Abstract
Context: Increased fat mass has been reported in children and adults born small for gestational age (SGA). However, the progression of anthropometric parameters have been poorly documented in SGA adults.
Objective: We hypothesized that SGA individuals would remain susceptible to gain more fat when adults beyond the period of postnatal catch-up growth.
Study Population and Design: From a community-based cohort, 389 subjects born full-term SGA (body weight < 10th percentile) were compared with 462 subjects born appropriate for gestational age (25th < body weight < 75th percentile). Anthropometric parameters were measured at 22 and 30 yr as well as body composition (by multifrequency bioelectrical impedancometry and skinfold thickness) at 30 yr.
Results: Both groups gained weight, body mass index (BMI), and waist circumference. Progression of BMI was significantly greater in SGA (1.8 ± 2.6 vs. 1.4 ± 2.6 kg/m2; P = 0.03). At 30 yr, the proportion of obese individuals was significantly increased in SGA (12.1 vs. 6.5%; P = 0.02), and percent body fat was significantly higher (23.5 ± 8.7 vs. 21.9 ± 8.0%; P = 0.01), the observation of which was confirmed by skinfold measures. Similarly, waist circumference gain was significantly greater in SGA (6.4 ± 7.6 vs. 5.5 ± 7.9, P = 0.04 when adjusted for gender and age).
Conclusion: Over 8-yr follow-up, adults born SGA gained more BMI than appropriate for gestational age, resulting in greater fat mass with more abdominal fat. These data suggest that the consequences of fetal growth restriction on body composition are evolving beyond the period of early postnatal catch-up.
Adults born small for gestational age have a higher body mass index than adults born appropriate for gestational age, reflecting a greater fat mass with more abdominal fat. The findings suggest a long-term effect of fetal programming in adults.
Individuals born small for gestational age (SGA) have an increased risk for later development of cardiovascular diseases and metabolic syndrome (1). The mechanism underlying this association remains unknown. However, several hypotheses have been proposed. In 1992 Hales and Barker (2) proposed the thrifty phenotype hypothesis. The growing fetus exposed to nutritional deprivation adopts strategies to aid survival. First, it diverts nutrients to the brain to preserve brain growth at the expense of body growth and the development of other organs such as pancreas, liver, and muscle. Second, fetal reprogramming occurs in a manner that is beneficial to survival under conditions of poor nutrition (3). However, when the organism is submitted to conditions of adequate nutrition in the postnatal period, the fetal reprogramming may result in the development of insulin resistance and type 2 diabetes. The catch-up growth hypothesis was also proposed by Cianfarani et al. in 1999 (4). Most SGA infants will catch up during the first years of life. This could represent the crucial time for the development of long-term consequences. Indeed, decreased body fat mass has been reported in SGA newborns (5,6) and several observations report that SGA infants who show early and complete catch-up growth would be at higher risk for the metabolic disorders (7,8). This catch-up growth encompasses growth in height and weight as well as fat mass (9,10).
During fetal growth restriction, the development of the adipose tissue is altered (5). Interestingly, most data on body composition in subjects born SGA found that, despite a similar body mass index (BMI), subjects born SGA had a greater percentage of body fat than subjects born appropriate for gestational age (AGA), either as young adults (19 and 22 yr old) or at an older age (64–72 yr) (11,12). Our group itself has also reported an increased percent body fat in a restricted number of young adults born SGA (13). Moreover, not only percentage of body fat but also fat distribution seems to be influenced by the fetal environment because some authors have reported that poor fetal growth is associated with increased abdominal fat later in life (14,15).
We investigated body composition in young adults during a prospective study using a community-based cohort, with individuals born either SGA or AGA. We hypothesized that, being born SGA, would have consequences on body fat in young adults. Subjects who have been exposed to fetal growth restriction would remain susceptible to gain more fat and in particular more abdominal fat in adulthood beyond the period of early catch-up.
Subjects and Methods
Participants were identified from a community-based cohort of young adults aimed at investigating the long-term consequences of being born SGA. Briefly, subjects were derived from a population-based registry of the metropolitan area of the city of Haguenau in France (7). This registry included information about all pregnancies and deliveries occurring in the maternity unit of the city hospital from 1971 to 1985. The SGA group included 734 singleton subjects born between 32 and 42 weeks of gestation with birth weight less than the 10th percentile for sex and gestational age according to the local growth standard curves. The AGA group was made up of singleton subjects born between 32 and 42 weeks of gestation, with birth size between the 25th and the 75th percentiles and who were the first babies in the registry born immediately after a subject born SGA. The AGA group included 886 individuals.
