Abstract
Context: Insulin resistance, impaired glucose tolerance, and type 2 diabetes are common in women with polycystic ovary syndrome (PCOS). Obstructive sleep apnea (OSA) has been linked to metabolic dysfunction. We studied women with and without PCOS to determine the extent to which OSA is responsible for insulin resistance and glucose intolerance in PCOS.
Methods: In a prospective design, 52 women with PCOS and 21 women without PCOS of similar age and body mass index had an overnight polysomnogram and a 75-g oral glucose tolerance test.
Results: Twenty-nine women (56%) with PCOS had OSA compared with four controls (19%) (adjusted odds ratio 7.1; 95% confidence interval, 1.7–45.7; P = 0.01). PCOS women with OSA were more insulin resistant than those without OSA [homeostasis model assessment (HOMA) index 5.7 ± 0.4 vs. 3.5 ± 0.4; P = 0.006] after controlling for age, body mass index, and ethnicity. Impaired glucose tolerance was found in 16 of 29 (55%) PCOS women with OSA and only six of 23 (26%) of those without OSA (unadjusted P = 0.049). Insulin resistance and glucose intolerance were highly correlated with the presence and severity of OSA. Among PCOS women with normal glucose tolerance, the presence of OSA was associated with a nearly 2-fold higher fasting insulin level and HOMA index. The severity of OSA was a highly significant predictor of the fasting concentrations of glucose and insulin as well as the 2-h glucose concentration and HOMA index.
Conclusions: OSA is a highly prevalent and important determinant of insulin resistance, glucose intolerance, and type 2 diabetes in PCOS.
Obstructive sleep apnea is highly prevalent among women with polycystic ovary syndrome, and is an important determinant of insulin resistance and glucose intolerance in this population.
Polycystic ovary syndrome (PCOS) is a disorder of chronic hyperandrogenemic anovulation that affects 5–8% of women of reproductive age (1). Insulin resistance is a hallmark of PCOS and plays an important role in its pathogenesis by contributing to both androgen overproduction and metabolic disturbances. Although the etiology of insulin resistance in PCOS remains unclear, it is well established that women with PCOS are more insulin resistant when compared with women without PCOS who are of similar age and body composition (2). Remarkably, nearly half of PCOS women in the United States will develop impaired glucose tolerance or type 2 diabetes before the end of their fourth decade of life (3,4).
Obstructive sleep apnea (OSA) is a disorder characterized by repetitive upper airway closures during sleep leading to oxygen desaturations and sleep fragmentation. OSA is most closely associated with risk factors of obesity and male gender (5). In large epidemiological studies in which the majority of the participants were middle-aged overweight men, the presence and severity of OSA was predictive of insulin resistance and glucose intolerance, independently of the degree of obesity (6,7,8). Some, but not all, studies have indicated that insulin resistance and glucose intolerance may improve with successful treatment of OSA (7,9,10,11).
In three previous reports, the prevalence of OSA in women with PCOS exceeded that observed in women without PCOS after controlling for age and body mass index (BMI) (12,13,14). We questioned whether insulin resistance and the risk of early-onset impaired glucose tolerance and type 2 diabetes among women with PCOS might be due, at least in part, to the presence of OSA. Given that OSA has not been previously accounted for in the assessment of metabolic alterations in this population, we designed the present study to test the hypothesis that the presence and severity of OSA are important determinants of insulin resistance and glucose tolerance in women with PCOS.
Subjects and Methods
Study design and population
Women with PCOS were consecutively recruited from the Endocrinology Clinics at the University of Chicago between February 1, 2004, and September 30, 2007. During the same period of time, overweight (BMI >25 kg/m2 but <30 kg/m2) and obese (BMI ≥30 kg/m2) women who were otherwise healthy were recruited through public advertisements in the local community. Sleep complaints or symptoms of OSA were not used as selection criteria for the study. Only women between 18 and 40 yr of age were recruited to reduce the impact of age upon ovarian function and glucose tolerance. Subjects were excluded if they smoked cigarettes; were diabetic or hypertensive; had a history of cardiac, psychiatric, neurological, or endocrine disease; or were taking any medications at the time of the study. All participants gave written informed consent. The study was approved by the University of Chicago Institutional Review Board.
