Abstract
Context: Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide are incretins secreted from enteroendocrine cells postprandially in part to regulate glucose homeostasis. Dysregulation of these hormones is evident in type 2 diabetes mellitus (T2DM). Two new drugs, exenatide (GLP-1 mimetic) and sitagliptin [dipeptidyl peptidase (DPP) 4 inhibitor], have been approved by regulatory agencies for treating T2DM. Liraglutide (GLP-1 mimetic) and vildagliptin (DPP 4 inhibitor) are expected to arrive on the market soon.
Evidence Acquisition: The background of incretin-based therapy and selected clinical trials of these four drugs are reviewed. A MEDLINE search was conducted for published articles using the key words incretin, glucose-dependent insulinotropic polypeptide, GLP-1, exendin-4, exenatide, DPP 4, liraglutide, sitagliptin, and vildagliptin.
Evidence Synthesis: Exenatide and liraglutide are injection based. Three-year follow-up data on exenatide showed a sustained weight loss and glycosylated hemoglobin (HbA1c) reduction of 1%. Nausea and vomiting are common. Results from phase 3 studies are pending on liraglutide. Sitagliptin and vildagliptin are orally active. In 24-wk studies, sitagliptin reduces HbA1c by 0.6–0.8% as monotherapy, 1.8% as initial combination therapy with metformin, and 0.7% as add-on therapy to metformin. Vildagliptin monotherapy lowered HbA1c by 1.0–1.4% after 24 wk. Their major side effects are urinary tract and nasopharyngeal infections and headaches. Exenatide and liraglutide cause weight loss, whereas sitagliptin and vildagliptin do not.
Conclusions: The availability of GLP-1 mimetics and DPP 4 inhibitors has increased our armamentarium for treating T2DM. Unresolved issues such as the effects of GLP-1 mimetics and DPP 4 inhibitors on β-cell mass, the mechanism by which GLP-1 mimetics lowers glucagon levels, and exactly how DPP 4 inhibitors lead to a decline in plasma glucose levels without an increase in insulin secretion, need further research.
The use of glucagon-like peptide 1 mimetics and dipeptidyl peptidase 4 inhibitors in treating type 2 diabetes mellitus in selected clinical trials is reviewed.
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), termed “incretins,” are enteroendocrine hormones released into the bloodstream from L and K cells dispersed throughout the gastrointestinal tract in response to ingested nutrients. They provide the additional stimulus to insulin secretion during oral glucose ingestion that is not present with iv glucose infusion (1,2). These incretins increase insulin secretion in a glucose-dependent manner through activation of their specific receptors on β-cells.
In newly diagnosed type 2 diabetes mellitus (T2DM) with relatively good glycemic control [glycosylated hemoglobin (HbA1c) ∼6.9%], both GIP and GLP-1 secretion in response to glucose and mixed meal challenges are the same or even increased when compared with healthy subjects (3,4). However, in long-standing T2DM with poor glycemic control (HbA1c ∼8–9%), the GLP-1 response is decreased, whereas GIP secretion is unchanged (5,6,7). In addition, insulin response to exogenous GLP-1 is 3- to 5-fold lower in T2DM. However, acute GLP-1 administration is able to increase insulin secretion to normal levels and to lower plasma glucose effectively (8,9). In contrast, exogenous GIP, even at supraphysiological doses, has markedly reduced insulinotropic actions with little or no glucose-lowering effects in T2DM (9,10). Therefore, therapeutic strategies for T2DM within the incretin field focused on the use of GLP-1, GLP-1 analogs, and GLP-1 receptor (GLP-1R) agonists or GLP-1 mimetics, and not GIP.
GLP-1, when administered at pharmacological doses, also has other noninsulinotropic effects beneficial for treating T2DM: suppression of glucagon secretion in the presence of hyperglycemia and euglycemia, but not hypoglycemia, leading to improved hepatic insulin resistance and glycemic control (11,12); slowing of gastric emptying and gut motility, causing delayed nutrient absorption and dampened postprandial glucose (PPG) excursion (13); and increasing the duration of postprandial satiety, leading to lower food intake, weight loss, and improved insulin resistance (14,15,16). More importantly, acute GLP-1 infusion normalized fasting plasma glucose (FPG) in patients with long-standing, uncontrolled T2DM who were no longer responsive to sulfonylureas or metformin (17).
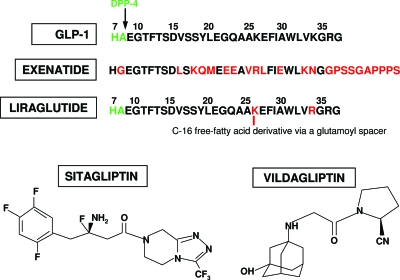
One major drawback of GLP-1 treatment is its short half-life (2 min) (18). GLP-1 is rapidly degraded by dipeptidyl peptidase (DPP) 4, which cleaves the N-terminal dipeptides (His7-Ala8) from GLP-1 (7–36) and renders the resulting major metabolite GLP-1 (9–36) inactive (Fig. 1) (19,20). In addition, neutral endopeptidase (NEP) 24.11 hydrolyzes GLP-1 at six different places (21). With short half-life, bolus sc injections resulted in only a transient effect on insulin secretion and plasma glucose levels (22).
Figure 1.
Structure of native GLP-1, exenatide, liraglutide, sitagliptin, and vildagliptin. The N-terminal dipeptide “HA” of GLP-1 is cleaved by DPP 4, and the remaining fragment does not increase insulin secretion. For exenatide the substitution of glycine for alanine at position 8 prevents the degradation by DPP 4. The free-fatty acid derivative that is attached to liraglutide is thought to promote noncovalent binding of liraglutide to albumin.
Nonetheless, in patients with T2DM, bolus sc administration of GLP-1 before breakfast, lunch, and dinner for 7 d significantly improved PPG and decreased plasma lipid levels (23). Overnight iv GLP-1 infusions lowered FPG and PPG to near-normal levels, markedly improved β-cell function, and restored first-phase insulin secretion, the absence of which is a hallmark of T2DM (24).
Continuous sc GLP-1 infusion via a pump for 6–12 wk improved glucose-induced insulin secretion, enhanced insulin-mediated glucose disposal, and increased insulin pulse mass and pulsatile insulin secretion in T2DM (25,26). Six weeks of GLP-1 infusion also restored first-phase insulin secretion in T2DM, therefore, demonstrating the insulinotropic potency of long-term GLP-1 treatment (15).
Recent animal studies suggest that exogenous GLP-1 has the ability to increase islet size, enhance β-cell proliferation, inhibit β-cell apoptosis, and regulate islet growth (27,28). These effects have tremendous implication in the treatment of T2DM because they directly address one of the fundamental defects in T2DM, i.e. β-cell failure.
Collectively, the aforementioned studies demonstrated the potential of using GLP-1-based therapy for treating T2DM. Two options for GLP-1-based therapies are GLP-1 mimetics resistant to DPP 4 activity, therefore, a longer half-life, and agents such as DPP 4 inhibitors, which increase plasma endogenous GLP-1 levels. In this review we will focus on: 1) exenatide (GLP-1 mimetic) and sitagliptin (DPP 4 inhibitor), which have been approved by regulatory agencies for treatment of T2DM, as well as liraglutide (GLP-1 mimetic) and vildagliptin (DPP 4 inhibitor), which are expected to arrive on the market soon; and 2) issues that are still open for debate regarding the actions of these agents.
GLP-1 Mimetics
Given that DPP 4 cleaves peptides with an alanine, proline, or hydroxyproline in the penultimate N-terminal position, various modifications of GLP-1 at His7, Ala8, or Glu9 have been investigated (29). Additional mid-chain modifications of GLP-1 to prevent NEP hydrolysis are also being investigated to provide longer plasma half-life. Exenatide and liraglutide are two compounds that exhibit these characteristics.
Exenatide
Exenatide (synthetic exendin-4) is the only GLP-1R agonist approved by regulatory agencies as an adjunct therapy to patients with T2DM not achieving satisfactory glycemic control. It is a 39-amino acid peptide produced in the salivary glands of the Gila monster (Heloderma suspectum) with 53% amino acid homology to full-length GLP-1. It binds more avidly to GLP-1R than GLP-1 in GLP-1R-expressing cells (30). There appears to be no specific exendin-4 receptor. Exendin-4 is not a substrate for DPP 4 because it has a Gly8 in place of an Ala8 (Fig. 1). In addition, it lacks some of the target bonds for NEP, and its secondary and tertiary structures may also prevent NEP hydrolysis. Exenatide, being a peptide, must be injected sc, and is eliminated by the kidneys through glomerular filtration (31). It has a mean half-life of 3.3–4 h, is detected in the plasma 15 h after sc injection, and has biological effect 8 h after dosing (32).
Selected clinical studies
Clinical trials investigating exenatide as adjuvant therapy to patients with T2DM not achieving adequate glycemic control on metformin and/or sulfonylurea, metformin and/or thiazolidinedione, as well as comparison trials with insulin glargine and biphasic insulin aspart, are summarized in Table 1 (33,34,35,36,37,38,39). With exenatide 10 μg twice daily as adjuvant therapy to oral hypoglycemic agents, a significant number of patients (32–62%) achieved HbA1c of 7% or less when compared with placebo (7–13%), glargine (48%), and biphasic insulin aspart (24%), and HbA1c reductions of 0.8–1.1% were sustained up to 3 yr. Progressive weight loss from 1.6–2.8 kg noted at 30 wk to 5.3 kg at 3 yr was also noted. Antiexenatide antibodies were detected in 41–49% of patients in the treatment arms but were not associated with glycemic control (33,34,35,36,37,38).
Table 1.
