Abstract
Background: Prolactin (PRL) is a multifunctional hormone produced in humans by both pituitary and extrapituitary sites, including adipose tissue.
Objectives: Our objectives were to: 1) compare PRL secretion by sc and visceral adipose explants and mature adipocytes from obese and nonobese patients; and 2) examine the effects of insulin and selected cytokines on PRL gene expression and release from primary adipocytes and LS14 adipocytes.
Design and Subjects: Adipose tissue was obtained from morbidly obese [body mass index (BMI) > 40 kg/m2] and nonobese (BMI <30 kg/m2) patients. Explants and isolated mature adipocytes were incubated for 10 d. Primary adipocytes or LS14 cells were used before or after differentiation and incubated with the test compounds for 24 h. PRL release was analyzed by a bioassay, and PRL expression was determined by real-time PCR.
Results: PRL release from explants and mature adipocytes increased in a time-dependent manner indicating removal from inhibition. Visceral explants from obese patients showed higher PRL release than that from sc explants; both types of explants from nonobese patients released similar amounts of PRL. Analysis of data from 50 patients revealed an inverse relationship between PRL release from sc depots and BMI. Insulin suppressed PRL expression and release from differentiated adipocytes but moderately stimulated PRL release from nondifferentiated cells. The cAMP elevating compound forskolin increased PRL release in both cell types.
Conclusions: PRL should be recognized as an important adipokine whose release is regulated by insulin and is affected by obesity in a depot-specific manner.
The release of prolactin from subcutaneous and visceral adipose explants and mature adipocytes, the effects of obesity, and the regulation of prolactin secretion by insulin and selected cytokines is discussed.
Prolactin (PRL) is a 23-kDa protein hormone that binds to a single-span membrane receptor, a member of the cytokine receptor superfamily, and acts via several interacting signaling pathways (1). PRL is a versatile hormone that affects multiple reproductive, immune, and metabolic functions and is also involved in breast and prostate tumorigenesis (2,3). In addition to being a circulating hormone of pituitary origin, PRL in humans is produced by nonpituitary sites, including the endometrium, decidua, lymphocytes, brain, breast, and prostate, where it acts as a cytokine (4). Although both pituitary and nonpituitary sites produce an identical PRL protein, the regulation of its production/release is dissimilar. Whereas pituitary PRL is driven by a proximal promoter that requires Pit-1 transcription factor for activation, nonpituitary PRL is transcribed from an alternative, superdistal promoter that is independent of Pit-1, does not respond to estrogen or neuropeptides, and is regulated in a cell-specific manner (5).
Compared with the well-established metabolic activities of GH, the role of PRL, its sister molecule, in adipose tissue functions has received less attention. Yet, both the long and short forms of the PRL receptor are expressed in rodent and human adipose tissue (6,7,8,9,10), and their expression increases during differentiation of 3T3-L1 cells (11) and rat epididymal preadipocytes (10). Accumulating evidence indicates that PRL participates in many aspects of adipose tissue biology, including adipogenesis (12,13), metabolic enzyme activity (7,14,15), lipolysis (10,16), and release of adipokines such as leptin, adiponectin, and IL-6 (10,16,17,18).
We previously reported that human breast adipose tissue produces and releases PRL (19). Subsequent studies revealed de novo synthesis of PRL by breast preadipocytes and stimulation of PRL expression and release by cAMP-activating compounds (20). We also generated a novel human adipocyte cell line, named LS14, which produces and responds to PRL (8). These findings raise the question whether PRL is synthesized by fat depots other than the breast and, if so, whether its release is affected by obesity. Given that PRL expression at nonpituitary sites is under tissue-specific regulation, we also ask whether regulators of metabolic and endocrine activities of adipocytes such as insulin and cytokines also affect PRL secretion in human adipocytes.
The objectives were to: 1) compare time-related secretion of PRL by sc and visceral (vis) adipose tissue explants from morbidly obese and nonobese patients; 2) determine whether insulin alters PRL gene expression and release in primary adipocytes and LS14 cells; and 3) compare the effects of insulin and selected cytokines on PRL release in proliferating vs. differentiated LS14 cells.