A complete medical history was obtained, and a physical examination was conducted in all subjects. Overnight laboratory polysomnography was performed to establish the presence and the severity of OSA. The following morning, a fasting blood sample was drawn for routine laboratory tests and the measurement of serum concentrations of total testosterone, free testosterone, SHBG, and dehydroepiandrosterone sulfate (DHEAS). A standard 75-g oral glucose tolerance test (OGTT) was performed to assess glucose tolerance.
A diagnosis of PCOS required 1) the presence of oligo/amenorrhea; 2) hyperandrogenemia, defined by a supranormal plasma free testosterone level (>10 pg/ml); 3) hyperandrogenism, as evidenced by infertility, hirsutism, acne, or androgenetic alopecia; and 4) exclusion of nonclassic 21-hydroxylase deficiency, congenital adrenal hyperplasia, Cushing’s syndrome, hypothyroidism, or significant elevations in serum prolactin (1).
All testing was performed in the follicular phase of the menstrual cycle in normally cycling women. Progesterone levels were measured on a fasting blood sample to confirm the phase of the menstrual cycle.
Polysomnography
Overnight polysomnography (Neurofax EEG 1100 digital acquisition system; Nihon Kohden, Foothill Ranch, CA) included recordings of two central and two occipital electroencephalogram channels, bilateral electrooculograms, submental electromyogram, leg movements by bilateral anterior tibialis electromyogram, electrocardiogram, oronasal airflow by thermistor, chest and abdominal wall motion by piezo electrodes, and arterial oxygen saturation by pulse oximeter. Sleep recordings were visually scored in 30-sec epochs in stages 1, 2, 3, and 4 of non-rapid eye movement sleep and in rapid eye movement sleep according to standard criteria (15). Obstructive respiratory events (i.e. apneas and hypopneas) and microarousals were scored according to established criteria (16,17). The apnea-hypopnea index (AHI) was calculated as the total number of obstructive respiratory events per hour of sleep, and a diagnosis of OSA was assigned if the AHI was equal to or higher than five events per hour. The severity of OSA was graded according to commonly used clinical criteria as mild (AHI > 5 but <15), moderate (AHI >15 but <30), or severe (AHI ≥30). The microarousal index was calculated as the total number of microarousals per hour of sleep.
OGTT
After an overnight 12-h fast, an iv catheter was placed into an antecubital vein for blood drawing. Baseline samples were obtained at 0 min for measurement of glucose and insulin concentrations. At time 0 min, 75 g glucose was administered orally, and blood samples were collected for the measurement of glucose and insulin concentrations at 30, 60, 90, and 120 min. A diagnosis of normal glucose tolerance, impaired glucose tolerance, or diabetes was assigned if the glucose level at 2 h was less than 140 mg/dl, between 140 and 200 mg/dl, or 200 mg/dl or more, respectively (18). Areas under the curve (AUC) for glucose and insulin responses were calculated for the first 2-h interval after glucose load using the trapezoidal rule. The degree of insulin resistance was quantified by homeostasis model assessment (HOMA) index using the following formula: (fasting serum insulin × fasting plasma glucose)/22.5 (19).
Assays
Plasma glucose was assayed by the glucose oxidase method, and serum insulin was assayed by a double-antibody RIA in blood samples collected during the OGTT. Total testosterone was measured using a kit from Diagnostic Products (Los Angeles, CA). The free fraction of plasma testosterone was measured by a competitive protein-binding assay as previously described (20). The intra- and inter-assay coefficients of variation (CV) are 3.8 and 8.7%, respectively. DHEAS was measured by RIA using a commercial kit (Diagnostics Systems Laboratories, Inc., Webster, TX). Hemoglobin A1C was measured by Bio-Rad Variant Classic boronate affinity-automated HPLC (Bio-Rad, Hercules, CA). The intraassay CV is 0.5–1.0%, and the interassay CV ranges from 2.2–2.4%.
