Abstract
Context: Risk factors for low testosterone and symptomatic androgen deficiency (AD) may be modifiable.
Objective: Our objective was to examine demographic, anthropometric, and medical correlates of low testosterone and symptomatic AD.
Design: Data were used from the Boston Area Community Health Survey, an epidemiological study conducted from 2002–2005.
Setting: Data were obtained from a community-based random sample of racially and ethnically diverse men.
Patients or other Participants: Data were available for 1822 men.
Main Outcome Measures: Multivariate logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for associations of covariates with 1) low testosterone and 2) symptomatic AD. The operational definition of low testosterone was serum total testosterone less than 300 ng/dl and free testosterone less than 5 ng/dl; symptomatic AD was defined as the additional presence of symptoms: any of low libido, erectile dysfunction, or osteoporosis or two or more of sleep disturbance, depressed mood, lethargy, or diminished physical performance.
Results: Factors associated with low testosterone included age (OR = 1.36; 95% CI= 1.11–1.66, per decade), low per-capita income ($6000 or less per household member vs. more than $30,000; OR = 2.86; 95% CI = 1.39–5.87), and waist circumference (per 10-cm increase; OR = 1.75; 95% CI = 1.45–2.12). Only age (OR = 1.36; 95% CI = 1.04–1.77), waist circumference (OR = 1.88; 95% CI = 1.44–2.47), and health status (OR = 0.21; 95% CI = 0.05–0.92, excellent vs. fair/poor) were associated with our construct of symptomatic AD. Of all variables, waist circumference was the most important contributor in both models.
Conclusions: Waist circumference is a potentially modifiable risk factor for low testosterone and symptomatic AD. Manifestation of symptoms may be a consequence of generally poor health status.
The most important correlate of low testosterone and symptomatic low testosterone is waist circumference, not age.
As the U.S. population increases in age and if the current secular trend of increasing obesity continues (1), androgen deficiency (AD), a construct consisting of low testosterone and related symptoms (2), is also likely to increase in prevalence. Although advanced age and large body size are considered well-established risk factors for low testosterone (3), prior studies that considered correlates of low testosterone or symptomatic AD were limited by a small sample size (4) or were based in medical care with unknown representation of the underlying general population (5,6). A recent analysis of a longitudinal, U.S. population-based study found that several medical factors (presence of diabetes or hypertension) and body mass index (BMI) were related to a decline in total testosterone among participants (7). Similarly, a prior community-based study of the influence on comorbidity, medications, body size, and lifestyle variables on sex hormone levels found that age, BMI, waist circumference, current smoking, general health status, and physical activity were the most important correlates of testosterone levels among men aged 40–80 yr in The Netherlands (8).
There is an interest in understanding the co-occurrence of symptoms of AD as well as low testosterone, because the clinical significance of low testosterone alone is unclear (9). To consider the association of various factors on testosterone levels and symptoms thought to be related to low testosterone, we used data from a recent epidemiological study of community-dwelling men to estimate the correlation of a wide variety of demographics, anthropometrics, comorbidities, and medication use to 1) low testosterone and low free testosterone and 2) symptomatic AD (low testosterone and low free testosterone plus relevant symptoms).
Subjects and Methods
Study design and data collection
The Boston Area Community Health (BACH) Survey is a population-based, cross-sectional observational study of male and female residents of the city of Boston, MA. Details of the study design and procedures are available elsewhere (10). A two-stage, stratified cluster sampling design was used for the purposes of recruiting approximately equal numbers of participants to prespecified age groups (30–39, 40–49, 50–59, and 60–79 yr), race and ethnic groups (Black, White, and Hispanic), and gender. Interviews were completed for 63.3% of eligible subjects, with a resulting study population of 2301 men and 3201 women comprised of 1766 Black participants, 1877 Hispanic participants, and 1859 White participants. After written informed consent, data were collected between April 2002 and June 2005 during a 2-h interview conducted by a trained, bilingual interviewer. A venous blood sample (20 ml) was also collected as close to awakening as possible (median time since awakening for men was 3 h 38 min). All protocols and informed consent procedures were approved by New England Research Institutes’ Institutional Review Board.