The initial observation took place when the mean age of the cohort was 22 yr. For the present study, subjects were eligible if there was duration of follow-up of at least 5 yr. The second observation was conducted between April 2005 and June 2007. Among 1040 eligible subjects for the prospective follow-up, two were excluded because of concomitant diseases (one cancer, one cystic fibrosis) and four subjects died during follow-up; 851 (389 SGA and 462 AGA) agreed to come for a second visit (rate of participation 76%). All eligible individuals included in the cohort were contacted by phone or mail. When subjects declined to participate, their current weight was recorded. We then compared the 851 subjects who participated to the second visit with the 189 subjects who did not (Table 1). The proportion of AGA and SGA and the sex ratio were comparable, and both groups shared the same anthropometric characteristics. BMI at the two time points and the distribution of overweight and obese people were not statistically different. Mean age in the individuals who dropped out was mildly but significantly increased.
Table 1.
Comparison between participants and nonparticipants at the second observation
| Nonparticipants (n = 189) | Study subjects (n = 851) | P | |
|---|---|---|---|
| Age at the first visit (yr) | 21.6 ± 3.1 | 22.3 ± 3.7 | 0.72 |
| BMI at the first visit (kg/m2) | 22.7 ± 4.3 | 22.1 ± 4.6 | 0.86 |
| BMI distribution at the first visit (%) | 0.28 | ||
| ≤25 | 72.9 | 79.4 | |
| 25 to <30 | 22.4 | 15.7 | |
| ≥30 | 4.7 | 5 | |
| Age at the second visit (yr) | 31.3 ± 3.1 | 30.5 ± 3.6 | 0.02 |
| BMI at the second visit (kg/m2) | 23.9 ± 4.6 | 24.1 ± 4.5 | 0.84 |
| BMI distribution at the second call (%) | 0.27 | ||
| ≤25 | 68.2 | 65.7 | |
| 25 to <30 | 18.8 | 25.3 | |
| ≥30 | 12 | 9.1 |
Results are expressed as mean ± sd or in frequency (%). P, Univariate analysis between the two groups using t test for quantitative variables and χ 2 for qualitative variables.
Clinical characteristics of the study population at birth are given in Table 2. Mean gestational age and sex distribution did not differ between the two study groups. According to the selection criteria, subjects born SGA were lighter, shorter, and thinner at birth than subjects born AGA.
Table 2.
Birth characteristics of the subjects
| SGA (n = 389) | AGA (n = 462) | |
|---|---|---|
| Gestational age (wk) | 39.5 ± 1.5 | 39.6 ± 1.4 |
| Birth weight (g) | 2617 ± 300 | 3391 ± 266 |
| Birth length (cm) | 47.7 ± 2.1 | 50.4 ± 1.1 |
| BMI (kg/m2) | 11.5 ± 1.0 | 13.3 ± 0.8 |
The values are given as mean ± sd.
Study design
Subjects attended the two medical visits at the hospital of the city of Haguenau. For both visits, the same three trained nurses recorded information about medical history using a standardized questionnaire. Body weight was measured with a portable scale and height with a wall-mounted stadiometer. Weight for height was assessed as BMI (kilograms per square meter). Waist circumference was measured at the level of the umbilicus and hip circumference at the level of the greater trochanter. Arm circumference was measured at midpoint between the lateral projection of the acromial process and the inferior border of the olecranon of the cubitus.
At the second visit, skinfold thickness was measured using Harpenden calipers. Skinfold measurements were taken from the following four sites: triceps (posterior aspect of the arm at midpoint between the lateral projection of the acromial process and the inferior border of the olecranon of the cubitus); biceps (anterior aspect of the arm, same level as triceps skinfold); subscapula (inferior to the inferior angle to the scapula, 45° angle); and suprailiac (horizontal skinfold at the midaxillary line immediately superior to the iliac crest). The right side of the body was used for all measurements. Two readings (to the nearest 0.1 mm) were obtained from each site and the mean value was used in calculations. The muscular circumference of the arm (MCA), in centimeters, was calculated from the measurement of the arm circumference and the thickness of the triceps skinfold using the formula proposed by Jelliffe (16): MCA = arm circumference (centimeters) − 3.14 × thickness of the triceps skinfold (centimeters).