Subjects and Methods
Subjects
Informed consent, approved by the Institutional Review Boards of the University of Cincinnati and The Christ Hospital, Cincinnati, was used to obtain surgical samples. For the study on PRL release from adipose explants, matched vis (omental) and sc samples were obtained from morbidly obese patients undergoing gastric bypass surgery. Samples from nonobese patients were obtained during abdominal surgery, including cholecystectomy, colectomy, colitis, hysterectomy, and partial gastrectomy; patients with malignancies were excluded. Subjects were divided into the following groups, with the number of patients, age (years; mean ± sd), and body mass index (BMI) (kilograms/meter2; mean ± sd) listed in parentheses, respectively: group 1, obese women (22, 44 ± 18.8, 48 ± 9.4); group 2, obese men (13, 42 ± 14.4, 50 ± 3.6); and group 3, nonobese men and women (15, 54 ± 19.4, 26 ± 3.9). Data from nonobese men (n = 10) and women (n = 5) were combined because they did not differ statistically. For studies using isolated adipocytes, sc adipose tissue was obtained from nonobese patients (BMI <30 kg/m2) undergoing abdominoplasty.
Explant preparation and incubation
Adipose tissue was placed in DMEM/F12 medium (CellGro, Manassas, VA), and visible connective tissue and blood vessels were removed. Explants (≈2 mm3) were placed into 48-well plates (40–60 mg/well, four wells per depot) containing DMEM/F12 supplemented with 1% ITS (insulin, transferrin, selenium, and linoleic acid; Becton Dickinson, San Jose, CA) and incubated at 37 C and 5% CO2 for 10 d. Conditioned media (CM) were collected and completely replaced on d 1, 3, 7, and 10, and aliquots were analyzed for PRL by the Nb2 bioassay. Weight of explants was determined at the end of the experiment. The PRL release rate is expressed as picograms per 100 mg per day.
Cell harvesting
Subcutaneous adipose tissue from nonobese patients undergoing abdominoplasty was used to prepare mature adipocytes and preadipocytes as described (8,20). Briefly, tissue fragments were placed into Hank’s balanced salt solution containing 2% fatty acid-free BSA, 200 nm adenosine, and 200 U/g type IV collagenase (Worthington, Lakewood, NJ). Tissue was digested at 37 C for 40–60 min, and the digest was filtered through a 150-μm mesh. Mature adipocytes were separated from the stromal vascular fraction by centrifugation. The mature adipocytes (100 μl of packed cells, at an average weight of 80 mg) were placed into wide-mouth polypropylene tubes and incubated under minimal disturbances for 10 d in the above explant media supplemented with 200 nm adenosine to reduce cell lysis. CM were collected from quadruplicate samples on the designated days. The release rate at each time point was calculated by dividing the total accumulated PRL by the number of days in culture.
The stromal vascular fraction containing the preadipocytes was centrifuged at 800 × g for 10 min, and the cells were resuspended in erythrocyte lysis buffer (154 mm ammonium chloride, 10 mm potassium carbonate, and 0.1 mm EDTA). After additional centrifugation, cells were plated into culture flasks and incubated in DMEM/F12 containing 10% fetal bovine serum and 50 μg/ml Primocin (Invivogen, San Diego, CA). After two or three passages, cells were stored frozen until used for differentiation.
Cell culture and treatments
LS14 cells were maintained as described (8). Briefly, cells were cultured in DMEM/F12 containing 5% fetal bovine serum (Cell Gro), 5% FetalClone III (Hyclone, Logan, UT), 15 μg/ml bovine pituitary extract (Invitrogen, Carlsbad, CA), 1% ITS+, 0.5 ng/ml basic fibroblast growth factor, 1 ng/ml epidermal growth factor, and 0.1 ng/ml TGFβ1 (Peprotech, Rocky Hill, NJ), and 50 μg/ml normocin (Invivogen). For studying PRL release, LS14 cells were used either before or after differentiation. Nondifferentiated LS14 cells were plated in the above media at 50,000 cells/well in 24-well plates. After 24 h, cells were rinsed and maintained for 24 h in DMEM/F12 containing 1% charcoal stripped serum (CSS). Cells were then incubated in the same media with IL-6, TNFα, TGFβ (Peprotech), insulin, IGF-I (Sigma, St. Louis, MO), or forskolin (LC Laboratories, Woburn, MA). After 24 h, CM were collected for PRL analysis by the bioassay.