Measurement of testosterone and AD symptoms
The measurement of testosterone and SHBG in BACH have been reported previously (11). Briefly, testosterone and SHBG were measured by competitive electrochemiluminescence immunoassay on the 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN). The lower limit of detection for testosterone was 2 ng/dl (0.07 nmol/liter), and the day-to-day imprecision values at concentrations of 0.24, 2.75, and 7.01 ng/ml (0.8, 9.5, and 24.3 nmol/liter) were 7.4, 2.2, and 1.7%, respectively; within-run values at the same concentrations were 4.6, 1.4, and 1.1%. Laboratory reference ranges were 260–801 ng/dl (9–27.8 nmol/liter) for testosterone and 14.5–48.4 nmol/liter for SHBG. Free testosterone was calculated using the mass action equations described by Södergard et al. (12), with association constants for testosterone from Vermeulen et al. (13). These calculations take into account the concentrations of serum total testosterone and SHBG; the possible binding of other steroids to SHBG was disregarded, and a fixed albumin concentration of 4.3 g/dl was assumed. The association constants of SHBG for testosterone were 1.0 × 109/mol and albumin for testosterone 3.6 × 104/mol (13). All assays used in the study have been approved by the Food and Drug Administration for clinical use. Testosterone values obtained in BACH are similar to those reported in other major observational studies of sex steroids in older men (14,15).
Symptoms related to AD that were available in the BACH Survey were as follows: low libido, erectile dysfunction (ED), osteoporosis, sleep disturbance, lethargy, depressed mood, and low physical performance. Among those with low total and low free testosterone (defined as less than 300 ng/dl or less than 10.4 nmol/liter for total testosterone and less than 5 ng/dl or less than 0.17 nmol/liter for free testosterone in accordance with the Endocrine Society’s Clinical Practice Guideline), participants were considered symptomatic if they had one or more of these symptoms considered specific for AD by the Clinical Practice Guideline (low libido, ED, or osteoporosis) or two more less specific symptoms (sleep disturbance, depressed mood, lethargy, or diminished physical performance) (2). Symptoms were assessed as follows. Men who reported that their level (degree) of sexual desire or interest over the past 4 wk was low or very low were defined as having low libido; men with a score of 17 or lower on the abridged International Index of Erectile Function (IIEF-5) were considered positive on ED (16). Men with a diagnosis of osteoporosis or a fracture of the hip, wrist, or spine after age 50 were considered positive on osteoporosis. For nonspecific symptoms, sleep disturbance and depressed mood over the last week were assessed using yes/no questions to “I felt depressed” and “My sleep was restless” in the abridged eight-item Center for Epidemiologic Studies Depression Scale (17). Lethargy was defined as a reply of some or none of the time to the query “Did you have a lot of energy?” considering the past 4 wk. Finally, low physical performance was considered present if the physical component score of the 12-item Short Form Health Survey (SF-12) fell into the lowest quintile (18).
Covariates
Per-capita income was defined as annual household income divided by household size and categorized into lower, middle, and upper, such that approximately 25, 50, and 25%, respectively, fell into each group. The use of medications over the past 4 wk was assessed using a combination of drug inventory and self-report methods. Comorbidities were defined as reporting yes to the question of “Have you ever been told by a healthcare provider that you have or had… . ”? Cardiac disease was defined as history of myocardial infarction and/or angina and/or coronary artery bypass or angioplasty. BMI (weight in kilograms divided by height in meters squared) was grouped into categories (<25, 25.0–29.9, ≥30), with weight and height measured by the interviewer. Waist circumference measurements were taken twice using a standardized protocol at the end of a normal expiration at the natural waist; in obese subjects, the smallest horizontal circumference was measured in the area between the ribs and the iliac crest, and the average of the two measures was used. Use of alcohol over the past month was defined as average drinks per day. Self-reported health status was measured using the 12-item Short Form Health Survey (18). Physical activity was defined using the Physical Activity Scale for the Elderly (PASE) (19). Because low physical function is part of the definition of symptomatic AD, PASE was used only as a covariate in models predicting low testosterone.
Analysis sample and statistical analysis
Of the 2301 male participants, blood samples were obtained on 1899 (82.5%). Twenty-eight subjects were excluded for reporting current treatment for cancer, and one subject was removed for reporting cancer but not current treatment status. Of the remaining 1870 men, we excluded eight men who were missing testosterone or SHBG, 29 men on medications known to affect sex steroid levels (including dehydroepiandrosterone and prescription testosterone), and nine men with extreme outlying values (≥4 sd from natural log-transformed mean) of testosterone or SHBG. Two men were missing per-capita income due to missing household size. Other missing data were replaced by plausible values using multiple imputation (20). This left 1822 men as a base analysis sample.
To account for the complex sampling design, analyses were conducted using SUDAAN (21). Prevalence estimates were weighted inversely proportional to the probability of selection (22). The distribution of covariates was examined within three groups (low testosterone and low free testosterone with AD symptoms, low testosterone/free testosterone without AD symptoms, and normal), and tests for significant differences by these groups were conducted (χ2 for categorical variables and Wald test for continuous variables).