Percentage of body fat mass was measured using a multifrequency bioelectrical-impedance meter. All measurements were carried out with subjects lying in a supine position on a flat, nonconductive bed by using tetrapolar technique (Quadscan 4000; Bodystat, Douglas, UK). The Bodystat QuadScan 4000 U has four electrodes. Two electrodes were placed on the right wrist, one just proximal to the third metacarpophalangeal joints (positive) and one on the wrist next to the ulnar head (negative). Two electrodes were placed on the right ankle with one just proximal to the third metatarsophalangeal joint (positive) and one between medial and lateral malleoli (negative). Multifrequency currents (5, 50, 100, and 200 kHz) were introduced from the positive leads and traveled throughout the body to the negative leads. Percent of body fat was calculated using the manufacturer’s software. In our hands, coefficients of variation of percent body fat measured at 12 and 35% were 2.3 and 0.6% for within-subject precision and 3.5 and 1.1% and intersubject precision, respectively.
The study protocol was reviewed and approved by the ethical committee of the Saint Louis medical school at the René Diderot University of Paris, and all subjects gave written consent.
Statistical analysis
All statistical analyses were performed using the SAS 9.1 software (SAS Inc., Cary, NC). Quantitative variables were expressed as means ± 1 sd and qualitative variables numbers (percentages).
Quantitative variables were compared using t test in univariate analyses and χ2 test was used for the comparison of class variables.
Linear regression was performed to explore the relationship between fetal environment and anthropometric data. We used two models. The first was a model adjusted on age at the second visit and gender. A second model was adjusted on change in BMI between 22 and 30 yr, gender, and age at the second observation. We checked the normality of residuals and the absence of heteroscedasticity for regression analyses. All tests were two tailed and an α-risk of 5% was considered a significant.
Results
Description of the two groups at 22 and 30 yr of age: (Table 3)
Table 3.
Clinical characteristics between SGA and AGA subjects over 8 yr of follow-up
| 22 yr of age
|
30 yr of age
|
|||||
|---|---|---|---|---|---|---|
| SGA (n = 389) | AGA (n = 462) | P value | SGA (n = 389) | AGA (n = 462) | P value | |
| Age (yr) | 22.1 ± 3.6 | 22.3 ± 3.6 | 0.37 | 29.9 ± 3.6 | 30.3 ± 3.6 | 0.08 |
| Gender | 0.25 | |||||
| Female | 214 (56.7%) | 236 (52.4%) | ||||
| Male | 175 (43.3%) | 226 (47.6%) | ||||
| Height (cm) | 167 ± 8.9 | 173 ± 9.0 | 0.0001 | 167 ± 8.8 | 172 ± 9 | 0.001 |
| Weight (kg) | 63.4 ± 13.7 | 67.4 ± 13.5 | 0.0001 | 68.3 ± 16.4 | 71.5 ± 15.3 | 0.003 |
| BMI (kg/m2) | 22.5 ± 4.2 | 22.6 ± 3.8 | 0.55 | 24.2 ± 5.2 | 24.0 ± 4.3 | 0.53 |
| Waist circumference (cm) | 75 ± 12 | 77 ± 10 | 0.02 | 82 ± 14 | 82 ± 13 | 0.68 |
| Women | 73.2 ± 10 | 74.0 ± 9 | 0.78 | 78.3 ± 12 | 78.3 ± 11 | 0.79 |
| Men | 82.6 ± 11 | 82.9 ± 10 | 0.39 | 90.4 ± 12 | 89.9 ± 11 | 0.61 |
| Waist/hip circumferences | 0,80 ± 0.08 | 0,80 ± 0.07 | 0.71 | 0,81 ± 0.08 | 0,82 ± 0.08 | 0.58 |
| BMI distribution | 0.20 | 0.02 | ||||
| <25 | 308 (79.2%) | 366 (79.2%) | 247 (63.5%) | 312 (67.5%) | ||
| 25 to <30 | 55 (14.1%) | 78 (16.9%) | 95 (24.4%) | 120 (26.0%) | ||
| >30 | 24 (6.2%) | 18 (3.9%) | 47 (12.1%) | 30 (6.5%) | ||
| Body fat (%) | 23.5 ± 8.8 | 21.9 ± 8.0 | 0.01 | |||
| MCA (cm) | 28.8 ± 4.1 | 29.0 ± 3.6 | 0.39 | |||
Results are expressed as mean ± sd or in frequency (%). P, Univariate analysis between the two groups using t test for quantitative variables and χ 2 for qualitative variables. MCA, Arm circumference (centimeters) − 3.14 × tricipital skinfold (centimeters).