Summaries of selected clinical trials on exenatide, liraglutide, sitagliptin, and vildagliptin
| Prior glycemic treatment | Length | Trial design | Intervention | No. of subjects randomized | No. of subjects completed | Baseline HbA1c(%) | Baseline FPG (mg/dl) | Δ HbA1c(%) baseline | Baseline HbA1c (%)d | % Achieved HbA1c ≤7% | Δ FPG (mg/dl) baseline | Δ Weight (lb) baseline |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exenatide | ||||||||||||
| Sulfonylurea (34) (trial A) | 30 wk | Randomized, triple-blind, placebo-controlled | Placebo plus sulfonylurea | 123 | 74 | 8.7 (1.2) | 194 (58) | +0.1 ± 0.1 | 9 | +7.2 ± 5.4 | −0.6 ± 0.3 | |
| Exenatide 5 μg bid plus sulfonylurea | 125 | 95 | 8.5 (1.1) | 180 (45) | −0.5 ± 0.1 | 33 | −5.4 ± 3.6 | −0.9 ± 0.3 | ||||
| Exenatide 10 μg bid plus sulfonylurea | 129 | 91 | 8.6 (1.2) | 178 (50) | −0.9 ± 0.1 | 41 | −10.8 ± 5.4 | −1.6 ± 0.3 | ||||
| Metformin (33) (trial B) | 30 wk | Randomized, triple-blind, placebo-controlled | Placebo plus metformin | 113 | 89 | 8.2 (1.0) | 170 (40) | +0.1 ± 0.1 | 13 | +14.4 ± 4.2 | −0.3 ± 0.3 | |
| Exenatide 5 μg bid plus metformin | 110 | 90 | 8.3 (1.1) | 176 (43) | −0.4 ± 0.1 | 32 | −7.2 ± 4.6 | −1.6 ± 0.4 | ||||
| Exenatide 10 μg bid plus metformin | 113 | 93 | 8.2 (1.0) | 168 (46) | −0.8 ± 0.1 | 46 | −10.1 ± 4.4 | −2.8 ± 0.5 | ||||
| Sulfonylurea/metformin (35) (trial C) | 30 wk | Randomized, double-blind, placebo-controlled | Placebo plus metformin/sulfonylurea | 247 | 188 | 8.5 (1.0) | 180 (49) | +0.2 ± 0.1 | 9 | +14.4 ± 3.6 | −0.9 ± 0.2 | |
| Exenatide 5 μg bid plus metformin/sulfonylurea | 245 | 206 | 8.5 (1.0) | 182 (52) | −0.6 ± 0.1 | 27 | −9.0 ± 3.6 | −1.6 ± 0.2 | ||||
| Exenatide 10 μg bid plus metformin/sulfonylurea | 241 | 199 | 8.5 (1.1) | 178 (43) | −0.8 ± 0.1 | 34 | −10.8 ± 3.6 | −1.6 ± 0.2 | ||||
| TZD with/without metformin (36) | 16 wk | Randomized, double-blind, placebo-controlled | Placebo plus TZD with/without metformin | 112 | 96 | 7.9 (0.8) | 159 (34) | +0.1 ± 0.1 | 16 | +1.8 ± 3.8 | −0.2 ± 0.3 | |
| Exenatide 10 μg bid plus TZD with/without metformin | 121 | 86 | 7.9 (0.9) | 164 (47) | −0.9 ± 0.1 | 62 | −28.6 ± 4.0 | −1.8 ± 0.3 | ||||
| Sulfonylurea/metformin (37) | 26 wk | Randomized, open-label, controlled | Exenatide 10 μg bid plus metformin/sulfonylurea | 282 | 228 | 8.2 (1.0) | 182 (47) | −1.1 | 46 | −25.7 | −2.3 | |
| Glargine approximately 25 U/d plus metformin/sulfonylurea | 267 | 242 | 8.3 (1.0) | 187 (52) | −1.1 | 48 | −51.5 | +1.8 | ||||
| Sulfonylurea/metformin (38) | 52 wk | Randomized, open-label, noninferiority | Exenatide 10 μg bid plus metformin/sulfonylurea | 253 | 199 | 8.6 (1.0) | 198 (49) | −1.0 ± 0.1 | 32 | −32.4 ± 3.6 | −2.5 ± 0.2 | |
| Biphasic aspart (30% insulin aspart) plus metformin/sulfonylurea | 248 | 223 | 8.6 (1.1) | 203 (50) | −0.9 ± 0.1 | 24 | −30.6 ± 3.6 | +2.9 ± 0.2 | ||||
| Metformin and/or sulfonylurea (39) | ≥3 yr | Trials A–C (above) and their open-label extensions were folded into one open-ended, open-label trial | Exenatide 10 μg bid plus metformin and/or sulfonylurea | 527 | 217 | 8.2 (1.0) | 172 (45) | −1.0 ± 0.1 | 46 | −23.5 ± 3.8 | −5.3 ± 0.4 | |
| (Continuous) | ||||||||||||
Table 1A.
(Continued)
| Prior glycemic treatment | Length | Trial design | Intervention | No. of subjects randomized | No. of subjects completed | Baseline HbA1c(%) | Baseline FPG (mg/dl) | Δ HbA1c(%) baseline | Baseline HbA1c (%)d | % Achieved HbA1c ≤7% | Δ FPG (mg/dl) baseline | Δ Weight (lb) baseline |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liraglutide | ||||||||||||
| Two or less oral hypoglycemic agents (except TZD) (44) | 5 wk | Randomized, double-blind (liraglutide titration 0.5–2.0 mg in 0.5 mg increment weekly) plus 2-wk run-in with metformin | Liraglutide plus metformin 1000 mg bid | 36 | 34 | 9.5 (1.0) | 238 (45) | −0.8a | −70.2a | −2.2 | ||
| Liraglutide plus placebo | 36 | 30 | 9.4 (0.8) | 239 (45) | −0.2a | −25.2a | −2.1 | |||||
| Metformin 1000 mg bid plus placebo | 36 | 25 | 9.4 (0.8) | 243 (52) | −1.7 | |||||||
| Randomized, open label | Metformin 1000 mg bid plus glimepiride 4 mg qd | 36 | 36 | 9.4 (1.2) | 234 (47) | −0.3b | −21.6b | +0.8 | ||||
| One oral hypoglycemic agent (45) | 14 wk | Randomized, double-blind, placebo-controlled (previous hypoglycemic agent discontinued) | Placebo | 40 | 29 | 8.2 (0.7) | 203 (40) | +0.3 | 5 | |||
| Liraglutide 0.65 mg qd | 40 | 35 | 8.1 (0.6) | 203 (49) | −1.0 | 38 | −48.6c | |||||
| Liraglutide 1.25 mg qd | 42 | 39 | 8.3 (0.8) | 214 (43) | −1.4 | 48 | −61.2c | |||||
| Liraglutide 1.90 mg qd | 41 | 37 | 8.5 (0.9) | 221 (56) | −1.5 | 46 | −61.2c | −3.0 | ||||
| Sitagliptin | ||||||||||||
| Diet or one oral hypoglycemic agent (72,77) | 24 wk | Randomized, double-blind, placebo-controlled (washout period) | Placebo | 253 | 216 | 8.0 (0.8) | 176.4 (41.4) | +0.2 | 17 | +5.4 | −1.1 ± 0.2 | |
| Sitagliptin 100 mg qd | 238 | 209 | 8.0 (0.9) | 171.0 (43.2) | −0.6 | 41 | −12.6 | −0.2 ± 0.2 | ||||
| Sitagliptin 200 mg qd | 250 | 214 | 8.1 (0.9) | 174.6 (45.0) | −0.8 | 45 | −16.2 | −0.1 ± 0.2 | ||||
| 30-wk extension | Randomized | Sitagliptin 100 mg qd | 188 | 7.9 | −0.6 | 41 | ||||||
| Sitagliptin 200 mg qd | 194 | 8.0 | −0.6 | 46 | ||||||||
| Diet and exercise (73,78) | 24 wk | Randomized, double-blind, placebo-controlled, parallel-group | Placebo | 176 | 127 | 8.7 (1.0) | 196.3 (47.4) | +0.2 | 9 | +5.8 | −0.9 | |
| Sitagliptin 100 mg qd | 179 | 142 | 8.9 (1.0) | 201.4 (49.4) | −0.7 | 20 | −17.5 | 0.0 | ||||
| Metformin 500 mg bid | 182 | 153 | 8.9 (1.0) | 205.2 (50.6) | −0.8 | 23 | −27.3 | −0.6 to −1.3 | ||||
| Metformin 1000 mg bid | 182 | 156 | 8.7 (0.9) | 197.0 (46.8) | −1.1 | 38 | −29.3 | −0.6 to −1.3 | ||||
| Sitagliptin 50 mg bid plus metformin 500 mg bid | 190 | 164 | 8.8 (1.0) | 203.9 (51.7) | −1.4 | 43 | −47.1 | −0.6 to −1.3 | ||||
| Sitagliptin 50 mg bid plus metformin 1000 mg bid | 182 | 164 | 8.8 (1.0) | 196.7 (48.2) | −1.9 | 66 | −63.9 | −0.6 to −1.3 | ||||
| 30-wk extension | Double-blind, active-controlled phase | Sitagliptin 100 mg qd | 100 | Mean HbA1c 8.7% | −0.8 | 23 | ||||||
| Metformin 500 mg bid | 122 | Mean HbA1c 8.7% | −1.0 | 25 | ||||||||
| Metformin 1000 mg bid | 137 | Mean HbA1c 8.7% | −1.3 | 44 | ||||||||
| Sitagliptin 50 mg bid plus metformin 500 mg bid | 148 | Mean HbA1c 8.7% | −1.4 | 48 | ||||||||
| Sitagliptin 50 mg bid plus metformin 1000 mg bid | 157 | Mean HbA1c 8.7% | −1.8 | 67 | ||||||||
| Pioglitazone (74) | 24 wk | Randomized, double-blind, placebo-controlled, parallel group | Placebo plus pioglitazone 30–45 mg qd | 178 | 158 | 8.0 (0.8) | 165.6 (39.9) | −0.2 | 23 | +1.0 | +1.5 | |
| Sitagliptin 100 mg qd plus pioglitazone 30–45 mg qd | 175 | 149 | 8.1 (0.8) | 168.3 (39.5) | −0.9 | 45 | −16.7 | +1.8 | ||||
| (Continuous) | ||||||||||||
Table 1B.
