with earlier IA development [hazard ratio (HR): 1.19; 95% confidence interval (CI): 0.94–1.51] (Table 1). The nonsignificant association between GI and IA development was not affected by the addition of the BMI z score to the model (GI HR without BMI z-score adjustment: 0.97, 95% CI 0.76–1.24; and GI HR with BMI z-score adjustment: 0.98, 95% CI 0.77–1.25). The nonsignificant association seen for GL also was not affected by the BMI z score (GL HR without BMI z-score adjustment: 0.95, 95% CI 0.74–1.23; and GL HR with BMI z-score adjustment: 0.96, 95% CI 0.75–1.24).
Development of type 1 diabetes in children with IA
After an average of 3.5 yr follow-up (range 0.03–9.23), 17 children were diagnosed with type 1 diabetes, at mean age 6.22 yr (Table 3). There were no differences in HLA DR,DQ genotype, family history of type 1 diabetes, ethnicity, maternal age, maternal education, or income between type 1 diabetes progressors and nonprogressors.
Table 3.
Descriptive characteristics of the type 1 diabetes analysis cohort and description of the reported childhood diet with time to type 1 diabetes development
| Progressed to type 1 diabetes (n = 17) | Has not progressed to type 1 diabetes (n = 72) | HR (95% CI) | |
|---|---|---|---|
| Variable | % | % | |
| High-risk genotype HLA-DR3/4,DQ8 | 58.8 | 29.2 | 2.03 (0.77–5.36) |
| Family history of type 1 diabetes | 70.6 | 61.1 | 1.55 (0.54–4.39) |
| Female | 58.8 | 51.4 | 1.93 (0.72–5.15)a |
| Non-Hispanic white ethnicity | 88.2 | 80.6 | 0.96 (0.21–4.36)a |
| Maternal education >12 yr | 88.2 | 86.1 | 1.08 (0.24–4.98)a |
| Annual income ≥$30,000 | 81.3 | 84.1 | 0.55 (0.15–2.11)a |
| Mean age (yr) at first autoantibody positive visit (sd) | 3.10 (1.70) | 5.15 (2.46) | 0.75 (0.56–0.99)a |
| Mean age (yr) at diagnosis or last visit (sd) | 6.22 (2.51) | 8.75 (2.58) | N/A |
| Mean BMI z score (75 subjects, 10 cases) (sd) | 0.40 (0.96) | 0.27 (0.96) | 0.93 (0.41–2.13)b |
| Mean total calories (kcal/d) (sd) | 1996.39 (572.98) | 2140.72 (752.12) | 0.88 (0.51 – 1.52)c |
| Mean total carbohydrates (g/d) (sd) | 269.72 (87.67) | 266.97 (93.95) | 1.44 (0.86–2.41)d |
| Mean total fiber (g/d) (sd) | 16.45 (6.82) | 17.88 (7.57) | 0.81 (0.52–1.28)d |
| Mean GI (sd) | 55.89 (3.16) | 54.57 (3.32) | 2.20 (1.17–4.15)d |
| Mean GL (sd) | 148.42 (50.82) | 144.49 (53.95) | 1.59 (0.96–2.64)d |
N/A, Not applicable.
Adjusted for HLA genotype and family history of type 1 diabetes.
Adjusted for age at first autoantibody positive visit, HLA genotype, and family history of type 1 diabetes.
Adjusted for age at first autoantibody positive visit, HLA genotype, and family history of type 1 diabetes. HR and 95% CI represent 1 sd change in the diet variable.
Diet variables were adjusted for energy intake using the residual method (19). HR and 95% CI represent 1 sd change in the diet variable. Models were adjusted for total calories, HLA genotype, age at first autoantibody positive visit, and family history of type 1 diabetes.