At the two time points, SGA subjects were smaller and lighter. Mean BMIs were not statistically different. Mean waist circumference was significantly larger in the AGA group at 22 yr, but this difference disappeared at 30 yr. Mixed-model analyses showed no statistically significant interactions between the effects of sex and percent body fat, waist circumference, or δ of BMI (P values for interaction >0.2). We therefore present a pooled analysis adjusted for gender and other covariates described in the Statistical analysis. At 30 yr, the exact same proportion of women (59%) has already been pregnant in the two groups and the average number of pregnancies was similar.
At 30 yr, percent body fat measured by bioelectrical impedance assay (BIA) was significantly higher in the SGA group. This result was comforted by the replication of the observation when using skinfolds measurement: the sum of the four skinfolds was significantly increased in SGA group (53.2 ± 24.3 vs. 49.1 ± 20.9 mm, P = 0.009).
MCA, indicative of body lean mass, was not significantly different between the groups.
The distribution of overweight and obese people, defined as a BMI between 25 and 30 kg/m2 and above 30 kg/m2, respectively, was not statistically different between the two groups at 22 yr old. However, 8 yr later, the proportion of obese individuals was about twice as high in the SGA, in comparison with the AGA group (12.3 vs. 6.5%, P = 0.02). After exclusion of obese subjects in the two groups (30 AGA subjects and 47 SGA subjects), percent body fat remained increased in SGA group, compared with the AGA group, although the difference was marginally significant (22.0 ± 7.4 vs. 21.1 ± 7.2; P = 0.07).
Progression of the anthropometric data during the follow-up
The progression of obesity was significantly increased in the SGA group. Between the two visits, the number of subjects who became obese was higher in the SGA group: 26 subjects in the SGA group (6.7%) vs. 18 in the AGA group (3.9%). In the meantime, six of 18 obese individuals in the AGA group vs. three of 24 in the SGA group lost weight and consequently left the obese group. These two trends explain the significant increase of obesity seen at 30 yr in the SGA group (P = 0.03).
Progression of BMI was significantly greater in the SGA group over the period of follow-up (Table 4). This difference between the two groups remained significant after adjustment on age at the second visit and gender (P = 0.02).
Table 4.
Progression of body size over 8 yr of follow-up in the two groups
| SGA (n = 389) | AGA (n = 462) | P univariate | P adjusteda | |
|---|---|---|---|---|
| Time of follow-up (yr) | 7.8 ± 2.8 | 8.1 ± 2.8 | 0.56 | |
| Diff. BMI | 1.8 ± 2.6 | 1.4 ± 2.6 | 0.019 | 0.03 |
| Difference in weight gain (kg) | 4.9 ± 7.2 | 4.1 ± 7.3 | 0.10 | 0.06 |
| Diff. WC (cm) | 6.4 ± 7.6 | 5.5 ± 7.9 | 0.07 | 0.04 |
Results are expressed in means ± sd. P values for t test. Diff. WC, Waist circumference at the second visit minus waist circumference at the first visit; Diff. BMI, BMI at the second visit minus BMI at the first visit.
Adjusted for gender and age at the second observation.
Both groups gained waist circumference and ratio of waist to hip circumferences. The gain in waist circumference between the two visits was greater in the SGA group (6.4 ± 7.6 vs. 5.5 ± 7.9, P = 0.07), the difference of which became statistically significant when adjusted for gender and age (P = 0.04); it remained so even after further adjustment on supra iliac skinfolds (P = 0.02).