(Continued)
| Prior glycemic treatment | Length | Trial design | Intervention | No. of subjects randomized | No. of subjects completed | Baseline HbA1c(%) | Baseline FPG (mg/dl) | Δ HbA1c(%) baseline | Baseline HbA1c (%)d | % Achieved HbA1c ≤7% | Δ FPG (mg/dl) baseline | Δ Weight (lb) baseline |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sitagliptin (continued) | ||||||||||||
| Metformin (75) | 24 wk | Randomized, double-blind, placebo-controlled, parallel group | Placebo plus metformin ≥1500 mg/d | 237 | 192 | 8.0 (0.8) | 172.8 (41.4) | 0.0 | 18 | +9.0 | ||
| Sitagliptin 100 mg qd plus metformin ≥1500 mg/d | 464 | 416 | 8.0 (0.8) | 169.2 (41.4) | −0.7 | 47 | −16.2 | |||||
| 30-wk extension | Double-blind, active-controlled (placebo switched to glipizide) | Glipizide 5–15 mg/d plus metformin ≥1500 mg/d | 157 | 7.9 | −0.9 | 61 | +1.5 | |||||
| Sitagliptin 100 mg qd plus metformin ≥1500 mg/d | 387 | 7.9 | −0.7 | 51 | −0.9 | |||||||
| Glimepiride or glimepiride/metformin (76) | 24 wk | Randomized, double-blind, placebo-controlled, parallel group | Placebo plus glimepiride ≥ 4 mg/d | 106 | 87 | 8.4 (0.8) | 184.9 (42.3) | +0.3 | 9 | +18.4 | 0.0 | |
| Placebo plus glimepiride ≥ 4 mg/d plus metformin ≥1500 mg/d | 113 | 92 | 8.3 (0.7) | 178.4 (42.6) | +0.3 | 1 | +12.9 | −0.7 | ||||
| Sitagliptin 100 mg qd plus glimepiride ≥4 mg/d | 106 | 83 | 8.4 (0.8) | 182.6 (33.1) | −0.3 | 11 | −0.88 | +1.1 | ||||
| Sitagliptin 100 mg qd plus glimepiride ≥4 mg/d plus metformin ≥1500 mg/d | 116 | 102 | 8.3 (0.7) | 179.4 (41.6) | −0.6 | 23 | −7.8 | +0.4 | ||||
| Metformin (80) | 52 wk | Randomized, double-blind, active-controlled, parallel-group, noninferiority trial | Sitagliptin 100 mg qd plus metformin ≥1500 mg/d | 588 | 386 | 7.5 (0.8) | 157.7 (33.7) | −0.7 | 63 | −10.1 | −1.5 | |
| Glipizide 5–20 mg/d plus metformin ≥1500 mg/d | 584 | 412 | 7.5 (0.9) | 159.1 (38.5) | −0.7 | 59 | −7.6 | +1.1 | ||||
| Vildagliptin | ||||||||||||
| Drug naive (91,92) | 52 wk | Randomized, double-blind, placebo-controlled, parallel group | Placebo | 150 | 131 | 6.8 (0.4) | 129.6 (21.6) | +0.1 ± 0.1 | +9.0 ± 1.8 | −0.2 ± 0.3 | ||
| Vildagliptin 50 mg qd | 156 | 133 | 6.7 (0.4) | 127.8 (21.6) | −0.2 ± 0.1 | +3.6 ± 1.8 | −0.5 ± 0.3 | |||||
| 4-wk washout, 52-wk extension | Placebo | 63 | 50 | 6.7 (0.4) | 126.0 (18.0) | +0.5 ± 0.1 | +21.6 ± 5.4 | −0.3 ± 0.4 | ||||
| Vildagliptin 50 mg qd | 68 | 58 | 6.6 (0.4) | 122.4 (18.0) | +0.1 ± 0.1 | +10.8 ± 5.4 | −1.1 ± 0.5 | |||||
| Drug naive (93) | 24 wk | Randomized, double-blind, placebo-controlled, parallel group | Placebo | 160 | 119 | 8.4 (0.8) | 178.2 (45.0) | −0.3 ± 0.1 | −1.8 | −1.4 ± 0.4 | ||
| Vildagliptin 50 mg qd | 163 | 130 | 8.2 (0.8) | 176.4 (43.2) | −0.8 ± 0.1 | −18.0 | −1.8 ± 0.4 | |||||
| Vildagliptin 50 mg bid | 152 | 128 | 8.6 (0.8) | 181.8 (39.6) | −0.8 ± 0.1 | −14.4 | −0.3 ± 0.4 | |||||
| Vildagliptin 100 mg qd | 157 | 134 | 8.4 (0.8) | 178.2 (41.4) | −0.9 ± 0.1 | −14.4 | −0.8 ± 0.4 | |||||
| Drug naive (94) | 52 wk | Randomized, double-blind, active-controlled, parallel group | Vildagliptin 50 mg bid | 526 | 378 | 8.7 (1.1) | 189.0 (52.2) | −1.0 ± 0.1 | −16.2 ± 1.8 | +0.3 ± 0.2 | ||
| Metformin 1000 mg bid | 254 | 191 | 8.7 (1.1) | 189.0 (52.2) | −1.4 ± 0.1 | −34.2 ± 3.6 | −1.9 ± 0.3 | |||||
| Drug naive (95) | 24 wk | Randomized, double-blind, active-controlled, parallel group | Vildagliptin 50 mg bid | 519 | 446 | 8.7 (1.1) | 185.4 (48.6) | −1.1 ± 0.1 | −23.4 ± 1.8 | −0.3 ± 0.2 | ||
| Rosiglitazone 8 mg qd | 267 | 232 | 8.7 (1.1) | 185.4 (52.2) | −1.3 ± 0.1 | −41.4 ± 3.6 | +1.6 ± 0.3 | |||||
| Drug naive (96) | 24 wk | Randomized, double-blind, active-controlled, parallel group | Pioglitazone 30 mg qd | 161 | 133 | 8.7 (1.0) | 189.0 (55.8) | −1.4 ± 0.1 | 43 | −34.2 ± 3.6 | +1.5 ± 0.3 | |
| Vildagliptin50 mg qd plus pioglitazone 15 mg qd | 144 | 115 | 8.8 (0.9) | 192.6 (48.6) | −1.7 ± 0.1 | 54 | −43.2 ± 3.6 | +1.4 ± 0.3 | ||||
| Vildagliptin100 mg qd plus pioglitazone 30 mg qd | 148 | 129 | 8.8 (1.1) | 196.2 (48.6) | −1.9 ± 0.1 | 65 | −50.4 ± 3.6 | +2.1 ± 0.3 | ||||
| Vildagliptin 100 mg qd | 154 | 136 | 8.6 (1.0) | 190.8 (48.6) | −1.1 ± 0.1 | 43 | −23.4 ± 3.6 | +0.2 ± 0.3 | ||||
| Drug naive (97) | 24 wk | Randomized, double-blind, active-controlled, parallel group | Vildagliptin 50 mg bid | 441 | 399 | 8.6 (0.9) | 180.0 (43.2) | −1.4 ± 0.1 | 46 | −21.6 ± 1.8 | −0.4 ± 0.1 | |
| Acarbose up to 100 mg tid | 220 | 192 | 8.6 (1.0) | 183.6 (45.0) | −1.3 ± 0.1 | 47 | −27.0 ± 3.6 | −1.7 ± 0.2 | ||||
| (Continuous) | ||||||||||||
Table 1C.