Cell differentiation
LS14 cells or primary sc preadipocytes (pools of frozen stocks from four or five patients) were plated in collagen-coated wells at 100,000 cells/well in 24-well plates. Cells were incubated in serum-free basal adipogenesis medium consisting of DMEM/F12 (1:1) supplemented with 33 μm biotin, 17 μm pantothenic acid, 1 μm human insulin, 10 μg/ml apotransferrin, and 1 nm T3 (all from Sigma), 2 μm rosiglitazone (Kemprotec, Middlesbrough, UK), 200 μm ascorbate phosphate, 4 μm oleic acid/BSA, 4 μm linoleic acid/BSA (USB, Cleveland, OH), 1 μm methoprene acid (RXR ligand; MPBio, Santa Ana, CA), and 1 μm T0901317 (LXR ligand; BioMol, Plymouth Meeting, PA). After 24 h, adipogenesis was induced by adding 250 μm isobutylmethyl-xanthine (BioMol). After 72 h, cells were incubated in basal adipogenesis medium without isobutylmethyl-xanthine, and media were replaced every fourth day. The progress of differentiation was monitored by Oil-red O staining (20). On d 10 of differentiation, approximately 70–80% of the cells had lipid accumulation. Differentiated cells were rinsed and incubated for 24 h in DMEM/F12 containing 1% CSS. After additional rinsing, cells were treated for 24 h with the various compounds under the same conditions as for nondifferentiated cells.
Nb2 bioassay for PRL
PRL concentrations in CM were determined by the rat Nb2 lymphoma bioassay (8,20). Briefly, serum-starved Nb2 cells were plated in 96-well plates (25,000 cells/well) and incubated with human PRL (hPRL) [NIDDK (National Institute of Diabetes & Digestive & Kidney Diseases)] in triplicate or CM aliquots in duplicate for 3 d. Cell proliferation was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. PRL concentration was calculated from a standard curve, with a lowest detectable level of 2 pg/well. To confirm assay specificity, Nb2 cells were incubated with hPRL, human GH (hGH), or CM aliquots alone or together with purified anti-hPRL antibodies, anti-hGH antibodies (NIDDK) or rabbit IgG. Nb2 cell growth was not affected by insulin or the tested cytokines (data not shown).
Resazurin viability assay
To measure the viability of mature adipocytes over the 10-d incubation period, resazurin (Sigma) was added to the cells at a final concentration of 50 μg/ml. After 2 h, media aliquots were transferred to black 96-well plates, and fluorescence was determined at 530 nm excitation 590 nm emission in a Gemini XLS microplate fluorometer (Molecular Devices, Sunnyvale, CA). Separate sets of adipocyte cultures were used for each time point.
Real-time PCR
Total RNA, isolated with Tri-reagent (MRC, Cincinnati, OH), was used to synthesize oligo dT primed cDNA (8). Real-time PCR was performed on 200 ng of cDNA using the following intron-spanning primers: 1) hPRL- TTCAGCGAATTCGATAAACGG (forward), and TGATACAGAGGCTCATTCCAG (reverse), with an expected product size of 181; and 2) β microglobulin (B2M)-TGCTCGCGC-TACTCTCTCTTT (forward), and TGTCGG-ATGGATGAAACCCAGA (reverse), with an expected product size of 114. Quantitative RT-PCR was performed in a SmartCycler I (Cepheid, Sunnyvale, CA) using Immolase heat-activated Taq DNA polymerase (Bioline, Randolph, MA) and SYBR Green I (Invitrogen). Cycle parameters were: 96 C for 6 min, followed by 40 cycles at 95 C for 15 sec, 57 C for 15 sec, and 72 C for 25 sec. Product purity was confirmed by melting curve analysis. Changes in gene expression were calculated from the cycle threshold, after correcting for cDNA amounts using B2M expression, according to Pfaffl et al. (21). Data are expressed as percentage of control.
Data analysis
When appropriate, values are expressed as the mean ± sem. Statistical analysis was done using either Student’s t test or one-way ANOVA followed by Fisher least significant difference post hoc analysis. P values < 0.05 were considered significant. Experiments were repeated three times.
Results
Validation of the bioassay specificity
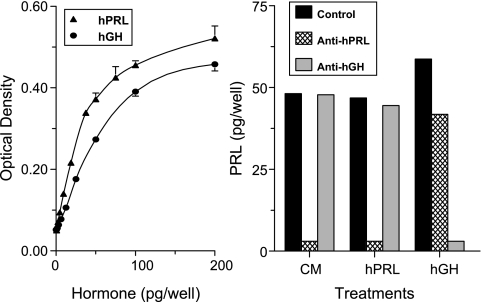
Rat Nb2 lymphocytes proliferate robustly in response to PRL and have served as a widely used bioassay for PRL, which is 40–50 times more sensitive than RIA (22). As expected, both hPRL and hGH induced dose-dependent stimulation of Nb2 cell proliferation (Fig. 1, left panel). To confirm that the mitogenic activity in CM is due to PRL only, CM aliquots from adipose explants were incubated with Nb2 cells in the absence and presence of antibodies against hPRL or hGH. Figure 1, right panel, shows that the mitogenic activity of hPRL and hGH is abolished by the corresponding antibodies, verifying their specificity for immunoneutralization. Only the anti-PRL antibodies blocked the mitogenic activity present in CM, confirming that it was entirely due to PRL.