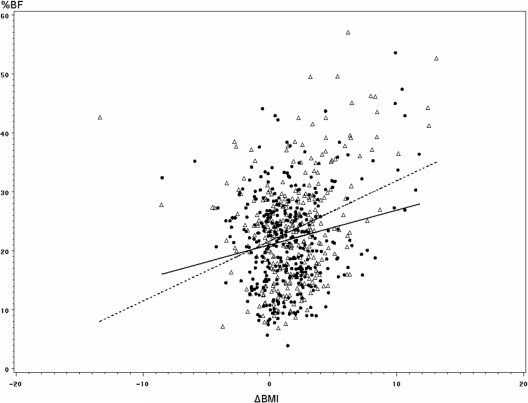
The effect of progression of BMI on body fat was tested between the two groups using a multivariate linear model with age and gender as covariates. Percent body fat was significantly related to birth status (SGA vs. AGA; P = 0.03), gender (P < 0.001), and the age at the second observation (P < 0.001). Interestingly, we found a significant difference between the two groups in the slopes of the regression lines of percent body fat over the change in BMI between the two visits (P = 0.02; Fig. 1), indicating that the effect of gain in BMI on percent of body fat was greater in SGA than AGA.
Figure 1.
Interaction between birth groups (SGA, ▵; AGA, •) and increase in BMI over time on body fat. % BF, Percent of body fat measured at 30 yr of age by bioelectrical impedancometry; ΔBMI, difference between BMI at 30 and 22 yr of age (kilograms per square meter); dotted line, regression line in SGA; solid line, regression line in AGA. There was a significant interaction between the groups and ΔBMI on percent of body fat (P = 0.02).
Discussion
In this follow-up study, we confirm that being born SGA has consequences during adulthood on body size and body composition. When the cohort was observed at the age of 22 yr, adults born SGA were shorter and lighter than controls, and their BMIs were not significantly different. Eight years later, at the mean age of 30 yr, SGA subjects show faster progression of BMI. Moreover, subjects born SGA have a 2-fold increase in the proportion of obesity when compared with subjects born AGA. At 30 yr, SGA subjects have higher body fat measured by both BIA and skinfolds. Progression of waist circumference was significantly higher in SGA subjects. After adjustment for the suprailiac skinfold, waist circumference remained significantly increased in the SGA group, suggesting an intraabdominal distribution of this excess of fat mass. Taken together, these data point to a more rapid progression of adiposity in SGA with abdominal deposition during early adulthood. Subjects born SGA are at higher risk to develop cardiovascular diseases and complications that encompass insulin resistance and metabolic syndrome (7,17,18,19) The excess of adiposity with a central distribution might contribute, at least in part, to the metabolic consequences of being born SGA (20).
We report here the results of a community-based cohort. The study subjects were young adults from the general population and not referred to a specialized clinic. The study population was from the east of France, an area with a rather high prevalence of obesity. Data established in 2000 (21) in the same area found 4.8% of obesity for 15- to 24-yr-olds and 10.2% prevalence of obesity in the 25- 34-yr-olds. Our data are well matched with these results.
Our results illustrate the consequences on body composition of having being exposed during fetal life to a restrictive environment. The consequences of being born SGA have been largely described in older populations. One interest of our results is the intermediate age of these subjects for the following reasons: they were old enough to put on weight with pregnancies or changes in lifestyle, but they were not yet old enough for the effect of aging to be evident.
Few data are available on the progression of body composition in adults previously exposed to fetal growth restriction. In a follow-up study of a cohort exposed in utero to the Dutch famine in 1944–1945, the authors found a doubling of the prevalence of overweight at age 19 yr with maternal exposure to famine (22). In a second study, at the age of 50 yr, exposure in utero to reduced nutrition was associated with increased weight and greater indexes of fat deposition at several sites in women (23). These results were later confirmed at the age of 59 yr, but the authors did not provide data on the progression itself of the anthropometric measurements with age (24).
The putative contribution of genetic factors has been sought for by several authors, but the results so far indicate a modest contribution. In the same Haguenau cohort, we have previously shown that a common variant of PPARG in the adipose tissue contributes to insulin resistance seen in adults born SGA; moreover, this observation was replicated in a Finnish birth cohort (25,26). Recently in a small sample of SGA newborns, it has been reported that the fat mass- and obesity-associated gene is associated with a larger development of fat mass over the first weeks of life (27). It remains to be seen whether gene-environment interactions might contribute to the accelerated progression of BMI in SGA adults.