(Continued)
| Prior glycemic treatment | Length | Trial design | Intervention | No. of subjects randomized | No. of subjects completed | Baseline HbA1c(%) | Baseline FPG (mg/dl) | Δ HbA1c(%) baseline | Baseline HbA1c (%)d | % Achieved HbA1c ≤7% | Δ FPG (mg/dl) baseline | Δ Weight (lb) baseline |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vildagliptin (continued) | ||||||||||||
| Sulfonylurea (98) | 24 wk | Randomized, double-blind, placebo-controlled | Placebo plus glimepiride 4 mg qd | 176 | 108 | 8.5 (1.0) | 185.4 (52.2) | +0.1 ± 0.1 | 12 | +3.6 ± 3.6 | −0.4 ± 0.3 | |
| Vildagliptin 50 mg qd plus glimepiride 4 mg qd | 170 | 113 | 8.5 (0.9) | 189.0 (54.0) | −0.6 ± 0.1 | 21 | −5.4 ± 3.6 | −0.1 ± 0.3 | ||||
| Vildagliptin 50 mg bid plus glimepiride 4 mg qd | 169 | 111 | 8.6 (1.0) | 189.0 (48.6) | −0.6 ± 0.1 | 25 | −7.2 ± 3.6 | +1.3 ± 0.3 | ||||
| Metformin (99) | 24 wk | Randomized, double-blind, placebo-controlled, parallel group | Placebo plus metformin ≥1500 mg/d | 182 | 152 | 8.3 (0.9) | 181.8 (43.2) | +0.2 ± 0.1 | ≤7.9 | 14 | ||
| 7.9–8.5 | 13 | |||||||||||
| >8.5 | 2 | |||||||||||
| Vildagliptin 50 mg qd plus metformin ≥1500 mg/d | 177 | 153 | 8.4 (0.9) | 174.6 (39.6) | −0.5 ± 0.1 | ≤7.9 | 50 | |||||
| 7.9–8.5 | 22 | |||||||||||
| >8.5 | 8 | |||||||||||
| Vildagliptin 50 mg bid plus metformin ≥1500 mg/d | 185 | 157 | 8.4 (1.0) | 178.2 (46.8) | −0.9 ± 0.1 | ≤7.9 | 54 | |||||
| 7.9–8.5 | 31 | |||||||||||
| >8.5 | 16 | |||||||||||
| TZD (100) | 24 wk | Randomized, double-blind, placebo-controlled, parallel group | Placebo plus pioglitazone 45 mg qd | 158 | 128 | 8.7 (1.2) | 181.8 (54.0) | −0.3 ± 0.1 | 15 | −9.0 ± 3.6 | ||
| Vildagliptin 50 mg qd plus pioglitazone 45 mg qd | 147 | 124 | 8.6 (1.0) | 185.4 (52.2) | −0.8 ± 0.1 | 29 | −14.4 ± 3.6 | |||||
| Vildagliptin 50 mg bid plus pioglitazone 45 mg qd | 158 | 124 | 8.7 (1.2) | 180.0 (59.4) | −1.0 ± 0.1 | 36 | −19.8 ± 3.6 | |||||
| Insulin (101) | 24 wk | Randomized, double-blind, placebo-controlled, parallel group | Placebo plus insulin | 152 | 124 | 8.4 (1.1) | 156.6 (55.8) | −0.2 ± 0.1 | −3.6 ± 7.2 | 0.6 ± 0.3 | ||
| Vildagliptin 50 mg bid plus insulin | 144 | 114 | 8.4 (1.0) | 167.4 (55.8) | −0.5 ± 0.1 | −14.4 ± 5.4 | 1.3 ± 0.3 | |||||
| Metformin (102) | 24 wk | Randomized, double-blind, active-controlled | Vildagliptin 50 mg bid plus metformin ≥1500 mg/d | 295 | 262 | 8.4 (1.0) | 196.2 (46.8) | −0.9 ± 0.1 | 27% | −25.2 ± 1.8 | +0.3 ± 0.2 | |
| Pioglitazone 30 mg qd plus metformin ≥1500 mg/d | 281 | 244 | 8.4 (0.9) | 198.0 (48.6) | −1.0 ± 0.1 | 36% | −37.8 ± 1.8 | +1.9 ± 0.2 |
Data are presented as mean (sd) or mean ± se. bid, Twice daily; qd, every day; tid, three times a day; TZD, thiazolidinedione.
Change relative to metformin therapy.
Change relative to liraglutide and metformin combination therapy.
Change relative to placebo.
This column only applies to the Vildagliptin trial (99).
Side effects
A metaanalysis on the randomized controlled trials with exenatide showed that severe hypoglycemia was rare. Mild to moderate hypoglycemia was 16 vs. 7% (exenatide vs. placebo) and more common with coadministration with a sulfonylurea. The most common side effects of exenatide were nausea (57%) and vomiting (17%). Nausea was usually mild to moderate in nature, and being most common during the initial 8 wk therapy and declined thereafter. Overall, 4% of patients withdrew from the studies because of gastrointestinal side effects (40).
Liraglutide
Liraglutide is a long-acting GLP-1 analog with a substitution of Lys34 with Arg34, and an attachment of a C-16 free-fatty acid derivative via a glutamoyl spacer to Lys26 (Fig. 1). The free-fatty acid derivative is thought to promote noncovalent binding of liraglutide to albumin, therefore, increasing plasma half-life through protection from renal clearance and slow absorption rate from injection site (41). Like GLP-1 and exenatide, liraglutide needs to be injected sc. After sc injection, maximum plasma concentrations are reached after 10–14 h, and it has a half-life of 11–13 h (42,43).
Selected clinical trials
In a 5-wk dose-escalation study, liraglutide/metformin combination was associated with a 0.8% reduction in HbA1c and a 70 mg/dl reduction in fasting glucose when compared with metformin alone. In addition, liraglutide/metformin significantly reduced fasting glucose (21.6 mg/dl) and body weight (2.9 kg) when compared with the metformin/glimepiride group, and liraglutide/placebo significantly reduced fasting glucose (25.2 mg/dl) when compared with the metformin/placebo group (Table 1) (44). In a 14-wk study of liraglutide vs. placebo, liraglutide significantly reduced HbA1c by 1.45, 1.40, and 0.98% in the 1.90, 1.25, and 0.65-mg groups, respectively, whereas placebo group had an increase of 0.29% in HbA1c. The percentages of patients that achieved HbA1c of 7% or less were 46, 48, 38, and 5 in the 1.9, 1.25, 0.65-mg groups and the placebo group, respectively (Table 1) (45). The results from phase 3 trials have not been presented at scientific meetings or published in peer-reviewed journals.
Side effects
Most frequently reported adverse events were nausea and vomiting, especially at the higher doses (40,45). There is also no development of antibodies noted in trials up to 14 wk (45,46,47).
Unresolved Issues Regarding GLP-1 and GLP-1 Mimetics
1. Does GLP-1 and GLP-1 mimetics have favorable effects on β-cell mass in humans?
Studies have shown that exenatide has favorable effects on parameters of β-cell function in humans using indirect measures such as first-phase insulin secretion and homeostasis model assessment β-cell index (48,49). In rodent studies, GLP-1 induced glucose sensitivity in glucose-resistant β-cells (50). Exenatide given to rodents in pharmacological doses appeared to have beneficial effects on β-cell mass not seen with other antidiabetic agents. However, whether exenatide has a favorable effect on β-cell mass in humans is unknown.
Exenatide prevented cytotoxic agent-induced apoptosis of rodent islets (51), and chronic treatment increased β-cell turnover in rodents (52). GLP-1 also inhibited nonchemically induced β-cell apoptosis in freshly isolated human islets (53). Both decreased apoptosis and increased β-cell turnover should and do lead to increases in islet size and β-cell numbers. The trophic effects of exenatide on β-cells in rodents are seen with concentrations not achieved in clinical practice. Although markers of β-cell function show improvement in humans with chronic exenatide use of up to 3 yr (39), this improvement in function may be due to the restoration of glucose-competence to β-cells and the insulinotropic, glucose-lowering, and weight-loss effects of exenatide, and not because of any direct effect of exenatide on β-cell mass.
2. What is the mechanism by which GLP-1 and GLP-1 mimetics lower glucagon secretion from α-cells?
Elevated fasting and postprandial plasma glucagon levels throughout the day are a feature of T2DM (54), and exenatide treatment lowers both (55). The ability for exenatide and GLP-1 to lower glucagon levels in patients with T2DM most likely contributes to its overall glucose-lowering effect. In addition, by virtue of enhancing endogenous insulin secretion concurrently with suppressing glucagon secretion, a more physiological insulin to glucagon ratio in the portal vein should be established, resulting in better suppression of hepatic glucose output. Whether GLP-1 and GLP-1 mimetics lower glucagon secretion from α-cells through direct or indirect mechanisms is still unclear.
The presence of GLP-1R on human α-cells has not been directly investigated. Overnight iv GLP-1 administration to fasted subjects with type 1 diabetes mellitus resulted in the lowering of plasma glucagon levels that was postulated to be a direct effect of GLP-1 on α-cells (56). However, given that plasma C-peptide levels were doubled by GLP-1 infusion, an indirect action mediated by the stimulatory effect of GLP-1 on residual neighboring β (or δ) cells resulting in intraislet paracrine inhibition of glucagon release is also plausible, though at considerably lower insulin concentrations than in healthy or T2DM subjects. A transgenic model of β-cell dysfunction also favors a paracrine effect of GLP-1 on glucagon secretion. Mice with a β-cell-specific mutation of the pdx-1 gene had defective insulin secretory and glucagon suppressive responses to exenatide, both of which were present in wild-type mice (57). This strongly suggests that a β-cell secreted factor is absolutely necessary for GLP-1-mediated suppression of glucagon secretion.
Rodent data on the presence or absence of GLP-1R on α-cells are not convincing either way. Neither GLP-1Rs nor their transcripts could be detected in purified rat α-cells (58,59). Direct GLP-1 application to rat α-cells did not alter glucagon secretion or cause an increase in cAMP levels. However, GLP-1R expression was detected by immunocytochemistry in a subpopulation (20%) of glucagon-positive cells in dispersed rat islets (60). Because this is a small number of cells and the cells were not obtained with precise methodology, such as laser-captured microscopy, contaminating cells may be the source of the GLP-1R expression.
Furthermore, GLP-1 was also recently reported to elicit an increase in the cAMP content and glucagon secretion in an α-cell line transfected with the GLP-1Rs (61). Therefore, if α-cells actually contain GLP-1Rs, increased glucagon secretion would be the expected response to elevated plasma GLP-1 levels or exenatide therapy. Finally, neuronal control of glucagon secretion through the autonomic nervous system is well recognized, and this pathway may be mediated by GLP-1. Therefore, GLP-1 and exenatide infusion may cause glucagon suppression in vivo via feedback from vagal afferents where neuronal networks are intact but not in vitro from dispersed α-cells or cell lines. Regardless, the mechanism underlying suppressed plasma glucagon levels by exenatide is an interesting area of research and may offer insights as to how glucagon secretion might be controlled in T2DM.
DPP 4 Inhibitors
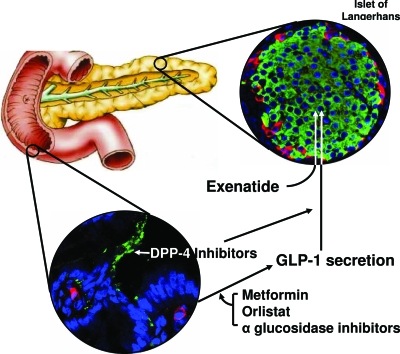
If pharmacological levels of exogenous GLP-1 can lower blood glucose in T2DM, it is logical to assume that supraphysiological levels of endogenous active GLP-1 (aGLP-1) can also lower blood glucose. No secretagogue of L cells has been specifically developed, though clear headway has been made in elucidating how food products bring about GLP-1 secretion from L cells (Fig. 2) (62,63,64). Compared with wild-type mice, DPP knockout mice have elevated fasting incretin levels, lower plasma glucose, and higher plasma insulin levels after a glucose challenge (65). There has been immense interest at disrupting DPP 4 activity in humans to increase plasma aGLP-1 levels. Sitagliptin and vildagliptin are two such DPP 4 inhibitors.