We used multivariate logistic regression models to estimate odds ratios (OR) and 95% confidence intervals (CI) for 1) low testosterone and low free testosterone levels (hereinafter, low testosterone) and 2) symptomatic AD (as described above). The final models for each outcome were built separately using a submodel approach. First, four submodels were constructed individually and consisted of variables related to demographics, body composition, lifestyle, and health status and medication use; all submodels were always adjusted for study design-related variables (race/ethnicity and age). Within each submodel, variables were then backwards eliminated using a significance level of P < 0.10. As an example, available body composition measures included weight, BMI, hip circumference, waist circumference, and the ratio of waist circumference to hip circumference. All were included in the body composition submodel and eliminated in the following order: weight (P = 0.89), waist-to-hip ratio (P = 0.84), BMI (P = 0.55), and finally hip circumference (P = 0.42). Variables that were significant in the submodels (i.e. waist circumference, P < 0.001) were then used to construct a final multivariate model.
We used the McFadden deviance R2 measure to estimate the contribution of each covariate to the model (23). The R2 quantity is computed as a ratio. Its numerator is itself the ratio of information provided by the model under consideration to the information provided by the null model with no regressors, whereas its denominator is the ratio of information provided by a model with perfect prediction of outcomes to the information in the null model. The deviance R2 may therefore be interpreted as an estimate of the information provided by the covariates expressed as a proportion of all possible information available. In this analysis, age was broken out separately to show its contribution, and race/ethnicity was used as a covariate only in the demographics submodel.
Finally, backwards elimination at Wald test significance level of P ≤ 0.15 was used to reach a parsimonious final model. Age and race/ethnicity remained in the final models, regardless of significance level.
Results
Of 1822 men in our sample, 9.2% (n = 181) had low testosterone (with or without symptoms), and 5.7% (n = 70) had symptomatic AD by our operational definitions. A full treatment of age-specific prevalence and the distribution of symptoms and testosterone levels in the BACH Survey are available elsewhere (24). Characteristics of the analysis sample by AD group are presented in Tables 1 and 2. Men with low testosterone regardless of symptoms were older than normal men, but symptomatic men were not very different in age from asymptomatic men with low testosterone (means 52.5 vs. 52.1 yr, respectively) (Table 1). Type of health insurance varied by androgen grouping, with symptomatic men most likely to report public health insurance and asymptomatic men most likely to report private health insurance. Education level also varied by androgen grouping, with symptomatic men reporting the lowest levels.
Table 1.
Demographics, body composition, and lifestyle factors according to AD group among men in the BACH Survey (n = 1822)
| Covariates | Percentages and means ± se
|
||
|---|---|---|---|
| Low testosteronea
|
Normal n = 1641 (90.8%) | ||
| Symptomatic n = 70 (5.7%) | Asymptomatic n = 111 (3.5%) | ||
| Age (yr)b,d | 52.5 ± 3.5 | 52.1 ± 1.8 | 46.5 ± 0.5 |
| Race and ethnicity (%) | |||
| Black | 27.3 | 23.8 | 28.5 |
| Hispanic | 30.5 | 40.2 | 35.0 |
| White | 42.2 | 35.9 | 36.5 |
| Type of health insurance (%)b,c,d,e | |||
| Private | 46.5 | 74.3 | 67.2 |
| Public | 33.0 | 6.4 | 17.6 |
| None | 20.5 | 19.3 | 15.1 |
| Education (yr)b,c | 13.0 ± 0.7 | 14.6 ± 0.6 | 14.9 ± 0.2 |
| Per capita income | |||
| ≤$6000/person | 16.7 | 17.4 | 12.0 |
| $6001–$30,000/person | 64.0 | 56.8 | 51.2 |
| >$30,000/person | 19.3 | 25.8 | 36.8 |
| Married or living with a partner (%)d,e | 64.5 | 69.1 | 53.9 |
| Weight (kg)b,c,d | 111.7 ± 10.9 | 93.4 ± 2.8 | 87.6 ± 0.7 |
| BMI categories (%)b,c,d | |||
| <25 | 10.6 | 16.4 | 27.7 |
| 25–29.9 | 22.0 | 35.0 | 41.6 |
| ≥30 | 67.4 | 48.6 | 30.7 |
| Waist circumference (cm)b,c,d | 116.4 ± 6.9 | 104.2 ± 2.2 | 96.4 ± 0.5 |
| Current smokerd | 23.4 | 16.6 | 33.1 |
| Alcohol use (%)b | |||
| None | 45.1 | 25.8 | 24.8 |
| <1/d | 39.8 | 37.4 | 40.4 |
| 1–2/d | 11.0 | 23.5 | 25.4 |
| ≥3/d | 4.1 | 13.3 | 9.4 |
| PASE (%)b,c,e | |||
| Low (0–99) | 59.1 | 13.5 | 23.5 |
| Medium (100–249) | 31.7 | 58.0 | 49.0 |
| High (≥250) | 9.2 | 28.5 | 27.5 |
All analyses were weighted inversely to the probability of selection.