Figure 1.
Verification of the Nb2 bioassay specificity. Left, Nb2 cell proliferation in response to hPRL and hGH. Rat Nb2 lymphocytes were incubated with increasing amounts of hPRL or hGH. After 3 d, cell proliferation was measured by the MTT method. Right, Confirmation of PRL identity in CM as determined by immunoneutralization. Aliquots of CM from adipose explants, hPRL (50 pg/well), and hGH (50 pg/well) were incubated with Nb2 cells in the presence of rabbit IgG (control, 5 μg/well), anti-hPRL IgG (5 μg/well), or anti-hGH antiserum (1:200).
Differential release of PRL from sc and vis adipose explants
A total of 22 obese women (BMI, 48 ± 9.4 kg/m2), 13 obese men (BMI, 50 ± 3.6 kg/m2), and 15 nonobese men and women (BMI, 26 ± 3.9 kg/m2) provided samples for this in vitro study. As illustrated in Fig. 2, left panels, PRL release rate from all types of explants showed a time-dependent rise, reaching peak levels on d 7 and declining thereafter. Samples from both obese women and men had significantly (P < 0.05) higher PRL release from vis than sc explants on all days except d 1. The most striking difference between the groups was a significant (P < 0.05) attenuation of PRL release from sc depots from morbidly obese patients, compared with nonobese patients. In addition, total PRL release (over the 10-d incubation period) from vis explants from obese men was significantly (P < 0.05) higher than that from obese women.
Figure 2.
Differential PRL release from vis and sc adipose explants and the effect of obesity. Three left panels, CM were collected from quadruplicate vis and sc explant cultures on the designated days and analyzed for PRL by the Nb2 bioassay. Values shown are means ± sem. *, P < 0.05 between paired sc and vis samples. Right panel, Time-dependent increase in PRL release from mature sc adipocytes from a nonobese patient. Metabolic activity was determined by the Resazurin fluorometric assay. RFU, relative fluorescent units. #, Significant difference (P < 0.05) compared with d 1. Each value is a mean ± sem of four replicates. This is a representative experiment that was repeated with mature adipocytes harvested from three different patients and shows similar results. BMI is expressed in kilograms per meter2 (mean ± sem).
Time-dependent increase in PRL release from mature adipocytes
We next examined the profile of PRL release from mature adipocytes. After finding that vis mature adipocytes from obese individuals do not withstand long-term incubation, probably due to their larger size and increased fragility (23), we used mature adipocytes isolated from sc abdominal depots of nonobese patients. As evident in Fig. 2, right panel, the pattern of PRL release from mature adipocytes resembles that seen in the corresponding explants. To examine viability of mature adipocytes throughout the incubation period, we used resazurin (Alamar Blue), a nonfluorescent dye that is converted by diaphorase to the fluorescent resorfin (24). The results show that the viability/metabolic activity of mature adipocytes increased from d 1 to 7, followed by a small decline thereafter (Fig. 2).
Obesity is associated with a lower in vitro PRL release from sc but not vis depots
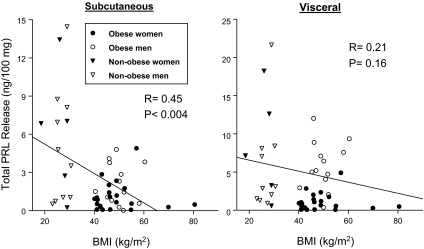
To determine whether the state of adiposity has an impact on PRL release from the two adipose depots, total PRL release over the 10-d incubation period from each patient was plotted against his/her BMI (Fig. 3). Correlation analysis revealed a highly significant (P < 0.004) inverse relationship between BMI and PRL release from sc explants but not from vis explants.
Figure 3.
Relationship between total PRL secreted from sc and vis explants and BMI. Results from 50 patients (female, male, obese, and nonobese) are shown. As determined by least squares regression analysis, an inverse correlation between PRL and BMI is highly significant (P < 0.004) for sc explants (left) but not for vis explants (right).