Figure 2.
Mechanism of action of sitagliptin, vildagliptin, and exenatide. GLP-1 is released from L cells (stained red) of the gut, and is subject to DPP 4 (stained green on endothelial cells of blood vessels of the gut) degradation in both gut and blood. Sitagliptin and vildagliptin inhibit DPP 4 action in blood and on endothelial cells. Metformin, orlistat, and α-glucosidase inhibitors increase GLP-1 secretion. Exenatide, a GLP-1R agonist, increases insulin secretion from β-cells (stained green) in islets of Langerhans. The α-cells in islets are stained red.
Sitagliptin
Sitagliptin, an organic molecule, appears to be selective for DPP 4 and not interact with other closely related proteases (Fig. 1) (66). Sitagliptin is rapidly absorbed, achieving peak plasma levels 1–6 h after dosing. Its half-life is 8–14 h with bioavailability of 87%, with or without food (67,68). About 80% of the dose is excreted unchanged by the kidney, with 15% of the bioavailable drug metabolized by CYP3A4 and CYP2C8 in the liver (67,69). At 100 mg daily, greater than 80% of plasma DPP 4 activity is inhibited over a 24-h period (67,70). A dose reduction to 50 mg is needed if creatinine clearance is less than 50 ml/min and to 25 mg if creatinine clearance is less than 30 ml/min (71).
Selected clinical studies
Five 24-wk trials in T2DM patients examined the following: sitagliptin monotherapy; comparison of sitagliptin monotherapy, metformin monotherapy, and initial combination therapy of sitagliptin and metformin; sitagliptin added to ongoing pioglitazone; sitagliptin added to ongoing metformin; and sitagliptin added to ongoing sulfonylurea and/or metformin (Table 1) (72,73,74,75,76). As initial therapy, sitagliptin/metformin combination therapy worked better than either sitagliptin or metformin monotherapy with an HbA1c reduction of 1.9% compared with 0.6–0.7% and 1.13% after 24 wk. As adjuvant therapy, sitagliptin in combination with metformin, glipizide, or pioglitazone yielded an HbA1c reduction of 0.6–0.7% when compared with placebo.
Preliminary results from 30-wk extension trials on sitagliptin monotherapy, initial sitagliptin combination therapy with or without metformin, and sitagliptin as adjuvant therapy to metformin showed that the reduction in HbA1c was sustained at wk 54 (77,78,79). A 52-wk trial on sitagliptin vs. glipizide as adjuvant therapy to metformin showed a reduction in HbA1c of 0.7% in both groups, however, the maximal HbA1c reduction was observed at 24–30 wk with a gradual increase in HbA1c from wk 30–52, which raises the issue of declining sitagliptin efficacy (80). Sitagliptin is reported to be weight neutral. Currently, there is an ongoing study on adding sitagliptin to exogenous insulin in patients with or without metformin treatment (81).
Side effects
A pooled analysis of 5141 patients in clinical trials for 2 yr or less showed that sitagliptin monotherapy or combination therapy (metformin, pioglitazone, sulfonylurea, or sulfonylurea and metformin) was well tolerated, and hypoglycemia occurred in the setting of combination therapy (82). The adverse events that were higher with sitagliptin compared with nonexposed groups included nasopharyngitis, contact dermatitis, and osteoarthritis. A systematic review and metaanalysis of incretin therapies showed that sitagliptin has no risk of gastrointestinal adverse events but has an increase risk for urinary track infection, headache, and especially nasopharyngitis (40), and may reflect a lack of DPP 4 activity required for immunosurveillance.
Vildagliptin
Vildagliptin, a selective, reversible, and competitive inhibitor of DPP 4, is a low molecular weight compound suitable for oral dosing (83,84). After dosing, vildagliptin is rapidly absorbed and achieves peak plasma levels in 1–2 h. Its half-life of 2 h is shorter than sitagliptin (85,86). Its bioavailability is 85% (87), and its pharmacokinetics is not affected by food (88). At 100 mg daily, it inhibits 98% of DPP 4 activities 45 min after dosing and 60% at 24 h. Approximately 85% of vildagliptin is metablolized in the liver to LAY151 by hydrolysis: LAY151 is inactive. The remaining 15% is eliminated unchanged by the kidneys (89). A study suggested that there was no significant difference in exposure to vildagliptin in patients with various degrees of hepatic impairment (89). In 2007, the Food and Drug Administration requested additional data on patients with renal impairment before granting final approval of vildagliptin (90).
Selected clinical trials
Six clinical trials evaluated vildagliptin as initial monotherapy in comparison to placebo, metformin, rosiglitazone, or acarbose, and also as initial combination therapy with pioglitazone in comparison to vildagliptin monotherapy in drug-naive patients with T2DM (Table 1) (91,92,93,94,95,96,97). Patients with worse glycemic control (HbA1c ∼8.4 vs. 6.7%) had bigger HbA1c reduction over 24 wk. Data from the extension study on the group with better glycemic control showed that maximum HbA1c reduction occurred around 24–30 wk, followed by a gradual increase thereafter until wk 108 (92). As monotherapy, vildagliptin 50 mg twice daily was as effective as rosiglitazone 8 mg once daily and acarbose 100 mg thrice daily in lowering HbA1c but not as effective as metformin 1000 mg twice daily (94,95,97). Initial combination therapy with vildagliptin and pioglitazone provided better glycemic control than either vildagliptin or pioglitazone monotherapy (96).
Vildagliptin is effective as adjuvant therapy when administered to patients inadequately controlled with sulfonylurea, metformin, thiazolidinedione, or insulin therapy with HbA1c reduction of 0.6, 0.9, 1.0, and 0.5%, respectively (98,99,100,101). In addition, vildagliptin and pioglitazone were equally effective as adjuvant therapy for patients who were inadequately controlled on metformin, in which HbA1c reductions of 0.9 and 1.0% were noted, respectively (102).
Side effects
The side effects from vildagliptin are comparable to that of sitagliptin. In a systematic review and metaanalysis of incretin therapies, vildagliptin has no risk of gastrointestinal adverse events but has an increase risk for urinary track infection and headache (40).
Unresolved Issues Regarding DPP 4 Inhibitors
1. Do DPP 4 inhibitors have favorable effects on β-cell mass in humans?
Exenatide appears to have beneficial effects on β-cell mass when given in pharmacological doses to rodents (51,52). The effect of DPP 4 inhibitors on β-cell mass is less clear. Three-month treatment of high-fat-fed diet streptozotocin-induced diabetic mice with des-fluoro-sitagliptin preserved β-cells from apoptosis with no increase in β-cell mass (103). β-Cells of DPP 4 knockout mice are also reported to be more resistant to the toxic effects of streptozotocin (104). But against DPP 4 inhibitors being trophic factors, 8-wk treatment with vildagliptin had no obvious effects on β-cell turnover or β-cell mass in mice (105).
2. Is the modest increase in aGLP-1 levels the sole modulator of glycemia using DPP 4 inhibitors?
DPP 4 inhibitors were developed to augment biologically active, endogenously secreted plasma GLP-1. In humans, sitagliptin, both after a single dose and after a once-daily dose for 10 d, resulted in about a 2-fold increase in aGLP-1 after meal (67,106). Furthermore, sitagliptin decreased total GLP-1 (tGLP-1) in the presence of increased aGLP-1 (107). However, whether the 2-fold increase in aGLP-1 is sufficient to explain the glucose-lowering effect with reduction of HbA1c in patients on chronic sitagliptin therapy is controversial.
If DPP 4 inhibitors did lower blood glucose as a direct consequence of increased aGLP-1 levels, plasma insulin levels would be expected to increase as well. However, fasting and postprandial plasma insulin and C-peptide levels were not different before and after 10 d DPP 4 inhibition in both healthy and T2DM subjects (106,108,109). Indeed, infusions of GLP-1 that result in comparable plasma aGLP-1 levels attained by DPP 4 inhibition do not induce insulin secretion in T2DM (10). Some reviewers noted that with DPP 4 inhibitors, the same amount of insulin is secreted at a lower glucose level, or insulinogenic index is improved (110). However, any treatment that lowers plasma glucose without increasing insulin secretion, such as weight loss, metformin, or α-glucosidase inhibitors, also improves insulinogenic indices (111,112).
Another surprising finding is that DPP 4 inhibition does not slow gastric emptying (108) when slowed gastric emptying is a consistent finding with exogenous GLP-1 and exenatide treatments (13,113). An explanation offered in some reviews is that the degree of elevation of aGLP-1 is not of sufficient magnitude to inhibit gastric emptying (110,114). However, by the same rationale, one can extrapolate that the elevation in aGLP-1 from DPP 4 inhibition is also not sufficient to bring about an increase in insulin secretion (108).
3. How might DPP 4 inhibition lead to a decline in plasma glucose levels without an increase in insulin secretion?
DPP 4 inhibition results in lower postprandial plasma glucagon levels (108,109,115). However, the reduced glucagon secretion is not evident in the fasting state when it would be most beneficial to decrease nocturnal hepatic glucose output. The postprandial glucagon suppressive effects of DPP 4 inhibitors, whereas significantly different from placebo, are small and short lived, and the levels are much higher than in nondiabetic subjects, therefore, unlikely to account for the full antihyperglycemic effect.