GI was positively associated with more rapid progression to type 1 diabetes in children that had already developed IA (HR: 2.20; 95% CI: 1.17–4.15; P = 0.02). In a similar multivariate survival model, GL was marginally associated with more rapid progression to type 1 diabetes (HR: 1.59; 95% CI: 0.96–2.64; P = 0.07) (Table 3). A higher BMI z score at the first autoantibody positive visit was not significantly associated with more rapid type 1 diabetes development in the 75 children for whom the BMI z score at the first autoantibody positive visit was available (HR: 0.93; 95% CI: 0.41–2.13). The association between GI and progression to type 1 diabetes was not affected by the addition of the BMI z score to the model (GI HR without BMI z-score adjustment: 2.55, 95% CI 1.18–5.53; and GI HR with BMI z-score adjustment: 2.58, 95% CI: 1.17–5.73). The association seen for GL was also not explained by the BMI z score (GL HR without BMI z-score adjustment: 2.12, 95% CI 1.03–4.36; and GL HR with BMI z-score adjustment: 2.13, 95% CI 1.03–4.40).
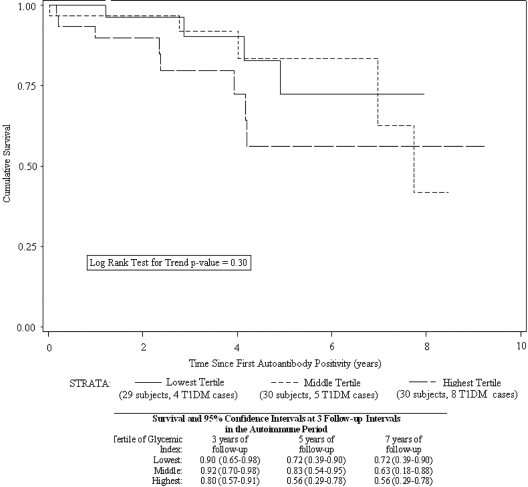
Tertile analysis revealed that time to type 1 diabetes development was not significantly shorter for children in the middle tertile of GI (HR: 2.66; 95% CI: 0.54–13.22) but was significantly shorter in the highest tertile of GI (HR: 7.97; 95% CI: 1.67–38.15), compared with the lowest tertile. In a similar tertile analysis of GL, time to type 1 diabetes development was not significantly shorter for children in the middle tertile (HR: 1.35; 95% CI: 0.33–5.54), nor in the highest tertile of GL (HR: 1.64; 95% CI: 0.49–5.47), compared with the lowest tertile. All models were adjusted for total energy intake, age at first autoantibody positive visit, HLA DR,DQ genotype, and family history of type 1 diabetes. The unadjusted, descriptive Kaplan-Meier curves by GI tertiles suggest that the tertiles are different (Fig. 2).
Figure 2.
Kaplan-Meier life table of dietary GI tertiles and time to development of type 1 diabetes (T1DM).
Discussion
We observed a novel association between higher GI of the diet at IA development and more rapid progression to type 1 diabetes, which was not explained by differences in the BMI z score. However, dietary GI and GL were not associated with IA development.
GI may be acting via two related pathways to drive β-cell decline in autoimmunity: β-cell stress and/or insulin resistance. Immediately after the consumption of a high GI meal, the body rapidly absorbs the food and enters a state of high blood glucose. This triggers high insulin production, resulting in a decrease in blood glucose levels, often below preprandial levels (hypoglycemia). This hypoglycemia triggers an increase in free fatty acids, and the body attains euglycemia (25). In contrast, consumption of a low GI meal results in the gradual absorption of glucose into the blood, lower overall insulin production (26), and no hypoglycemia or compensatory increase in free fatty acids (25). Frequent postprandial hyperglycemia may lead to a state of chronic oxidative stress (27). Long-term oxidative stress may compound the glucotoxic effects of hyperglycemia (25) and contribute to β-cell failure, although we have no direct evidence that study participants actually had postprandial hyperglycemia.