During childhood, Ibanez et al. (10) studied the impact of growth catch-up on the development of features of the metabolic syndrome. Twenty-nine SGA children were compared with 22 AGA children during a prospective study. A greater accumulation of body fat mass was observed in SGA children between 2 and 4 yr of age despite all children having already completed catch-up growth between birth and 2 yr of age. In a previous study (9), we found an inverse relationship between thinness at birth and BMI at 22 yr. In the present study, at 30 yr of age, the accelerated development of body size with a central distribution of fat in subjects born SGA was no longer statistically correlated with the intensity of fetal growth restriction (data not shown). Altogether, these observations suggest that being born SGA have different consequences on body composition, varying with the period of development. First, in early infancy, children born SGA will follow a catch-up growth pattern for both height and weight, and this compensatory phenomenon is directly correlated with the degree of fetal growth restriction. Later in life when adults, individuals born SGA will experiment gain in weight, which mostly affects fat mass. At that time, accretion of fat mass is related to but not tightly correlated to the magnitude of fetal growth restriction. This accumulation of body fat might be programmed by fetal life and would contribute to the further development of metabolic and cardiovascular consequences largely described in the literature.
This study has some limitations. The methods we used for the measurement are less precise than dual-energy x-ray absorptiometry, but dual-energy x-ray absorptiometry turned out not to be applicable on such a large study population. Indeed, modest differences were observed between the SGA and AGA groups, but these differences are at the same order of magnitude as the coefficients of variation for BIA. Moreover, all our results with respect to BMI, body fat, and distribution point to the same direction, and it is well recognized that changes resulting from restrictive fetal environment are modest in magnitude and even more so in population-based studies such as the present one (28). We also confirm previous data showing a higher fat mass in subjects born SGA with a central distribution using other methods (11).
To conclude, being born SGA affects body composition when adults: subjects born SGA show an accelerated gain in BMI and waist circumference, which in turn results in higher body fat content with a central distribution. Our data suggest that the excess of fat in SGA-born subjects could result in part from a long-term and persistent effect programmed by fetal life.
Acknowledgments
The authors are grateful to Christine Trabant, Monique Grasser, and Sandrine Wendling for their nursing skills and the nursing and medical staff of the Clinical Investigation Centre 92-02 (Robert Debré Hospital).
Footnotes
This work was partly supported by a grant from the Agence National de la Recherche (ANR-06-Physio 037) and by Institut National de la Santé et de la Recherche Médicale.
Disclosure Statement: T.M., S.D., P.A., and C.A. have nothing to declare. C.L.-M. received lecture fees from Lilly, Novo, and Pfizer.
First Published Online July 15, 2008
Abbreviations: AGA, Appropriate for gestational age; BIA, bioelectrical impedance assay; BMI, body mass index; MCA, muscular circumference of the arm; SGA, small for gestational age.