The following is speculation by the authors. Many endogenous compounds are subject to DPP 4 modification, resulting in their activation or inactivation, and any of these unknown qualities might have effects on glucose homeostasis (116,117). If indeed the glucose-lowering effects of DPP 4 inhibition are mediated by GLP-1, one would expect to see maximum clinical effects of one dose of DPP 4 inhibitor on PPG and insulin levels immediately after a meal when GLP-1 secretion is at its maximum. However, this is not the case because no clinical effects on glucose, insulin, glucagon, or C-peptide levels over a 2-h post-meal period were observed after one dose of sitagliptin (67). However, after 4 wk sitagliptin, PPG levels were significantly reduced over a 24-h period in the treatment group, but insulin and C-peptide levels were comparable between treatment and placebo groups (118). This phenomenon may signify accumulation, over time, of one or more DPP 4 products that have effects on glucose uptake.
GLP-1 is known to have effects on the gut-hepatoportal-brain neural axis. Sitagliptin should directly inhibit DPP 4 activity at the level of the vascular endothelium in the gut, resulting in greater activation by GLP-1 of sensory neurons originating in the nodose ganglion, where GLP-1R gene expression has been shown to occur (119,120). It should also cause higher aGLP-1 levels to enter the portal system after eating with subsequent activation of the vagal hepatic nerves (121). GLP-1R mRNA is present on nerve terminals of the portal vein in rodents (120), and there are GLP-1-modifiable glucose sensors in the hepatoportal bed (122). Dog studies had shown that direct infusion of GLP-1 into the portal vein results in increased glucose uptake (123,124). Against gut-neuronal pathways being the likely cause of the improved glucose homeostasis with DPP 4 inhibition is this – gastric emptying is not altered. GLP-1 is thought to influence gastric emptying through interacting with afferent sensory neurons. Therefore, if DPP 4 inhibition were of such magnitude as to influence neuronal pathways through greater GLP-1R activation, one would also expect to see effects on gastric emptying, which is not the case.
4. Was the development of DPP 4 inhibitors, which are not specific for GLP-1 and actually resulted in decreased tGLP-1 secretion, really needed to increase plasma aGLP-1 levels?
There are other hypoglycemic agents that cause a minor increase in plasma GLP-1 levels but were thought to not contribute to their antihyperglycemic effect. Three-day treatment with phenformin resulted in elevated levels of gut-derived glucagon-like immunoreactivity (measured before a RIA specific for GLP-1 was available) both during fasting and in response to intraduodenal glucose infusions in T2DM (125). One-week metformin treatment in healthy subjects resulted in dramatic increases in postprandial glucagon-like immunoreactivity levels when compared with baseline (126). Furthermore, a 2-wk course of metformin in obese nondiabetic volunteers resulted in a statistically significant increase in aGLP-1 levels during an oral glucose load performed under euglycemic-hyperinsulinemic clamp when compared with baseline (127). aGLP-1 levels during both fasting and after the oral glucose load did not change after a single 850 mg dose of metformin but were significantly increased after 4 wk metformin in obese patients with and without T2DM (128). Subsequently, metformin was found to inhibit DPP 4 activity in patients with T2DM (129). Similarly, metformin was found to decrease DPP 4 activity, increase aGLP-1 levels, and improve insulin secretory capability to exogenous GLP-1 administration in diabetic mice (130). However, on a molar basis, specific DPP 4 inhibitors are 15–20 times more effective at reducing DPP 4 activity than metformin. A recent study of healthy subjects showed the following: both postprandial tGLP-1 and aGLP-1 levels were increased 2-fold with metformin; aGLP-1 levels were increased 2-fold but tGLP-1 levels were diminished by a third with sitagliptin; and aGLP-1 levels were increased 4-fold and tGLP-1 increased by 1.6-fold with metformin/sitagliptin (107).
These data suggest that metformin and sitagliptin increase aGLP-1 levels through different mechanisms. Most likely metformin increases GLP-1 levels through both inhibition of DPP 4 and secretion from L cells. The mechanism by which metformin might increase GLP-1 secretion is speculative. Biguanides have inhibited glucose absorption (131,132). We hypothesize that this decrease in glucose absorption would prolong exposure of the sweet taste receptors on intestinal L cells (recently found to be the modulators of GLP-1 secretion from L cells) to glucose, resulting in the prolonged activation of the sweet taste receptors and secretion of GLP-1 (Fig. 2) (62).
Although metformin increases GLP-1 secretion, it is still unclear whether this increase has any glucose-lowering effect. It is well accepted that metformin lowers glucose levels by suppressing hepatic glucose output, mediated through kinase LKB1 in the liver (133,134). Therefore, it is also reasonable to ask whether sitagliptin, which increases aGLP-1 by the same amount as metformin, is actually lowering glucose through aGLP-1. However, given the synergistic effect of metformin and sitagliptin, both in terms of increase in aGLP-1 levels and lowering of HbA1c (0.8% with sitagliptin alone, 1.3% with metformin alone, and 1.8% with metformin/sitagliptin), combination therapy might actually have a meaningful impact in glucose lowering through the GLP-1 mechanism (73,78,107).
Summary
Exenatide, as adjuvant therapy in T2DM, led to sustained HbA1c reduction of 1.0%, and improved β-cell function and weight loss. It is inconvenient to use, but long-acting forms with once-weekly injection, such as long-acting release exenatide formulation are under development (135). Liraglutide lowered HbA1c by 1.5% in a 14-wk study, but phase 3 studies are not yet available in peer-reviewed journals.
The advantage of DPP 4 inhibitors is their availability in oral form. Sitagliptin monotherapy led to HbA1c reduction of 0.6–0.7% after 54 wk. Vildagliptin monotherapy lowered HbA1c by 0.9–1.4% after 24 wk. However, patients with mild T2DM on low-dose vildagliptin showed a return of HbA1c to pretreatment levels after 108 wk. A similar trend was seen in sitagliptin. Long-term data on sitagliptin and vildagliptin are needed to evaluate whether their glucose-lowering effects are sustained. Both DPP 4 inhibitors are weight neutral, and their effects on other DPP 4 substrates need further research.
A better understanding of the effects of GLP-1 and GLP-1 mimetics on β-cell mass in humans and the mechanism of action by which they lower glucagon secretion from α-cells are needed. Finally, more work is needed to elucidate how DPP 4 inhibitors improve insulin sensitivity in humans.
Footnotes
Address all correspondence and requests for reprints to: Josephine M. Egan, M.D., F.R.C.P.I., National Institute on Aging, National Institutes of Health, 5600 Nathan Shock Drive, Baltimore, Maryland 21224-6825. E-mail: eganj@grc.nia.nih.gov.
The writing of this special feature was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute on Aging.
The views expressed in this manuscript are those of the authors and do not reflect those of the NIH.
Disclosure Summary: The authors have nothing to declare.
First Published Online July 15, 2008
Abbreviations: aGLP-1, Active glucagon-like peptide-1; DPP, dipeptidyl peptidase; FPG, fasting plasma glucose; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; GLP-1R, glucagon-like peptide-1 receptor; HbA1c, glycosylated hemoglobin; NEP, neutral endopeptidase; PPG, postprandial glucose; tGLP-1, total glucagon-like peptide-1; T2DM, type 2 diabetes mellitus.
References
- Elrick H, Stimmler L, Hlad Jr CJ, Arai Y 1964 Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 24:1076–1082 [DOI] [PubMed] [Google Scholar]
- McIntyre N, Holdsworth CD, Turner DS 1965 Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab 25:1317–1324 [DOI] [PubMed] [Google Scholar]
- Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM 2006 Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290:E550–E559 [DOI] [PubMed] [Google Scholar]
- Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ 2008 Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 57:678–687 [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ 2001 Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50:609–613 [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ 2003 Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 88:2706–2713 [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ 2001 Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717–3723 [DOI] [PubMed] [Google Scholar]
- Kjems LL, Holst JJ, Volund A, Madsbad S 2003 The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 52:380–386 [DOI] [PubMed] [Google Scholar]
- Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, Minaker KL, Habener JF, Andersen DK 1994 The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7–37) in normal and diabetic subjects. Regul Pept 51:63–74 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W 1993 Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hufner M, Schmiegel WH 2002 Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 87:1239–1246 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W 1993 Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36:741–744 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA 2003 Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 88:2719–2725 [DOI] [PubMed] [Google Scholar]
- Verdich C, Flint A, Gutzwiller JP, Naslund E, Beglinger C, Hellstrom PM, Long SJ, Morgan LM, Holst JJ, Astrup A 2001 A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 86:4382–4389 [DOI] [PubMed] [Google Scholar]
- Zander M, Madsbad S, Madsen JL, Holst JJ 2002 Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet 359:824–830 [DOI] [PubMed] [Google Scholar]
- Naslund E, Gutniak M, Skogar S, Rossner S, Hellstrom PM 1998 Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr 68:525–530 [DOI] [PubMed] [Google Scholar]
- Willms B, Idowu K, Holst JJ, Creutzfeldt W, Nauck MA 1998 Overnight GLP-1 normalizes fasting but not daytime plasma glucose levels in NIDDM patients. Exp Clin Endocrinol Diabetes 106:103–107 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Nauck MA, Kranz D, Holst JJ, Deacon CF, Gaeckler D, Schmidt WE, Gallwitz B 2004 Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes 53:654–662 [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, McIntosh CH, Pederson RA 1995 Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136:3585–3596 [DOI] [PubMed] [Google Scholar]
- Hansen L, Deacon CF, Orskov C, Holst JJ 1999 Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140:5356–5363 [DOI] [PubMed] [Google Scholar]