Inhibition of PRL gene expression and release by insulin
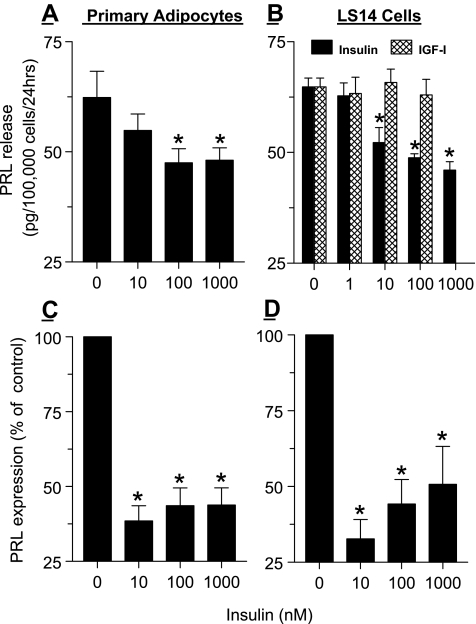
We next examined whether PRL gene expression and release from adipocytes is affected by insulin. Differentiated sc adipocytes from nonobese patients as well as differentiated LS14 cells were incubated with 10–1000 nm insulin for 24 h, followed by analysis of PRL release by the Nb2 bioassay and PRL mRNA levels by real-time PCR. As shown in Fig. 4, A and C, insulin suppressed PRL release by ≈30% and PRL gene expression by more than 60% in primary adipocytes. Figure 4, B and D, shows a similar inhibitory effect of insulin on PRL release and gene expression in LS14 cells, confirming comparable responsiveness to insulin by the two cell types. Because insulin at high concentrations binds to the IGF receptor, we also tested the effects of increasing doses of IGF-I. As evident in Fig. 4B, IGF-I did not alter PRL release from LS14 cells.
Figure 4.
Inhibition of PRL gene expression and release by insulin from differentiated primary sc adipocytes (A and C) and LS14 cells (B and D). Primary preadipocytes (pooled from several patients) and LS14 cells were induced to differentiated as detailed in Subjects and Methods. On d 10–12 of differentiation, cells were first incubated for 24 h in DMEM/F10 medium containing 1% CSS, and then incubated for an additional 24 h with various doses of insulin or IGF-I. CM were analyzed for PRL by the Nb2 bioassay (A and B), and isolated RNA was analyzed for PRL expression by real-time PCR (C and D). Each value is the mean ± sem of four to six replicates. *, P < 0.05 change with respect to controls. Shown are representative experiments, each repeated three times.
Differential responsiveness of proliferating and differentiated LS14 cells to insulin and cytokines
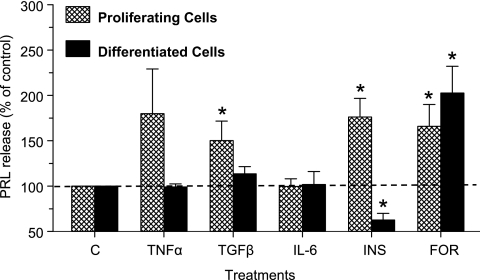
We then questioned whether PRL release from LS14 cells is regulated by cytokines that are produced by adipose tissue and/or involved in its regulation. We also compared the responses of proliferating vs. differentiated LS14 cells to the test compounds. Because basal PRL release was similar in both types of cells (∼140 pg/100,000 cells/24 h), results are expressed as percentage of control for a clearer comparison. Figure 5 shows that TGFβ and insulin had a stimulatory effect and an inhibitory effect, respectively, on PRL release from differentiated cells, whereas TNFα and IL-6 were ineffective. TGFβ also increased PRL release from the proliferating cells, whereas the increase in PRL release by TNFα did not reach statistical significance. In contrast to its suppressive effect on PRL release from differentiated cells, insulin caused a significant (P < 0.05) stimulation of PRL release from proliferating cells. Forskolin, a potent activator of adenylate cyclase, stimulated PRL release from both proliferating and differentiated LS14 cells (Fig. 5).
Figure 5.
Differential responsiveness of proliferating and differentiated LS14 cells to compounds that affect PRL release. Cells were first incubated in DMEM/F12 containing 1% CSS for 24 h and then with TNFα (1 ng/ml), TGFβ (10 ng/ml), IL-6 (10 ng/ml), insulin (INS; 100 nm) or forskolin (FOR; 10 μm) for an additional 24 h. CM were analyzed for PRL by the Nb2 bioassay. Each value is a mean ± sem of three independent experiments. *, P < 0.05 change with respect to controls.
Discussion
We are reporting that PRL is released by human vis and sc adipose explants, mature adipocytes, differentiated primary sc preadipocytes, and LS14 cells. PRL release in vitro shows a time-dependent increase, suggesting removal from inhibitory controls. These studies also establish depot-specific profile of PRL release in vitro, with obesity associated with a lower capacity of sc fat to release PRL. Insulin suppressed PRL gene expression and release from differentiated adipocytes but moderately stimulated PRL release from nondifferentiated cells. TGFβ stimulated PRL release from nondifferentiated cells, whereas the cAMP-elevating compound forskolin caused an increase in PRL release in both cell types.