Statistical analysis
Group data are expressed as mean ± sem. Using logistic regression, the odds ratio (with 95% confidence intervals) for having OSA in PCOS women compared with control women was calculated after adjustment for age, BMI, and ethnicity-based diabetes risk (Whites, low risk; African-Americans and Hispanics, high risk; referred to as ethnicity in the remainder of the text) (21). The main objective of the present study was to determine the impact of OSA on insulin resistance and glucose tolerance in women with PCOS. To this effect, we compared 1) PCOS women without OSA vs. control women without OSA and 2) PCOS women with OSA vs. PCOS women without OSA. Group differences were tested by ANOVA after adjustment for age, BMI, and ethnicity. Categorical data were compared with Fisher’s exact test. The relationships between the severity of OSA and the metabolic variables were analyzed by ANOVA with age, BMI, ethnicity, and markers of severity of OSA (i.e. AHI or microarousal index or lowest oxygen desaturation as continuous variables) as predictors. All statistical calculations were performed using JMP statistical software for Macintosh (SAS Institute, version 6.0.3). All reported P values are two sided.
Results
Fifty-two women with PCOS and 21 control women participated in the study. Both groups were young (controls, 30.7 ± 1.1 yr; PCOS, 29.7 ± 0.7 yr; P = 0.42). The majority of women in both groups were obese. The control group was comprised of two overweight and 19 obese women. The mean BMI among controls was 36.0 ± 1.5 kg/m2 (range, 27.7–48.8 kg/m2). The PCOS group had two lean, two overweight, and 48 obese women. The mean BMI in the PCOS group was 39.2 ± 1.0 kg/m2 (range, 23.2–58.8 kg/m2). The control group had a higher proportion of women of African-American or Hispanic descent (86 vs. 62%; P = 0.054) who have a higher risk of insulin resistance and type 2 diabetes than White women (21).
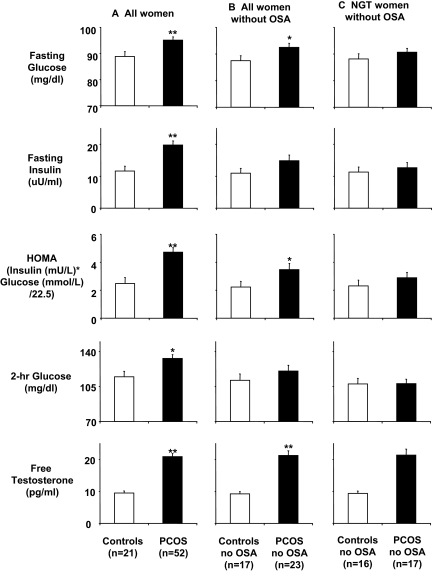
Figure 1A compares metabolic variables in all women. As expected, PCOS women were more insulin resistant than controls, as reflected by a higher HOMA index (adjusted P = 0.0002). They also had higher fasting concentrations of glucose (adjusted P = 0.0021) and insulin (adjusted P = 0.0001) and 2-h glucose levels after oral glucose administration (adjusted P = 0.0081).
Figure 1.
Metabolic and hormonal variables in study subjects. A, All PCOS women (n = 52) vs. controls (n = 21); B, PCOS women without OSA (n = 23) vs. controls without OSA (n = 17); C, normal glucose tolerant (NGT) PCOS women without OSA (n = 17) vs. normal glucose tolerant controls without OSA (n = 16). *, P < 0.05; **, P < 0.01.
Twenty-nine of the 52 PCOS women (56%) had OSA. In contrast, only four of 21 control women (19%) had OSA. After adjustment for age, BMI, and ethnicity, the risk of OSA in PCOS women was more than 7-fold higher than in controls (odds ratio, 7.7; 95% confidence interval, 1.7–45.7; P = 0.01).
Insulin resistance and glucose tolerance in PCOS and control women without OSA
PCOS women without OSA were similar in age (PCOS, 27.3 ± 0.7 yr; controls, 29.9 ± 1.3 yr; P = 0.09) and BMI (PCOS, 35.3 ± 1.4 kg/m2; controls, 35.3 ± 1.6 kg/m2; P = 0.97) to control women without OSA. There was a trend for a greater proportion of women with high ethnicity-based diabetes risk in the control group (88 vs. 61%; P = 0.08). There were no significant differences in sleep variables between these two groups (Table 1).
Table 1.