- Hupe-Sodmann K, McGregor GP, Bridenbaugh R, Goke R, Goke B, Thole H, Zimmermann B, Voigt K 1995 Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7–36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept 58:149–156 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Wollschlager D, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Willms B 1996 Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7–36 amide]) in patients with NIDDM. Diabetologia 39:1546–1553 [DOI] [PubMed] [Google Scholar]
- Juntti-Berggren L, Pigon J, Karpe F, Hamsten A, Gutniak M, Vignati L, Efendic S 1996 The antidiabetogenic effect of GLP-1 is maintained during a 7-day treatment period and improves diabetic dyslipoproteinemia in NIDDM patients. Diabetes Care 19:1200–1206 [DOI] [PubMed] [Google Scholar]
- Rachman J, Gribble FM, Barrow BA, Levy JC, Buchanan KD, Turner RC 1996 Normalization of insulin responses to glucose by overnight infusion of glucagon-like peptide 1 (7–36) amide in patients with NIDDM. Diabetes 45:1524–1530 [DOI] [PubMed] [Google Scholar]
- Meneilly GS, Greig N, Tildesley H, Habener JF, Egan JM, Elahi D 2003 Effects of 3 months of continuous subcutaneous administration of glucagon-like peptide 1 in elderly patients with type 2 diabetes. Diabetes Care 26:2835–2841 [DOI] [PubMed] [Google Scholar]
- Meneilly GS, Veldhuis JD, Elahi D 2005 Deconvolution analysis of rapid insulin pulses before and after six weeks of continuous subcutaneous administration of glucagon-like peptide-1 in elderly patients with type 2 diabetes. J Clin Endocrinol Metab 90:6251–6256 [DOI] [PubMed] [Google Scholar]
- Brubaker PL, Drucker DJ 2004 Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 145:2653–2659 [DOI] [PubMed] [Google Scholar]
- Egan JM, Bulotta A, Hui H, Perfetti R 2003 GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet β cells. Diabetes Metab Res Rev 19:115–123 [DOI] [PubMed] [Google Scholar]
- Green BD, Gault VA, O'Harte FP, Flatt PR 2004 Structurally modified analogues of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) as future antidiabetic agents. Curr Pharm Des 10:3651–3662 [DOI] [PubMed] [Google Scholar]
- Thorens B, Porret A, Buhler L, Deng SP, Morel P, Widmann C 1993 Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes 42:1678–1682 [DOI] [PubMed] [Google Scholar]
- Simonsen L, Holst JJ, Deacon CF 2006 Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia 49:706–712 [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, Baron AD 2005 Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 62:173–181 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD 2005 Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28:1092–1100 [DOI] [PubMed] [Google Scholar]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD 2004 Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27:2628–2635 [DOI] [PubMed] [Google Scholar]
- Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD 2005 Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 28:1083–1091 [DOI] [PubMed] [Google Scholar]
- Zinman B, Hoogwerf BJ, Duran Garcia S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG 2007 The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 146:477–485 [DOI] [PubMed] [Google Scholar]
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG 2005 Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 143:559–569 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M 2007 A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 50:259–267 [DOI] [PubMed] [Google Scholar]
- Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME, Maggs DG 2008 Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 24:275–286 [DOI] [PubMed] [Google Scholar]
- Amori RE, Lau J, Pittas AG 2007 Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 298:194–206 [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Knudsen SM, Wilken M, Colding-Joergensen M, Plum A, Ribel U, Agersoe H, Hansen K 2003 Plasma protein binding of NN2211, a long-acting derivative of GLP-1, is important for its efficacy. Diabetes 52:A321–A322 [Google Scholar]
- Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thogersen H, Wilken M, Agerso H 2000 Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 43:1664–1669 [DOI] [PubMed] [Google Scholar]
- Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M 2002 The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 45:195–202 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Hompesch M, Filipczak R, Le TD, Zdravkovic M, Gumprecht J 2006 Five weeks of treatment with the GLP-1 analogue liraglutide improves glycaemic control and lowers body weight in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes 114:417–423 [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courreges JP, Verhoeven R, Buganova I, Madsbad S 2007 Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 30:1608–1610 [DOI] [PubMed] [Google Scholar]
- Feinglos MN, Saad MF, Pi-Sunyer FX, An B, Santiago O 2005 Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with type 2 diabetes. Diabet Med 22:1016–1023 [DOI] [PubMed] [Google Scholar]
- Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR 2004 Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care 27:1335–1342 [DOI] [PubMed] [Google Scholar]
- Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL, Fineman MS, Kim DD, Nauck MA 2005 Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 90:5991–5997 [DOI] [PubMed] [Google Scholar]
- Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, Kim D, Baron AD 2003 Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care 26:2370–2377 [DOI] [PubMed] [Google Scholar]
- Holz 4th GG, Kuhtreiber WM, Habener JF 1993 Pancreatic β-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7–37). Nature 361:362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ 2003 Glucagon-like peptide-1 receptor signaling modulates β cell apoptosis. J Biol Chem 278:471–478 [DOI] [PubMed] [Google Scholar]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA 2007 Growth and regeneration of adult β cells does not involve specialized progenitors. Dev Cell 12:817–826 [DOI] [PubMed] [Google Scholar]
- Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R 2003 Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 144:5149–5158 [DOI] [PubMed] [Google Scholar]
- Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB 1987 Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 64:106–110 [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD 2003 Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88:3082–3089 [DOI] [PubMed] [Google Scholar]
- Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA 1996 Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7–36) amide in type I diabetic patients. Diabetes Care 19:580–586 [DOI] [PubMed] [Google Scholar]
- Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ 2005 β-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 54:482–491 [DOI] [PubMed] [Google Scholar]
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB 2005 β-cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54:1808–1815 [DOI] [PubMed] [Google Scholar]
- Moens K, Heimberg H, Flamez D, Huypens P, Quartier E, Ling Z, Pipeleers D, Gremlich S, Thorens B, Schuit F 1996 Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes 45:257–261 [DOI] [PubMed] [Google Scholar]
- Heller RS, Kieffer TJ, Habener JF 1997 Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing α-cells of the rat endocrine pancreas. Diabetes 46:785–791 [DOI] [PubMed] [Google Scholar]
- Doyle ME, Egan JM 2007 Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 113:546–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM 2007 Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104:15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G 2005 Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11:90–94 [DOI] [PubMed] [Google Scholar]
- Katsuma S, Hirasawa A, Tsujimoto G 2005 Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329:386–390 [DOI] [PubMed] [Google Scholar]
- Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N 2000 Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA 97:6874–6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H, Hickey GJ, Kowalchick JE, Leiting B, Lyons K, Marsilio F, McCann ME, Patel RA, Petrov A, Scapin G, Patel SB, Roy RS, Wu JK, Wyvratt MJ, Zhang BB, Zhu L, Thornberry NA, Weber AE 2005 (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 48:141–151 [DOI] [PubMed] [Google Scholar]
- Herman GA, Stevens C, Van Dyck K, Bergman A, Yi B, De Smet M, Snyder K, Hilliard D, Tanen M, Tanaka W, Wang AQ, Zeng W, Musson D, Winchell G, Davies MJ, Ramael S, Gottesdiener KM, Wagner JA 2005 Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 78:675–688 [DOI] [PubMed] [Google Scholar]
- Bergman A, Ebel D, Liu F, Stone J, Wang A, Zeng W, Chen L, Dilzer S, Lasseter K, Herman G, Wagner J, Krishna R 2007 Absolute bioavailability of sitagliptin, an oral dipeptidyl peptidase-4 inhibitor, in healthy volunteers. Biopharm Drug Dispos 28:315–322 [DOI] [PubMed] [Google Scholar]
- Vincent SH, Reed JR, Bergman AJ, Elmore CS, Zhu B, Xu S, Ebel D, Larson P, Zeng W, Chen L, Dilzer S, Lasseter K, Gottesdiener K, Wagner JA, Herman GA 2007 Metabolism and excretion of the dipeptidyl peptidase 4 inhibitor [14C]sitagliptin in humans. Drug Metab Dispos 35:533–538 [DOI] [PubMed] [Google Scholar]
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H 2006 Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 49:2564–2571 [DOI] [PubMed] [Google Scholar]
- Bergman AJ, Cote J, Yi B, Marbury T, Swan SK, Smith W, Gottesdiener K, Wagner J, Herman GA 2007 Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care 30:1862–1864 [DOI] [PubMed] [Google Scholar]
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE 2006 Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 29:2632–2637 [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE 2007 Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 30:1979–1987 [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P, Sitagliptin Study 019 Group 2006 Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 28:1556–1568 [DOI] [PubMed] [Google Scholar]
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G 2006 Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 29:2638–2643 [DOI] [PubMed] [Google Scholar]
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P 2007 Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 9:733–745 [DOI] [PubMed] [Google Scholar]
- Aschner P, Lunceford J, Williams-Herman D 2007 Once-daily sitagliptin monotherapy provides effective glycemic control over 54 weeks and is well tolerated in patients with type 2 diabetes. Diabetes 56:A553 [Google Scholar]
- Williams-Herman D, Johnson JR, Lunceford JK 2007 Initial combination therapy with sitagliptin and metformin provides effective and durable glycaemic control over 1 year in patients with type 2 diabetes (T2DM): a pivotal phase III clinical trial. Diabetologia 50:S52 [Google Scholar]
- Karasik A, Wu M, Williams-Herman D, Meininger G 2007 Sitagliptin added to ongoing metformin therapy provides sustained glycemic control over 54 weeks, with a low incidence of hypoglycemia and with weight loss. Diabetes 56:A139 [Google Scholar]
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, Sitagliptin Study 024 Group 2007 Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 9:194–205 [DOI] [PubMed] [Google Scholar]