Low testosterone was defined as serum total testosterone less than 300 ng/dl (<10.4 nmol/liter) and free testosterone less than 5 ng/dl (<0.17 nmol/liter). Symptomatic low testosterone was the additional presence of one specific symptom or two or more non-specific symptoms.
P value from logistic regression models or from χ2 test for overall comparison was <0.05.
P value from logistic regression models or from χ2 test for comparison of symptomatic low testosterone men with normal men was <0.05.
P value from logistic regression models or from χ2 test for comparison of asymptomatic low testosterone men with normal men was <0.05.
P value from logistic regression models or from χ2 test for comparison of symptomatic low testosterone men with asymptomatic low testosterone men was <0.05.
Table 2.
Health status and medication use according to AD group among men in the BACH Survey (n = 1822)
| Covariates | Low testosteronea
|
Normal n = 1641 (90.8%) | |
|---|---|---|---|
| Symptomatic n = 70 (5.7%) | Asymptomatic n = 111 (3.5%) | ||
| Cardiac disease (%)b,c,e,g | 25.7 | 10.5 | 7.6 |
| High blood pressure (%)c,e,g | 44.5 | 36.3 | 24.8 |
| Diabetes (type I or II) (%)e | 14.8 | 18.1 | 8.4 |
| Asthma (%) | 14.9 | 17.5 | 14.6 |
| Chronic lung disease (%)f,g | 9.9 | 2.5 | 5.9 |
| Arthritis (%)e | 25.3 | 26.0 | 15.9 |
| High cholesterol (%)d,e | 47.8 | 38.0 | 36.3 |
| Prostate or testicular cancer (%) | 1.8 | 0.2 | 1.4 |
| Other cancer (%) | 2.3 | 7.2 | 2.7 |
| HIV or AIDS (%) | 4.4 | 1.7 | 1.3 |
| Corticosteroids (%) | 11.3 | 12.2 | 7.5 |
| β−Blockers (%)c,e,g | 25.7 | 11.0 | 8.0 |
| Anticonvulsants (%)g | 7.2 | 2.0 | 4.7 |
| Diuretics (%)c,e,f | 23.5 | 15.0 | 5.7 |
| Angiotensin-converting enzyme inhibitors (%)c,e,g | 22.1 | 11.4 | 9.8 |
| Self-reported health status (%)c,e,g | |||
| Excellent | 6.6 | 18.6 | 22.1 |
| Very good | 15.8 | 41.7 | 35.9 |
| Good | 40.6 | 29.0 | 28.7 |
| Fair/poor | 37.0 | 10.8 | 13.3 |
All analyses weighted inversely to the probability of selection.
Low testosterone was defined as serum total testosterone less than 300 ng/dl (<10.4 nmol/liter) and free testosterone less than 5 ng/dl (<0.17 nmol/liter). Symptomatic low testosterone was the additional presence of one specific symptom or two or more nonspecific symptoms.
Cardiac disease (history of myocardial infarction and/or angina and/or coronary artery bypass or angioplasty).
P value from χ2 test for overall comparison was <0.05.
Self-report of high cholesterol or serum cholesterol ≥240 mg/dl.
P value from χ2 test for comparison of symptomatic low testosterone men with normal men was <0.05.
P value from χ2 test for comparison of asymptomatic low testosterone men with normal men was <0.05.
P value from χ2 test for comparison of symptomatic low testosterone men to asymptomatic low testosterone men was <0.05.
Men with symptomatic AD had a waist circumference 20 cm larger on average compared with normal men and were also larger considering BMI and weight than both asymptomatic and normal men. Two thirds of the symptomatic men were obese, compared with 48.6% of the asymptomatic low testosterone group and 30.7% of the normal men. Men with asymptomatic low testosterone were least likely to smoke cigarettes, whereas men with symptomatic low testosterone were most likely to abstain from drinking alcohol. Men with symptoms had a much lower (i.e. less active) PASE physical activity score compared with the other two groups. Men with symptoms had higher levels of cardiac disease and high blood pressure when compared with the other groups and were also more likely to be on relevant medications (Table 2). Men with symptoms had the highest proportion of fair or poor self-reported health, whereas this proportion was similar between the asymptomatic and normal group.