References
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM 1993 Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36:62–67 [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ 1992 Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601 [DOI] [PubMed] [Google Scholar]
- Lucas A, Fewtrell MS, Cole TJ 1999 Fetal origins of adult disease-the hypothesis revisited. BMJ 319:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfarani S, Germani D, Branca F 1999 Low birthweight and adult insulin resistance: the “catch-up growth” hypothesis. Arc Dis Child 81:F71–F73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzi G, Zanardo V, Caretta F, Inelmen EM, Rubaltelli F 1981 Intrauterine growth and adipose tissue development. Am J Clin Nutr 34:1785–1790 [DOI] [PubMed] [Google Scholar]
- Hediger ML, Overpeck MD, Kuczmarski RJ, McGlynn A, Maurer KR, Davis WW 1998 Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics 102:E60 [DOI] [PubMed] [Google Scholar]
- Jaquet D, Deghmoun S, Chevenne D, Collin D, Czernichow P, Levy-Marchal C 2005 Dynamic change in adiposity from fetal to postnatal life is involved in the metabolic syndrome associated with reduced fetal growth. Diabetologia 48:849–855 [DOI] [PubMed] [Google Scholar]
- Ekelund U, Ong KK, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S 2007 Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab 92:98–103 [DOI] [PubMed] [Google Scholar]
- Ezzahir N, Alberti C, Deghmoun S, Zaccaria I, Czernichow P, Levy-Marchal C, Jaquet D 2005 Time course of catch-up in adiposity influences adult anthropometry in individuals who were born small for gestational age. Pediatr Res 58:243–247 [DOI] [PubMed] [Google Scholar]
- Ibanez L, Ong K, Dunger DB, de Zegher F 2006 Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab 91:2153–2158 [DOI] [PubMed] [Google Scholar]
- Rasmussen EL, Malis C, Jensen CB, Jensen JE, Storgaard H, Poulsen P, Pilgaard K, Schou JH, Madsbad S, Astrup A, Vaag A 2005 Altered fat tissue distribution in young adult men who had low birth weight. Diabetes Care 28:151–153 [DOI] [PubMed] [Google Scholar]
- Kensara OA, Wootton SA, Phillips DI, Patel M, Jackson AA, Elia M 2005 Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr 82:980–987 [DOI] [PubMed] [Google Scholar]
- Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C 2001 Relatively low serum leptin levels in adults born with intra-uterine growth retardation. Int J Obes Relat Metab Disord 25:491–495 [DOI] [PubMed] [Google Scholar]
- Kuh D, Hardy R, Chaturvedi N, Wadsworth ME 2002 Birth weight, childhood growth and abdominal obesity in adult life. Int J Obes Relat Metab Disord 26:40–47 [DOI] [PubMed] [Google Scholar]
- Garnett SP, Cowell CT, Baur LA, Fay RA, Lee J, Coakley J, Peat JK, Boulton TJ 2001 Abdominal fat and birth size in healthy prepubertal children. Int J Obes Relat Metab Disord 25:1667–1673 [DOI] [PubMed] [Google Scholar]
- Jelliffe DB 1966 The assessment of the nutritional status of the community (with special reference to field surveys in developing regions of the world). Monogr Ser World Health Organ 53:3–271 [PubMed] [Google Scholar]
- Desai M, Hales CN 1997 Role of fetal and infant growth in programming metabolism in later life. Biol Rev Camb Philos Soc 72:329–348 [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen TJ, Osmond C, Barker DJ 2003 Pathways of infant and childhood growth that lead to type 2 diabetes. Diabetes Care 26:3006–3010 [DOI] [PubMed] [Google Scholar]
- Poulsen P, Vaag A 2006 The intrauterine environment as reflected by birth size and twin and zygosity status influences insulin action and intracellular glucose metabolism in an age- or time-dependent manner. Diabetes 55:1819–1825 [DOI] [PubMed] [Google Scholar]
- Yajnik CS 2002 The lifecycle effects of nutrition and body size on adult adiposity, diabetes and cardiovascular disease. Obes Rev 3:217–224 [DOI] [PubMed] [Google Scholar]
- Charles MA, Basdevant A, Eschwege E 2002 [Prevalence of obesity in adults in France: the situation in 2000 established from the OBEPI Study]. Ann Endocrinol (Paris) 63:154–158 [PubMed] [Google Scholar]
- Ravelli GP, Stein ZA, Susser MW 1976 Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 295:349–353 [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP 1999 Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 70:811–816 [DOI] [PubMed] [Google Scholar]
- Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal-de Bruin K, Lumey LH 2007 Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr 85:869–876 [DOI] [PubMed] [Google Scholar]
- Eriksson JG 2008 The role of genes in growth and later health. Nestle Nutrition Workshop Series 61:69–77 [DOI] [PubMed] [Google Scholar]
- Jaquet D, Tregouet DA, Godefroy T, Nicaud V, Chevenne D, Tiret L, Czernichow P, Levy-Marchal C 2002 Combined effects of genetic and environmental factors on insulin resistance associated with reduced fetal growth. Diabetes 51:3473–3478 [DOI] [PubMed] [Google Scholar]
- Lopez-Bermejo A, Petry CJ, Diaz M, Sebastiani G, de Zegher F, Dunger DB, Ibanez L 2008 The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J Clin Endocrinol Metab 93:1501–1505 [DOI] [PubMed] [Google Scholar]
- Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A 2007 Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab 92:804–810 [DOI] [PubMed] [Google Scholar]