Alternatively, a high GI diet may lead to insulin resistance (28) via chronic high blood glucose and increased free fatty acid levels, although this association has not been replicated (29,30). A state of insulin resistance would create a higher demand for insulin after a high GI meal, due to the increased level of insulin required for glucose transport, thus exacerbating the demands on the β-cell. Furthermore, insulin resistance has preceded type 1 diabetes development (31,32,33). Increased β-cell activity has been shown to put the β-cell at increased risk for autoimmune attack (9), and apoptosis (8). In summary, a high GI diet may place additional stresses (high insulin demand, oxidative stress and insulin resistance) on β-cells already under autoimmune attack. This may explain why we observed a higher GI diet to be associated with more rapid progression to type 1 diabetes, and not associated with IA development.
A high GL diet may be high in carbohydrates but low in GI (or vice versa), thus high GL does not necessarily trigger high postprandial spikes in blood glucose and the attending physiological responses. This may explain why the association for GL is not as strong as the association for GI. Alternatively, this could reflect differences in reporting accuracy. Although the agreement between the FFQ and a 4-d weighed food record has been good for GI, the agreement between the two methods for GL is only fair (34).
BMI z scores, a relative measure of body size, were not associated with progression to type 1 diabetes in children with IA, nor did they explain the association observed between GI and progression to type 1 diabetes. In the DAISY we do not measure insulin resistance, and, thus, could not investigate whether the association between higher GI and progression to type 1 diabetes may be acting through diet-induced insulin resistance. Our findings suggest that increased insulin demand on the β-cells due to diet makes the β-cells more active and, thus, may speed progression to type 1 diabetes (7). Because total caloric intake was not associated with progression to type 1 diabetes, our results suggest that the quality of dietary carbohydrates, rather than the quantity of food consumed, is the dietary factor driving progression to type 1 diabetes.
We note that it is possible that the association seen between high GI diets at autoimmunity development and more rapid progression to type 1 diabetes development may be due to the high GI diet increasing HbA1c levels (35), independent of the disease process. High HbA1c (>6.2%) is one of the factors that triggers physician referral for diagnosis in the DAISY, and may simply “unmask” type 1 diabetes earlier due to its ability to increase HbA1c levels (15).
Some type 1 diabetes patients report symptoms of hypoglycemia months or years before symptoms of hyperglycemia and the diagnosis of diabetes. This phenomenon is poorly documented, except for case reports of the presence of autoantibodies to the insulin receptor (36). Although it is plausible that IA may lead to inappropriate insulin release and symptomatic hypoglycemia that makes prediabetic children crave glucose, it is unlikely that this would explain our findings because our dietary data end at the time of first autoantibody positivity.
The strengths of this study are the prospective cohort design, with frequent follow-up intervals and collection of exposure variables before disease onset. The main limitation of this study is the parental report of diet, which may be inaccurate or imprecise. However, diet data for the type 1 diabetes analysis are free from recall bias because none of the families knew the child’s disease outcome at the time of diet survey completion.
We note that we have refrained from analyzing autoantibody levels as continuous variables for the following reason. In the DAISY we have observed that levels of autoantibodies often oscillate widely. It is not uncommon for a child to “lose” his/her autoantibodies (autoantibody levels decrease below the cutoff for positivity), and/or develop other autoantibodies, before type 1 diabetes development. It would be extremely difficult and potentially misleading to statistically model these oscillations and interpret the results.
Due to this concern, we have chosen to use a relatively inclusive and robust definition of IA. We feel that using a cutoff for positivity of an autoantibody measurement indicates that a child is having an abnormal autoimmune response and that the autoantibody data cannot reliably be interpreted beyond this definition. In addition, the traditional method for reporting islet autoantibody data is to dichotomize such data based on predetermined cutoffs for positivity, which makes our definition more clinically relevant to the field.
The lack of association between GI and IA development, coupled with the associations between GI and more rapid progression to type 1 diabetes, suggest that a diet high in GI may exacerbate the type 1 diabetes disease process but is unlikely to initiate the process. These findings are preliminary. Additional studies examining the dietary GI in children with IA should be conducted to replicate our novel findings in other populations, and elucidate further the mechanism using direct measures of insulin resistance and β-cell stress. The results of these studies may ultimately prove helpful in delaying the progression of type 1 diabetes in children with IA.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants R01-DK49654 and DK32493, and the Diabetes Endocrine Research Center, Clinical Investigation & Bioinformatics Core P30 DK 57516.