Multivariate models
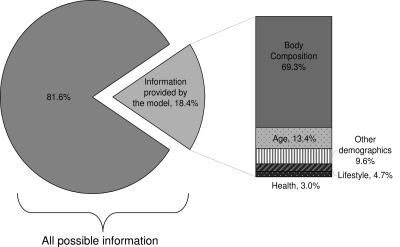
For each of the submodels comprised of thematically grouped sets of covariates, the proportion of information contributed by the variables in the model compared with a perfect prediction model was 0.184, or 18.4%, for low testosterone (Fig. 1). Of the 18.4%, the body composition cluster (containing the waist circumference variable) accounted for 69.3%, without adjustment for any other variables. Consequent addition of age to the model accounted for the next highest percentage (13.4%). We experimented with different sequences of addition, but regardless of order, body composition consistently explained the highest proportion of information in predicting low testosterone.
Figure 1.
Information provided by variables in multivariate models for low total and free testosterone. The model containing all the relevant variables from submodels accounted for a total of 18.4% of information compared with a theoretical model containing all possible predictors (as measured by the deviance R2). The proportionate contribution of covariate groups are depicted at the right. Covariate groups were added one at a time (from highest contributor to lowest). Of the contributing groups, the body composition group containing the variable for waist circumference was by far the most important.
Following backwards selection of candidate variables chosen in the submodeling approach, the final multivariate model for low testosterone is shown in Table 3. A 10-yr increase in age was associated with a 36% increase in odds of low testosterone, whereas a 10-cm increase in waist circumference was associated with a 75% increase in odds of low testosterone. Using diuretics or being married/partnered were also associated with having low testosterone, whereas current smoking was inversely associated. The final deviance R2 for the parsimonious final model was 17.9%.
Table 3.
Final parsimonious logistic regression model for factors correlated with low testosterone/free testosterone among men in the BACH Survey (n = 1822)
| Covariates | OR | 95% CI | P value |
|---|---|---|---|
| Race | 0.89 | ||
| Black | 0.95 | 0.55–1.63 | |
| Hispanic | 0.85 | 0.45–1.61 | |
| White | Referent | ||
| Age, per 10-yr increase | 1.36 | 1.11–1.66 | <0.01 |
| Income per capita | 0.02 | ||
| ≤$6000 | 2.86 | 1.39–5.87 | |
| $6001–$30,000 | 1.61 | 0.91–3.63 | |
| >$30,000 | Referent | ||
| Waist circumference, per 10-cm increase | 1.75 | 1.45–2.12 | <0.001 |
| Married or living with a partner | 0.14 | ||
| Yes | 1.50 | 0.88–2.58 | |
| No | Referent | ||
| Current smoker | 0.13 | ||
| Yes | 0.61 | 0.32–1.15 | |
| No | Referent | ||
| Diuretics | 0.09 | ||
| Yes | 1.81 | 0.91–3.63 | |
| No | Referent |
All analyses weighted inversely to the probability of selection. Deviance R2 of final model was 0.1794. Low testosterone was defined as serum total testosterone less than 300 ng/dl (<10.4 nmol/liter) and free testosterone less than 5 ng/dl (<0.17 nmol/liter).
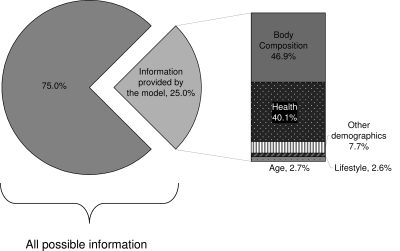
For symptomatic AD, the proportion of information contributed by the variables in the model compared with a perfect prediction model was 0.25, or 25%, in the submodels, and the body composition cluster again accounted for the largest proportion of information (46.9%) (Fig. 2). Adjusted for body composition, the health cluster contributed the next highest (40.1%). As with the model for low testosterone, our finding that waist circumference was the most important was robust to different sequences of model additions and adjustments.
Figure 2.
Information provided by variables in multivariate models for symptomatic AD. The model containing all the relevant variables from submodels accounted for a total of 25.0% of information compared with a theoretical model containing all possible predictors (as measured by the deviance R2).The proportionate contribution of covariate groups are depicted at the right. Covariate groups were added one at a time (from highest contributor to lowest). Of the contributing groups, the body composition group containing the variable for waist circumference was once again the most important.
In the final parsimonious model for symptomatic AD, better self-reported health status was inversely associated with the outcome (Table 4). Similar to the model for low testosterone, a 10-yr increase in age was associated with a 36% increase in odds of symptomatic AD, whereas a 10-cm increase in waist circumference was associated with an 88% increase. The total deviance R2 accounted for by the final parsimonious model was 22.8%.