In addition to terminally differentiated mature adipocytes, adipose tissue contains preadipocytes, fibroblasts, stem cells, endothelial cells, and lymphocytes/macrophages. Some adipokines, e.g., leptin and adiponectin, originate almost exclusively from adipocytes, whereas others, e.g., TNFα and IL-6, are primarily produced by the stromal-vascular fraction (25). Our data reveal a similar profile of PRL release from explants and isolated mature adipocytes (Fig. 2), suggesting that adipocytes, which constitute the largest cellular fraction in adipose tissue, serve as a major source of adipose PRL. However, we cannot rule out the possibility that other cells within adipose tissue, e.g., preadipocytes (20), endothelial cells (26), fibroblasts (27), and lymphocytes (28), contribute to the overall PRL output by adipose tissue and may also produce factors that affect PRL release by the adipocytes.
As reported by Fain et al. (25), the release of adiponectin, leptin, and TNFα from human adipose explants was dramatically reduced over time in culture, whereas that of plasminogen activator inhibitor-1, prostaglandin E2, and IL-8 increased. We found a significant rise in PRL release from both explants and mature adipocytes throughout the first 7 d in culture. As confirmed by using resazurin, this rise in PRL release is not due to loss of cell viability.
Notably, a time-dependent increase in PRL release in culture is seen in incubated lactotrophs (5), decidual (29) and myometrial (30) cells, dermal fibroblasts (27), and breast adipose explants (19). Thus, PRL production in all these sites appears to be under tonic inhibition by an inhibiting factor(s) that is either absent or becomes inactivated in culture. Although dopamine is well established as the physiological inhibitor of pituitary PRL production (5), the identity of the inhibitor(s) in nonpituitary PRL-producing cells and whether it is a common factor or site-specific await determination.
The lower PRL release from sc explants of morbidly obese individuals suggests depot-specific control of PRL production during obesity. Unexpectedly, total PRL release was higher in explants from obese men than obese women, in contrast to its circulating levels, which are generally higher in women than men.
The factors that determine the different capacities of the two fat depots to release PRL, how they are influenced by obesity, and the physiological significance of this difference are unknown. We found that PRL release from differentiated adipocytes is inhibited by insulin (Fig. 4) and is stimulated by cAMP-elevating compounds such as forskolin and isoproterenol, a potent β-adrenergic agonist (20). Insulin and catecholamines, which are important regulators of glucose uptake, lipolysis, and adipokine release, undergo acute fluctuations in their serum levels in response to food intake and stress. It remains to be determined whether adipose PRL in vivo responds to acute changes in the circulating levels of these hormones or is maintained at relatively stable levels.
According to our calculation, PRL production by each individual adipocyte is four to five orders of magnitude lower than that from a pituitary lactotroph. Yet, when considering the size of the two organs (1 g for the pituitary vs. >50 kg for adipose tissue in morbidly obese individuals), total PRL production by adipose tissue could approach that of total pituitary output. This raises the relevant question whether adipose-derived PRL contributes to circulating PRL levels. However, upon measuring serum PRL in 16 patients before surgery, we found no clear correlation between BMI and circulating PRL levels (Hugo, E. R., and N. Ben-Jonathan, unpublished observations).
We previously reported that hPRL, but not hGH or other pituitary hormones, binds to heparin (31). Such binding protects growth factors and adipokines from degradation, increases their local concentration, and augments their binding to cognate membrane receptors (32). Unlike the tightly packed pituitary epithelial cells, adipocytes are embedded in loose connective tissue that is highly enriched in heparan sulfate proteoglycans (33). Hence, we speculate that when released in large amounts by lactotrophs, PRL is deposited directly into the circulation. On the other hand, when produced at much lower levels by adipocytes, most of the locally produced PRL is retained near the producing cells, making adipose PRL a true autocrine/paracrine factor. A recent study reported moderately higher serum PRL levels in premenopausal women with vis obesity (BMI, 33 kg/m2) than in lean (BMI, 21 kg/m2) controls (34). However, the authors attributed this difference to obesity-related alterations in the neuroendocrine system. Still, it is possible that adipose tissue is the source of some excess circulating PRL levels in extreme obesity, and this should be examined by comparing serum PRL levels in morbidly obese vs. nonobese patients, especially men.