Disclosure Statement: The authors have nothing to declare.
First Published Online August 5, 2008
Abbreviations: BMI, Body mass index; CI, confidence interval; DAISY, Diabetes Autoimmunity Study in the Young; FFQ, food frequency questionnaire; GI, glycemic index; GL, glycemic load; HbA1c, glycosylated hemoglobin; HLA, human leukocyte antigen; HR, hazard ratio; IA, islet autoimmunity; SR, Standard Reference.
References
- Onkamo P, Vaananen S, Karvonen M, Tuomilehto J 1999 Worldwide increase in incidence of type I diabetes—the analysis of the data on published incidence trends. Diabetologia [Erratum (2000) 43:685] 42:1395–1403 [DOI] [PubMed] [Google Scholar]
- Gale EA 2005 Spring harvest? Reflections on the rise of type 1 diabetes. Diabetologia 48:2445–2450 [DOI] [PubMed] [Google Scholar]
- Vehik K, Hamman RF, Lezotte D, Norris, JM, Klingensmith G, Bloch C, Rewers M, Dabelea D 2007 Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care 30:503–509 [DOI] [PubMed] [Google Scholar]
- Pundziute-Lyckå A, Dahlquist G, Nyström L, Arnqvist H, Björk E, Blohmé G, Bolinder J, Eriksson JW, Sundkvist G, Ostman J, Swedish Childhood Diabetes Study Group 2002 The incidence of type I diabetes has not increased but shifted to a younger age at diagnosis in the 0–34 years group in Sweden 1983–1998. Diabetologia 45:783–791 [DOI] [PubMed] [Google Scholar]
- 2000 Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet [Erratum (2000) 356:1690] 355:873–876 [PubMed] [Google Scholar]
- Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS 1996 Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 45:926–933 [DOI] [PubMed] [Google Scholar]
- Dahlquist G 2006 Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia 49:20–24 [DOI] [PubMed] [Google Scholar]
- Grill V, Bjorklund A 2001 Overstimulation and β-cell function. Diabetes 50(Suppl 1):S122–S124 [DOI] [PubMed] [Google Scholar]
- Bjork E, Kampe O, Andersson A, Karlsson FA 1992 Expression of the 64 kDa/glutamic acid decarboxylase rat islet cell autoantigen is influenced by the rate of insulin secretion. Diabetologia 35:490–493 [DOI] [PubMed] [Google Scholar]
- Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie Jr RS, Hamman RF, Klingensmith G, Eisenbarth GS, Erlich HA 1996 Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 39:807–812 [DOI] [PubMed] [Google Scholar]
- Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, Rewers M 2003 Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 290:1713–1720 [DOI] [PubMed] [Google Scholar]
- Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, Eisenbarth GS 1996 Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 81:4264–4267 [DOI] [PubMed] [Google Scholar]
- Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, Eisenbarth GS, Rewers M, Diabetes Autoimmunity Study in the Young 2004 Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 89:3896–3902 [DOI] [PubMed] [Google Scholar]
- Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS 2000 Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA 97:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stene LC, Barriga K, Hoffman M, Kean J, Klingensmith G, Norris JM, Erlich HA, Eisenbarth GS, Rewers M 2006 Normal but increasing hemoglobin A1c levels predict progression from islet autoimmunity to overt type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). Pediatr Diabetes 7:247–253 [DOI] [PubMed] [Google Scholar]
- Parrish LA, Marshall JA, Krebs NF, Rewers M, Norris JM 2003 Validation of a food frequency questionnaire in preschool children. Epidemiology 14:213–217 [DOI] [PubMed] [Google Scholar]
- Brady H, Lamb MM, Sokol RJ, Ross CA, Seifert JA, Rewers MJ, Norris JM 2007 Plasma micronutrients are associated with dietary intake and environmental tobacco smoke exposure in a paediatric population. Public Health Nutr 10:712–718 [DOI] [PubMed] [Google Scholar]
- Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC 1997 Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 277:472–477 [DOI] [PubMed] [Google Scholar]
- Willett W 1998 Nutritional epidemiology. 2nd ed. New York: Oxford University Press [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG 2001 Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol 15:88–94 [DOI] [PubMed] [Google Scholar]
- Slyper AH 2004 The pediatric obesity epidemic: causes and controversies. J Clin Endocrinol Metab 89:2540–2547 [DOI] [PubMed] [Google Scholar]
- Hypponen E, Virtanen SM, Kenward MG, Knip M, Akerblom H, Childhood Diabetes in Finland Study Group 2000 Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care 23:1755 [DOI] [PubMed] [Google Scholar]
- Dabelea D, D'Agostino Jr RB, Mayer-Davis EJ, Pettitt DJ, Imperatore G, Dolan LM, Pihoker C, Hillier TA, Marcovina SM, Linder B, Ruggiero AM, Hamman RF 2006 Testing the accelerator hypothesis: body size, β-cell function, and age at onset of type 1 (autoimmune) diabetes. Diabetes Care 29:290–294 [DOI] [PubMed] [Google Scholar]
- Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, Orton HD, Barón AE, Clare-Salzler M, Chase HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M 2007 ω-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 298:1420–1428 [DOI] [PubMed] [Google Scholar]
- Ludwig DS 2002 The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287:2414–2423 [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Collier GR, Ocana A, Rao AV, Buckley G, Lam Y, Mayer A, Thompson LU 1987 Metabolic effects of a low-glycemic-index diet. Am J Clin Nutr 46:968–975 [DOI] [PubMed] [Google Scholar]
- Hu Y, Block G, Norkus EP, Morrow JD, Dietrich M, Hudes M 2006 Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am J Clin Nutr 84:70–76 [DOI] [PubMed] [Google Scholar]
- McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF 2004 Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 27:538–546 [DOI] [PubMed] [Google Scholar]
- Liese AD, Schulz M, Fang F, Wolever TM, D'Agostino Jr RB, Sparks KC, Mayer-Davis EJ 2005 Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care 28:2832–2838 [DOI] [PubMed] [Google Scholar]
- Lau C, Faerch K, Glümer C, Tetens I, Pedersen O, Carstensen B, Jørgensen T, Borch-Johnsen K, Inter99 study 2005 Dietary glycemic index, glycemic load, fiber, simple sugars, and insulin resistance: the Inter99 study. Diabetes Care [Erratum (2005) 28:2340–2341] 28:1397–1403 [DOI] [PubMed] [Google Scholar]
- Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC 2004 Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 47:1661–1667 [DOI] [PubMed] [Google Scholar]
- Bingley PJ, Mahon JL, Gale EA, European Nicotinamide Diabetes Intervention Trial Group 2008 Insulin resistance and progression to type 1 diabetes in the European Nicotinamide Diabetes Intervention Trial (ENDIT). Diabetes Care 31:146–150 [DOI] [PubMed] [Google Scholar]
- Xu P, Cuthbertson D, Greenbaum C, Palmer JP, Krischer JP, Diabetes Prevention Trial-Type 1 Study Group 2007 Role of insulin resistance in predicting progression to type 1 diabetes. Diabetes Care 30:2314–2320 [DOI] [PubMed] [Google Scholar]
- Barclay AW, Flood VM, Brand-Miller JC, Mitchell P 2008 Validity of carbohydrate, glycaemic index and glycaemic load data obtained using a semi-quantitative food-frequency questionnaire. Public Health Nutr 11:573–580 [DOI] [PubMed] [Google Scholar]
- Opperman AM, Venter CS, Oosthuizen W, Thompson RL, Vorster HH 2004 Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 92:367–381 [DOI] [PubMed] [Google Scholar]
- Maron R, Elias D, de Jongh BM, Bruining GJ, van Rood JJ, Shechter Y, Cohen IR 1983 Autoantibodies to the insulin receptor in juvenile onset insulin-dependent diabetes. Nature 303:817–818 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.