Table 4.
Final parsimonious logistic regression model for factors correlated with symptomatic androgen deficiency among men in the BACH Survey (n = 1822)
| Covariates | Symptomatic AD
|
||
|---|---|---|---|
| OR | 95% CI | P value | |
| Race | 0.59 | ||
| Black | 0.79 | 0.41–1.52 | |
| Hispanic | 0.69 | 0.32–1.46 | |
| White | Referent | ||
| Age, per 10-yr increase | 1.36 | 1.04–1.77 | 0.02 |
| Waist circumference, per 10-cm increase | 1.88 | 1.44–2.47 | <0.001 |
| Self-reported health status | 0.02 | ||
| Excellent | 0.21 | 0.05–0.92 | |
| Very good | 0.29 | 0.11–0.75 | |
| Good | 0.52 | 0.27–0.99 | |
| Fair/poor | Referent | ||
All analyses weighted inversely to the probability of selection. Deviance R2 of the final model was 0.2282. Symptomatic AD was defined as serum total testosterone less than 300 ng/dl (<10.4 nmol/liter) and free testosterone less than 5 ng/dl (<0.17 nmol/liter) and the presence of one specific or two or more nonspecific symptoms.
Discussion
In this population-based study, we considered the correlation of a wide range of demographic factors, anthropometrics, lifestyle factors, common comorbidities, and medications with low testosterone and symptomatic low testosterone. Among those with low testosterone, we compared asymptomatic and symptomatic men and found that there were more comorbidities among symptomatic men and that education level was lower, self-reported health status was poorer, and body size was larger, although not all differences were statistically significant. After multivariate adjustment, we found that factors that appeared to increase the odds (at P < 0.15) of having low testosterone/free testosterone (regardless of symptoms) were age, waist circumference, being married or living with a partner, being in the lowest category of per-capita income, and using diuretics, whereas current smoking was inversely associated. Physical activity had a borderline association (P = 0.16, data not shown), before its elimination from the final model, with lower levels of activity associated with lower testosterone. For symptomatic AD, only age, waist circumference, and self-reported health status were associated in the final model. Although our study cannot determine causality due to its cross-sectional design, our results are compatible with the conclusion that manifestation of symptoms may be a consequence of generally poor health status.
Our analysis also suggests a potential modifiable risk factor, waist circumference. In both models, waist circumference was a correlate of strong magnitude, even after adjustment for age and other factors, and contributed the most information to the model. Larger body size is a well-established risk factor for low testosterone (3). In recent studies, a one-unit increase in BMI was associated with a nearly 2% decline in testosterone in the Massachusetts Male Aging Study (7), whereas both waist circumference and BMI were associated with lower levels of testosterone in a recent study of diabetic men (6) and in the CARDIA study of young men (25). We considered both BMI and waist circumference in our study, and the latter was more strongly associated for both of our outcomes; this finding was also present in the Tromsø study (26). Suggesting modifiability, rapid weight loss, and weight maintenance for 1 yr among abdominally obese men have been found to increase testosterone levels in a small study (27). Waist circumference, a proxy for visceral adiposity, may decrease testosterone through the increased metabolic activity of visceral fat and the hypothesized hypogonadal-obesity cycle (28). However, the association may be bidirectional; those with low testosterone at baseline may be more at risk for developing visceral fat or central adiposity in later years (29,30). In our cross-sectional study, we capture prevalence and are unable to determine causality, but our study contributes knowledge about the strength of the association and its relative contribution in the presence of other risk factors.
In our model for low testosterone/free testosterone, having a lower socioeconomic status, as measured by categories of income per household member, was associated with having low testosterone, as in a recent study that considered symptomatic AD (4). Socioeconomic status may be functioning as a marker for poorer health access, increased stress, adverse health behaviors, and impoverished neighborhood environment. As with prior studies using the BACH data, race/ethnicity was not associated with low testosterone (11) or with symptoms (24). Our finding that men who are married or living with a partner have lower testosterone has been observed previously in a cohort of male Air Force veterans (31). Conversely, loss of spouse was associated with decreased testosterone in the longitudinal Massachusetts Male Aging Study (7). Why marital status may influence testosterone levels is not well understood, but future studies should include consideration of marital status until its influence (as a marker for lifestyle, diet, etc.) is better understood. We did not find that being married/living with a partner influenced having symptomatic AD, however.