- Merck Clinical Trials.gov Identifier: NCT00395343 Sitagliptin added-on to insulin study. http://www.clinicaltrials.gov/ct2/show/NCT00395343?term=NCT00395343&rank=1 [Google Scholar]
- Stein PP, Williams-Herman D, Khatami H, Meininger G, Round E, Sheng D, Sanchez M, Lunceford J, Kaufman KD, Amatruda JM 2007 Sitagliptin, a selective DPP-4 inhibitor, is well tolerated in patients with type 2 diabetes: pooled analysis of 5141 patients in clinical trials for up to 2 years. Diabetes 56:A142 [Google Scholar]
- Villhauer EB, Brinkman JA, Naderi GB, Burkey BF, Dunning BE, Prasad K, Mangold BL, Russell ME, Hughes TE 2003 1-[[(3-hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J Med Chem 46:2774–2789 [DOI] [PubMed] [Google Scholar]
- Barlocco D 2004 LAF-237 (Novartis). Curr Opin Investig Drugs 5:1094–1100 [PubMed] [Google Scholar]
- He YL, Serra D, Wang Y, Campestrini J, Riviere GJ, Deacon CF, Holst JJ, Schwartz S, Nielsen JC, Ligueros-Saylan M 2007 Pharmacokinetics and pharmacodynamics of vildagliptin in patients with type 2 diabetes mellitus. Clin Pharmacokinet 46:577–588 [DOI] [PubMed] [Google Scholar]
- He YL, Sabo R, Campestrini J, Wang Y, Riviere GJ, Nielsen JC, Rosenberg M, Ligueros-Saylan M, Howard D, Dole WP 2008 The effect of age, gender, and body mass index on the pharmacokinetics and pharmacodynamics of vildagliptin in healthy volunteers. Br J Clin Pharmacol 65:338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YL, Sadler BM, Sabo R, Balez S, Wang Y, Campestrini J, Laurent A, Ligueros-Saylan M, Howard D 2007 The absolute oral bioavailability and population-based pharmacokinetic modelling of a novel dipeptidylpeptidase-IV inhibitor, vildagliptin, in healthy volunteers. Clin Pharmacokinet 46:787–802 [DOI] [PubMed] [Google Scholar]
- Sunkara G, Sabo R, Wang Y, He YL, Campestrini J, Rosenberg M, Howard D, Dole WP 2007 Dose proportionality and the effect of food on vildagliptin, a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. J Clin Pharmacol 47:1152–1158 [DOI] [PubMed] [Google Scholar]
- He YL, Sabo R, Campestrini J, Wang Y, Ligueros-Saylan M, Lasseter KC, Dilzer SC, Howard D, Dole WP 2007 The influence of hepatic impairment on the pharmacokinetics of the dipeptidyl peptidase IV (DPP-4) inhibitor vildagliptin. Eur J Clin Pharmacol 63:677–686 [DOI] [PubMed] [Google Scholar]
- 2007 Norvatis. Galvus-FDA Action Investor Call [Google Scholar]
- Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Jauffret S, Foley JE 1 February 2008 Efficacy and tolerability of vildagliptin in drug-naive patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab 10:675–682 [DOI] [PubMed] [Google Scholar]
- Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Wang Y, Dunning BE, Foley JE 18 March 2008 Evidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab, in press [DOI] [PubMed] [Google Scholar]
- Dejager S, Razac S, Foley JE, Schweizer A 2007 Vildagliptin in drug-naive patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 39:218–223 [DOI] [PubMed] [Google Scholar]
- Schweizer A, Couturier A, Foley JE, Dejager S 2007 Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naive patients with type 2 diabetes. Diabet Med 24:955–961 [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A 2007 Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Care 30:217–223 [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Baron MA, Camisasca RP, Cressier F, Couturier A, Dejager S 2007 Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab 9:175–185 [DOI] [PubMed] [Google Scholar]
- Pan C, Yang W, Barona JP, Wang Y, Niggli M, Mohideen P, Wang Y, Foley JE 2008 Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med 25:435–441 [DOI] [PubMed] [Google Scholar]
- Garber AJ, Foley JE, Banerji MA, Ebeling P, Gudbjornsdottir S, Camisasca RP, Couturier A, Baron MA 18 February 2008 Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab, in press [DOI] [PubMed] [Google Scholar]
- Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ 2007 Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 30:890–895 [DOI] [PubMed] [Google Scholar]
- Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S 2007 Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 9:166–174 [DOI] [PubMed] [Google Scholar]
- Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S 2007 Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 50:1148–1155 [DOI] [PubMed] [Google Scholar]
- Bolli G, Dotta F, Rochotte E, Cohen SE 2008 Efficacy and tolerability of vildagliptin vs. pioglitazone when added to metformin: a 24-week, randomized, double-blind study. Diabetes Obes Metab 10:82–90 [DOI] [PubMed] [Google Scholar]
- Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, Feng Y, Zhu L, Li C, Howard AD, Moller DE, Thornberry NA, Zhang BB 2006 Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic β-cell mass and function in a rodent model of type 2 diabetes. Diabetes 55:1695–1704 [DOI] [PubMed] [Google Scholar]
- Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, Liu F, Woods J, Zycband E, Moller DE, Thornberry NA, Zhang BB 2003 Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA 100:6825–6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock G, Baggio LL, Longuet C, Drucker DJ 2007 Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes 56:3006–3013 [DOI] [PubMed] [Google Scholar]
- Bergman AJ, Stevens C, Zhou Y, Yi B, Laethem M, De Smet M, Snyder K, Hilliard D, Tanaka W, Zeng W, Tanen M, Wang AQ, Chen L, Winchell G, Davies MJ, Ramael S, Wagner JA, Herman GA 2006 Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther 28:55–72 [DOI] [PubMed] [Google Scholar]
- Migoya EM, Miller J, Larson P, Tanen M, Hilliard D, Deacon C, Gutierrez M, Stoch A, Herman GA, Stein PP, Holst JJ, Wagner JA 2007 Sitagliptin, a selective DPP-4 inhibitor, and metformin have complementary effects to increase active GLP-1 concentrations. Diabetes 56:A74 [Google Scholar]
- Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan ML, Dunning BE, Foley JE, Rizza RA, Camilleri M 2007 Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes 56:1475–1480 [DOI] [PubMed] [Google Scholar]
- Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A 2004 Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 89:2078–2084 [DOI] [PubMed] [Google Scholar]
- Holst JJ, Deacon CF 2005 Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 48:612–615 [DOI] [PubMed] [Google Scholar]
- Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, Andres R, Saudek C, Edelstein SL, Arakaki R, Murphy MB, Shamoon H 2005 Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Leiter LA, Mihic M, Nathan DM, Palmason C, Cohen RM, Wolever TM 1996 The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care 19:1190–1193 [DOI] [PubMed] [Google Scholar]
- Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR 2001 Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 281:E155–E161 [DOI] [PubMed] [Google Scholar]
- Nauck MA, El-Ouaghlidi A 2005 The therapeutic actions of DPP-IV inhibition are not mediated by glucagon-like peptide-1. Diabetologia 48:608–611 [DOI] [PubMed] [Google Scholar]
- Mari A, Sallas WM, He YL, Watson C, Ligueros-Saylan M, Dunning BE, Deacon CF, Holst JJ, Foley JE 2005 Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed β-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab 90:4888–4894 [DOI] [PubMed] [Google Scholar]
- De Meester I, Durinx C, Bal G, Proost P, Struyf S, Goossens F, Augustyns K, Scharpe S 2000 Natural substrates of dipeptidyl peptidase IV. Adv Exp Med Biol 477:67–87 [DOI] [PubMed] [Google Scholar]
- Drucker DJ 2007 Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 30:1335–1343 [DOI] [PubMed] [Google Scholar]
- Brazg R, Xu L, Dalla Man C, Cobelli C, Thomas K, Stein PP 2007 Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and β-cell function in patients with type 2 diabetes. Diabetes Obes Metab 9:186–193 [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K 2004 Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci 110:36–43 [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA 2007 Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148:4965–7493 [DOI] [PubMed] [Google Scholar]
- Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A 1996 Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol 271(5 Pt 1):E808–E813 [DOI] [PubMed] [Google Scholar]
- Burcelin R, Da Costa A, Drucker D, Thorens B 2001 Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 50:1720–1728 [DOI] [PubMed] [Google Scholar]
- Ionut V, Hucking K, Liberty IF, Bergman RN 2005 Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia 48:967–975 [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Moore MC, Shiota M, Gustavson SM, Snead WL, Neal DW, Cherrington AD 2003 Effect of intraportal glucagon-like peptide-1 on glucose metabolism in conscious dogs. Am J Physiol Endocrinol Metab 284:E1027–E1036 [DOI] [PubMed] [Google Scholar]
- Czyzyk A, Heding LG, Malczewski B, Miedzinska E 1975 The effect of phenformin upon the plasma pancreatic and gut glucagon-like immunoreactivity in diabetics. Diabetologia 11:129–133 [DOI] [PubMed] [Google Scholar]
- Molloy AM, Ardill J, Tomkin GH 1980 The effect of metformin treatment on gastric acid secretion and gastrointestinal hormone levels in normal subjects. Diabetologia 19:93–96 [DOI] [PubMed] [Google Scholar]
- Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Messeri G, Rotella CM 2001 Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care 24:489–494 [DOI] [PubMed] [Google Scholar]
- Mannucci E, Tesi F, Bardini G, Ognibene A, Petracca MG, Ciani S, Pezzatini A, Brogi M, Dicembrini I, Cremasco F, Messeri G, Rotella CM 2004 Effects of metformin on glucagon-like peptide-1 levels in obese patients with and without type 2 diabetes. Diabetes Nutr Metab 17:336–342 [PubMed] [Google Scholar]
- Lindsay JR, Duffy NA, McKillop AM, Ardill J, O'Harte FP, Flatt PR, Bell PM 2005 Inhibition of dipeptidyl peptidase IV activity by oral metformin in type 2 diabetes. Diabet Med 22:654–657 [DOI] [PubMed] [Google Scholar]
- Green BD, Irwin N, Duffy NA, Gault VA, O'harte FP, Flatt PR 2006 Inhibition of dipeptidyl peptidase-IV activity by metformin enhances the antidiabetic effects of glucagon-like peptide-1. Eur J Pharmacol 547:192–199 [DOI] [PubMed] [Google Scholar]
- Caspary WF, Creutzfeldt W 1971 Analysis of the inhibitory effect of biguanides on glucose absorption: inhibition of active sugar transport. Diabetologia 7:379–385 [DOI] [PubMed] [Google Scholar]
- Czyzyk A, Tawecki J, Sadowski J, Ponikowska I, Szczepanik Z 1968 Effect of biguanides on intestinal absorption of glucose. Diabetes 17:492–498 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Barzilai N, Simonson DC 1991 Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab 73:1294–1301 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC 2005 The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310:1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, Taylor K 2007 Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 30:1487–1493 [DOI] [PubMed] [Google Scholar]