The effect of insulin on PRL release varies with the producing site. Whereas insulin has no consistent effect on pituitary PRL (4), it stimulates PRL release from the decidua (35), inhibits PRL in differentiated adipocytes, and causes moderate stimulation in nondifferentiated cells (Figs. 4 and 5). Because preadipocytes represent only a small fraction of the total cell population within adipose tissue, the overall effect of insulin on PRL is likely inhibitory. Future studies should examine which signaling pathway(s) mediate the effect of insulin on PRL expression/release in adipocytes and what is the molecular mechanism underlying the switch from stimulation to inhibition that occurs during adipocyte differentiation.
Finally, this study focused on the regulation of adipocyte PRL production rather than on its local functions. Unraveling the spectrum of PRL functions in adipose tissue is complicated by the fact that human adipocytes are exposed to PRL from two sources, pituitary and local, which are differentially regulated. On the other hand, the exclusive source of PRL in rodents is the pituitary gland, with very low PRL production in few extrapituitary sites, and none in adipose tissue (36). This imposes certain limitations on the use of live rodents or murine adipocyte cell lines for understanding PRL homeostasis in human adipose tissue and adipocytes. Our previous report (8) and the present data demonstrate that LS14 cells share many characteristics with primary human adipocytes. Given their homogeneity and limitless supply, LS14 cells should serve as an excellent in vitro model for further investigations on potential reciprocal interactions between PRL, adipokines, and metabolic hormones.
Footnotes
These studies were supported by National Institutes of Health Grants ES012212 and CA096613; Department of Defense Grant BC05725; Susan G. Komen Breast Cancer Foundation Grant BCRT87406 (to N.B.-J.); and National Research Service Award fellowship F31 DK761852 (to D.C.B.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 22, 2008
Abbreviations: BMI, Body mass index; CM, conditioned media; CSS, charcoal stripped serum; hGH, human GH; hPRL, human PRL; PRL, prolactin; vis, visceral.
References
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA 1998 Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268 [DOI] [PubMed] [Google Scholar]
- Goffin V, Binart N, Touraine P, Kelly PA 2002 Prolactin: the new biology of an old hormone. Annu Rev Physiol 64:47–67 [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Furth PA, Hankinson SE, Schuler LA 2003 The role of prolactin in mammary carcinoma. Endocr Rev 24:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW 1996 Extrapituitary prolactin: distribution, regulation, functions and clinical aspects. Endocr Rev 17:639–669 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hnasko RM 2001 Dopamine as a prolactin inhibitor. Endocr Rev 22:724–763 [DOI] [PubMed] [Google Scholar]
- Ling C, Hellgren G, Gebre-Medhin M, Dillner K, Wennbo H, Carlsson B, Billig H 2000 Prolactin (PRL) receptor gene expression in mouse adipose tissue: increases during lactation and in PRL-transgenic mice. Endocrinology 141:3564–3572 [DOI] [PubMed] [Google Scholar]
- Ling C, Svensson L, Oden B, Weijdegard B, Eden B, Eden S, Billig H 2003 Identification of functional prolactin (PRL) receptor gene expression: PRL inhibits lipoprotein lipase activity in human white adipose tissue. J Clin Endocrinol Metab 88:1804–1808 [DOI] [PubMed] [Google Scholar]
- Hugo ER, Brandebourg TD, Comstock CE, Gersin KS, Sussman JJ, Ben- Jonathan N 2006 LS14: a novel human adipocyte cell line that produces prolactin. Endocrinology 147:306–313 [DOI] [PubMed] [Google Scholar]
- Freemark M 2001 Ontogenesis of prolactin receptors in the human fetus: roles in fetal development. Biochem Soc Trans 29:38–41 [DOI] [PubMed] [Google Scholar]
- Brandebourg TD, Bown JL, Ben-Jonathan N 2007 Prolactin upregulates its receptors and inhibits lipolysis and leptin release in male rat adipose tissue. Biochem Biophys Res Commun 357:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor D, Arumugam R, Freemark M 2006 Growth hormone and prolactin receptors in adipogenesis: STAT-5 activation, suppressors of cytokine signaling, and regulation of insulin-like growth factor I. Horm Res 66:101–110 [DOI] [PubMed] [Google Scholar]
- Stewart WC, Baugh Jr JE, Floyd ZE, Stephens JM 2004 STAT 5 activators can replace the requirement of FBS in the adipogenesis of 3T3–L1 cells. Biochem Biophys Res Commun 324:355–359 [DOI] [PubMed] [Google Scholar]