Use of diuretics, but not hypertension, was found to be associated in the final model for low testosterone. This could be considered another modifiable risk factor if the use of effective safe alternative treatments for hypertension were clinically appropriate. We found no specific comorbidity was associated in either final model. Self-reported health may be standing in for a multitude of existing diagnosed or undiagnosed comorbidities in the model for symptomatic AD, however.
There are potential limitations to this analysis. Although mass spectrometry methods for assessing testosterone may eventually be considered the gold standard, a recent review has noted that accuracy of platform assays for testosterone levels in the physiological male range is not problematic (32). Although the deviance R2 reported from our models may appear modest, it has been pointed out that this is typical in logistic regression analyses of epidemiological data (33). We were unable to include impaired fasting glucose as a potential correlate, and those with undiagnosed diabetes would not be captured in our study; these might have added to the information provided by the model had we been able to include them. Lean mass vs. fat mass as components of body composition may also have been explanatory. We included self-report of physician diagnosis for many comorbidities; in the case of diabetes in this analysis, we were able to confirm that over 80% had a record of relevant medications, suggesting the self-report was valid. We did not have complete medical information to ascertain primary or secondary hypogonadism status, but based on gonadotropin levels of the 70 men with symptomatic low testosterone, approximately 17% clearly had primary hypogonadism with elevated LH and/or FSH, approximately 11% appeared to have secondary with inappropriately low levels of LH and/or FSH, and about 72% were indeterminate. Of the 111 men with asymptomatic low testosterone, approximately 35% were clearly primary, about 8% appeared to be secondary, and approximately 57% were indeterminate. Finally, regarding our definition of symptomatic AD, we used symptoms that were available in the BACH Survey, but not all symptoms recommended in the Endocrine Society Guidelines were available (2). As such, our combination of symptom measures has not been independently validated as a reliable measure of AD. We examined the distribution of our identified risk factors at lower thresholds of total testosterone (<260 and <200 ng/dl) and found that the distribution of risk factors was largely consistent, suggesting that the less than 300 ng/dl threshold for total testosterone used in this study was capturing symptoms as well as more stringent cutoff points (data not shown).
Strengths of our study include the wide range of lifestyle, anthropometric, and medical covariates we were able to consider. A further strength is the diversity of race, ethnicity, and socioeconomic status among participants, allowing us to consider low testosterone and symptoms among persons who do not present to care. In addition, the generalizability of BACH to the U.S. population is known. BACH men were similar to men in the National Health and Nutrition Examination Survey, National Health Interview Survey, and Behavioral Risk Factor Surveillance Survey with respect to the distributions of common comorbidities (except asthma, more common in BACH) (10,34).
Available data suggest sales of prescription testosterone are on the rise (35), despite debate over the appropriateness of its use (36,37) and the absence of changes in approved indications for testosterone treatment (38). There is ongoing controversy over the male menopause construct (39) and safety concerns regarding use of testosterone (9,40). A large proportion of aged men will always have contraindications to treatment with testosterone (e.g. a history of prostate cancer, the most common malignancy in men) (41). As such, the modifiable risk factor for low testosterone and symptomatic AD suggested by this study (waist circumference) will have enduring relevance. Given the multitude of health benefits associated with maintaining a healthy weight, there is little risk in recommending this course of action.
Footnotes
Address reprint requests: John B. McKinlay, Ph.D., Senior Vice President, New England Research Institutes, Nine Galen Street, Watertown, Massachusetts 02472. E-mail: bach@neriscience.com.
Analyses for the current manuscript were supported by an unrestricted educational grant to New England Research Institutes from GlaxoSmithKline. The BACH study is supported by DK 56842 from the U.S. National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure Statement: S.A.H., G.R.E., A.B.A., T.G.T., and J.B.M. have nothing to declare. R.V.C. and R.E.W. are employees of GlaxoSmithKline and have equity interest in GlaxoSmithKline.
First Published Online July 9, 2008
Abbreviations: AD, Androgen deficiency; BMI, body mass index; CI, confidence interval; ED, erectile dysfunction; OR, odds ratio; PASE, Physical Activity Scale for the Elderly.