Polysomnographic, hormonal, and metabolic variables of the three groups of subjects
| Variable | Study groups
|
Group comparisons, P values
|
|||
|---|---|---|---|---|---|
| Control women without OSA (n = 17) | PCOS women without OSA (n = 23) | PCOS women with OSA (n = 29) | PCOS women without OSA vs. control women without OSA | PCOS women without OSA vs. PCOS women with OSA | |
| Total sleep time (min) | 445.5 ± 5.2 | 427.3 ± 6.4 | 422.7 ± 6.8 | 0.09 | 0.74 |
| AHI (per hour of sleep) | 2.3 ± 0.3 | 2.0 ± 0.4 | 19.4 ± 2.0 | 0.97 | <0.0001 |
| Microarousal index (per hour of sleep) | 14.9 ± 2.0 | 10.5 ± 0.9 | 22.3 ± 1.6 | 0.13 | <0.0001 |
| Minimum oxygen saturation (%) | 90.9 ± 0.7 | 92.0 ± 0.5 | 85.1 ± 1.3 | 0.70 | 0.002 |
| Total testosterone (pg/ml) | 40.5 ± 3.3 | 76.3 ± 6.4 | 67.9 ± 4.3 | <0.0001 | 0.19 |
| Free testosterone (pg/ml) | 9.2 ± 0.7 | 21.3 ± 1.5 | 20.6 ± 1.3 | <0.0001 | 0.32 |
| SHBG (nm) | 26.8 ± 3.6 | 16.6 ± .1.9 | 12.3 ± 1.2 | 0.0003 | 0.53 |
| DHEAS (μg/dl) | 120.9 ± 17.1 | 152.6 ± 15.0 | 121.2 ± 12.2 | 0.47 | 0.60 |
| Hemoglobin A1C (%) | 5.4 ± 0.1 | 5.5 ± 0.1 | 5.6 ± 0.1 | 0.27 | 0.39 |
| Fasting glucose (mg/dl) | 87.5 ± 1.9 | 92.5 ± 1.5 | 97.2 ± 1.8 | 0.02 | 0.11 |
| 2-h glucose (mg/dl) | 111.3 ± 6.4 | 120.6 ± 5.7 | 143.3 ± 4.4 | 0.09 | 0.16 |
| AUC glucose | 13528 ± 553 | 15681 ± 589 | 17550 ± 449 | 0.006 | 0.07 |
| Fasting insulin (μU/ml) | 10.9 ± 1.5 | 14.9 ± 1.8 | 23.7 ± 1.5 | 0.06 | 0.01 |
| AUC insulin | 8326 ± 882 | 10572 ± 1409 | 16802 ± 1589 | 0.13 | 0.11 |
| HOMA index (mg/dl·μU·ml) | 2.2 ± 0.4 | 3.5 ± 0.4 | 5.7 ± 0.4 | 0.01 | 0.006 |
Data are mean ± sem. P values are adjusted for age, BMI, and ethnicity-based diabetes risk.
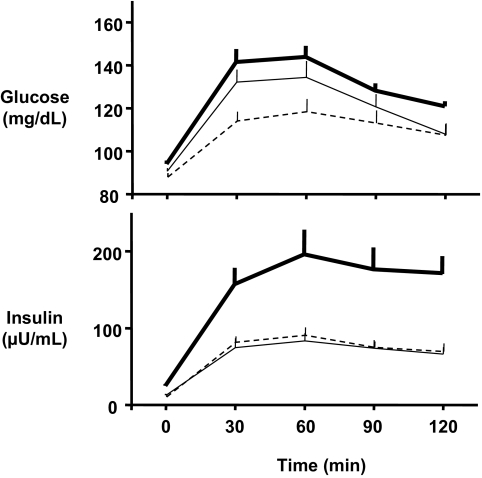
Impaired glucose tolerance was found in six (26%) of 23 non-apneic PCOS but in only one (6%) of 17 non-apneic control women (unadjusted P = 0.21, Fisher’s exact test; P adjusted for ethnicity = 0.11). Sleep and hormonal and metabolic variables for PCOS women without OSA and their controls are shown in Table 1. Non-apneic PCOS women had higher glucose levels (fasting and during the OGTT) and higher HOMA indices than control women (Fig. 1B). However, these differences in metabolic variables between non-apneic PCOS and non-apneic control women were nearly entirely due to the contribution of women with impaired glucose tolerance. Indeed, when only women with normal glucose tolerance were compared, there were no significant differences in metabolic variables between PCOS and control women (Fig. 1C). Specifically, there was no significant difference in insulin resistance, as assessed by the HOMA index, between non-apneic PCOS women (n = 17; 2.9 ± 0.4 mg/dl·μU·ml) and control women (n = 16; 2.3 ± 0.4 mU/liter × mmol/liter; adjusted P = 0.25). Figure 2 shows that for non-apneic women with normal glucose tolerance, insulin values during the OGTT are virtually identical in PCOS and control women. The plasma glucose concentrations at time 0, 60, 90, and 120 min after glucose ingestion were also not significantly different between non-apneic PCOS women and controls; at 30 min, the difference approached (P = 0.06) but did not reach significance. The difference in area under the glucose curve was also not significant (P = 0.10) between these two groups of women with normal glucose tolerance.