- Nanbu-Wakao R, Fujitani Y, Masuho Y, Muramatu M, Wakao H 2000 Prolactin enhances CCAAT enhancer-binding protein-beta (C/EBP β) and peroxisome proliferator-activated receptor gamma (PPAR γ) messenger RNA expression and stimulates adipogenic conversion of NIH-3T3 cells. Mol Endocrinol 14:307–316 [DOI] [PubMed] [Google Scholar]
- White UA, Coulter AA, Miles TK, Stephens JM 2007 The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes 56:1623–1629 [DOI] [PubMed] [Google Scholar]
- Hogan JC, Stephens JM 2005 The regulation of fatty acid synthase by STAT5A. Diabetes 54:1968–1975 [DOI] [PubMed] [Google Scholar]
- Lapensee CR, Horseman ND, Tso P, Brandebourg TD, Hugo ER, Ben- Jonathan N 2006 The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology 147:4638–4645 [DOI] [PubMed] [Google Scholar]
- Ling C, Billig H 2001 PRL receptor-mediated effects in female mouse adipocytes: PRL induces suppressors of cytokine signaling expression and suppresses insulin-induced leptin production in adipocytes in vitro. Endocrinology 142:4880–4890 [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE 2003 Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 52:268–276 [DOI] [PubMed] [Google Scholar]
- Zinger M, McFarland M, Ben-Jonathan N 2003 Prolactin expression and secretion by human breast glandular and adipose tissue. J Clin Endocrinol Metab 88:689–696 [DOI] [PubMed] [Google Scholar]
- McFarland-Mancini M, Hugo E, Loftus J, Ben-Jonathan N 2006 Induction of prolactin expression and release in human preadipocytes by cAMP activating ligands. Biochem Biophys Res Commun 344:9–16 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L 2002 Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DM, Sensui N, Haisenleder DH, Gala RR 1982 Rat lymphoma cell bioassay for prolactin: observations on its use and comparison with radioimmunoassay. Life Sci 31:3063–3070 [DOI] [PubMed] [Google Scholar]
- van Harmelen V, Skurk T, Röhrig K, Lee YM, Halbleib M, Aprath-Husmann I, Hauner H 2003 Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord 27:889–895 [DOI] [PubMed] [Google Scholar]
- Perrot S, Dutertre-Catella H, Martin C, Rat P, Warnet JM 2003 Resazurin metabolism assay is a new sensitive alternative test in isolated pig cornea. Toxicol Sci 72:122–129 [DOI] [PubMed] [Google Scholar]
- Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW 2004 Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145:2273–2282 [DOI] [PubMed] [Google Scholar]
- Corbacho AM, Macotela Y, Nava G, Torner L, Duenas Z, Noris G, Morales MA, Martinez de la EG, Clapp C 2000 Human umbilical vein endothelial cells express multiple prolactin isoforms. J Endocrinol 166:53–62 [DOI] [PubMed] [Google Scholar]
- Richards RG, Hartman SM 1996 Human dermal fibroblast cells express prolactin in vitro. J Invest Dermatol 106:1250–1255 [DOI] [PubMed] [Google Scholar]
- Pellegrini I, Lebrun JJ, Ali S, Kelly PA 1992 Expression of prolactin and its receptor in human lymphoid cells. Mol Endocrinol 6:1023–1031 [DOI] [PubMed] [Google Scholar]
- Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S 1997 Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 6:301–307 [DOI] [PubMed] [Google Scholar]
- Stewart EA, Rein MS, Friedman AJ, Zuchowski L, Nowak RA 1994 Glycoprotein hormones and their common α-subunit stimulate prolactin production by explant cultures of human leiomyoma and myometrium. Am J Obstet Gynecol 170:677–683 [DOI] [PubMed] [Google Scholar]
- Khurana S, Kuns R, Ben-Jonathan N 1999 Heparin-binding property of human prolactin: a novel aspect of prolactin biology. Endocrinology 140:1026–1029 [DOI] [PubMed] [Google Scholar]
- Klagsbrun M 1990 The affinity of fibroblast growth factors (FGFs) for heparin; FGF-heparan sulfate interactions in cells and extracellular matrix. Endocrinology 2:857–863 [DOI] [PubMed] [Google Scholar]
- Kolset SO, Salmivirta M 1999 Cell surface heparan sulfate proteoglycans and lipoprotein metabolism. Cell Mol Life Sci 56:857–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok P, Roelfsema F, Frolich M, Meinders AE, Pijl H 2004 Prolactin release is enhanced in proportion to excess visceral fat in obese women. J Clin Endocrinol Metab 89:4445–4449 [DOI] [PubMed] [Google Scholar]
- Handwerger S, Markoff E, Richards R 1991 Regulation of the synthesis and release of decidual prolactin by placental and autocrine/paracrine factors. Placenta 12:121–130 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Lapensee CR, LaPensee EW 2008 What can we learn from rodents about prolactin in humans? Endocr Rev 29:1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]