References
- US Department of Health and Human Services 2003 National Health and Nutrition Examination Survey data briefs: healthy weight, overweight, and obesity among U.S. adults. Atlanta, GA: Centers for Disease Control and Prevention [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM 2006 Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 91:1995–2010 [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A 2005 The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26:833–876 [DOI] [PubMed] [Google Scholar]
- Wong SY, Chan DC, Hong A, Woo J 2006 Prevalence of and risk factors for androgen deficiency in middle-aged men in Hong Kong. Metabolism 55:1488–1494 [DOI] [PubMed] [Google Scholar]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C 2006 Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 60:762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor D, Aldred H, Clark S, Channer KS, Jones TH 2007 Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 30:911–917 [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB 2007 The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 92:549–555 [DOI] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT 2003 Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol 149:583–589 [DOI] [PubMed] [Google Scholar]
- Harman SM 2005 Testosterone in older men after the Institute of Medicine Report: where do we go from here?. Climacteric 8:124–135 [DOI] [PubMed] [Google Scholar]
- McKinlay JB, Link CL 2007 Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol 52:389–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB 2006 Serum androgen levels in Black, Hispanic, and White men. J Clin Endocrinol Metab 91:4326–4334 [DOI] [PubMed] [Google Scholar]
- Södergard R, Backstrom T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB 2002 Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87:589–598 [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR 2001 Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed] [Google Scholar]
- Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM 1999 Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 11:319–326 [DOI] [PubMed] [Google Scholar]
- Turvey CL, Wallace RB, Herzog R 1999 A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr 11:139–148 [DOI] [PubMed] [Google Scholar]
- Ware Jr J, Kosinski M, Keller SD 1996 A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233 [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA 1993 The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162 [DOI] [PubMed] [Google Scholar]
- Schafer JL1997 Analysis of incomplete multivariate data. London: Chapman and Hall [Google Scholar]
- Research Triangle Institute 2004 SUDAAN language manual release 9.0. Research Triangle Park, NC: Research Triangle Institute [Google Scholar]
- Cochran W1977 Sampling techniques. 3rd ed. New York: John Wiley and Sons [Google Scholar]
- Menard S 2000 Coefficients of determination for multiple logistic regression analysis. Am Statistician 54:17–24 [Google Scholar]
- Araujo AB, Esche GR, Kupelian V, O'Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB2007 Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 92:4241–4247 [DOI] [PubMed] [Google Scholar]
- Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, Liu K 2002 Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev 11:1041–1047 [PubMed] [Google Scholar]
- Svartberg J, von Muhlen D, Sundsfjord J, Jorde R 2004 Waist circumference and testosterone levels in community dwelling men. The Tromso study. Eur J Epidemiol 19:657–663 [DOI] [PubMed] [Google Scholar]
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A 2004 Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 6:208–215 [DOI] [PubMed] [Google Scholar]
- Cohen PG 1999 The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt: a major factor in the genesis of morbid obesity. Med Hypotheses 52:49–51 [DOI] [PubMed] [Google Scholar]
- Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY 2000 Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord 24:485–491 [DOI] [PubMed] [Google Scholar]
- Khaw KT, Barrett-Connor E 1992 Lower endogenous androgens predict central adiposity in men. Ann Epidemiol 2:675–682 [DOI] [PubMed] [Google Scholar]
- Mazur A, Michalek J 1998 Marriage, divorce, and male testosterone. Social Forces 77:315–330 [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H 2007 Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 92:405–413 [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S2000 Applied logistic regression. 2nd ed. New York: John Wiley and Sons [Google Scholar]
- New England Research Institutes 2006 Can the BACH data be generalized to the U.S. population? Comparison of BACH batch 1–4 data with data from three national surveys (NHIS, NHANES, and BRFSS). Watertown, MA: New England Research Institutes [Google Scholar]
- Tan RS, Salazar JA 2004 Risks of testosterone replacement therapy in ageing men. Expert Opin Drug Saf 3:599–606 [DOI] [PubMed] [Google Scholar]
- Morgentaler A 2007 Guideline for male testosterone therapy: a clinician’s perspective. J Clin Endocrinol Metab 92:416–417 [DOI] [PubMed] [Google Scholar]
- Wu FC 2007 Guideline for male testosterone therapy: a European perspective. J Clin Endocrinol Metab 92:418–419 [DOI] [PubMed] [Google Scholar]
- Handelsman DJ 2004 Trends and regional differences in testosterone prescribing in Australia, 1991–2001. Med J Aust 181:419–422 [DOI] [PubMed] [Google Scholar]
- McKinlay JB, Travison TG, Araujo AB, Kupelian V 2007 Male menopause-time for a decent burial? Menopause 14:973–975 [DOI] [PubMed] [Google Scholar]
- Brand TC, Canby-Hagino E, Thompson IM 2007 Testosterone replacement therapy and prostate cancer: a word of caution. Curr Urol Rep 8:185–189 [DOI] [PubMed] [Google Scholar]
- Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, Wingo PA, Howe HL, Ries LA, Miller BA, Jemal A, Ahmed F, Cobb N, Kaur JS, Edwards BK 2007 Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 110:2119–2152 [DOI] [PubMed] [Google Scholar]