Figure 2.
Mean (± sem) concentrations of glucose (top) and insulin (bottom) in response to a 75-g oral glucose challenge. All subjects have normal glucose tolerance. Solid heavy lines depict PCOS women with OSA, solid thin lines depict PCOS women without OSA, and dashed lines depict control women without OSA. Plasma glucose concentrations at time 0, 60, 90, and 120 min after glucose ingestion did not differ between PCOS women and controls without OSA; at 30 min, the difference approached (P = 0.06) but did not reach significance. Insulin values during the OGTT are virtually identical in PCOS and control women without OSA.
Insulin resistance and glucose tolerance in PCOS women with and without OSA
PCOS women with OSA were slightly older (31.6 ± 1.0 vs. 27.3 ± 0.7 yr; P = 0.002) and had a higher BMI (42.2 ± 1.1 vs. 35.3 ± 1.4 kg/m2; P < 0.001) than PCOS women without OSA. The proportion of women with elevated ethnicity-based diabetes risk was similar in the two groups (62 vs. 61%). Both groups of PCOS women had the same degree of hyperandrogenism, as reflected by nearly identical concentrations of free testosterone (Table 1).
On average, the severity of OSA among PCOS women was in the moderate range, as reflected by a mean AHI of 19.4 ± 2.0 (Table 1). Twelve (41%) of the 29 PCOS women had mild OSA (mean AHI, 11.4 ± 8.3; range, 6.3–14.6); 14 (49%) had moderate OSA (mean AHI, 20.9 ± 1.2; range, 15.1–27.7), and three (10%) had severe OSA (mean AHI, 44.9 ± 3.9; range, 37.5–50.6). The presence of OSA was associated with lower minimum oxygen saturation during sleep and a higher degree of sleep fragmentation, as quantified by an elevated microarousal index (Table 1). There were no significant differences in total sleep time or in sleep stage distribution between PCOS women with and without OSA.
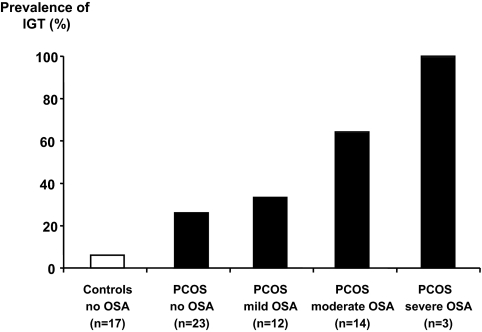
Impaired glucose tolerance was found in 16 (55%) of 29 PCOS women with OSA but in only six (26%) of 23 of those without OSA (unadjusted P = 0.049, Fisher’s exact test; P adjusted for BMI = 0.09). As illustrated in Fig. 3, the prevalence of impaired glucose tolerance increased in direct proportion to the severity of OSA.
Figure 3.
Prevalence of impaired glucose tolerance (IGT) among control women without OSA, PCOS women without OSA, and PCOS women with mild, moderate, and severe OSA. The prevalence of impaired glucose tolerance increased in direct proportion to the severity of OSA.
Measures of insulin resistance were markedly higher in PCOS women with OSA than in PCOS women without OSA. Indeed, both the fasting insulin concentration and the HOMA index were higher in the PCOS women with OSA (Table 1). Levels of glucose, both fasting and after glucose ingestion, were not significantly different between apneic and non-apneic PCOS women, although trends were apparent.
Among PCOS women with normal glucose tolerance, the presence of OSA was associated with a nearly 2-fold higher fasting insulin level (24.3 vs. 12.7 μU/ml; P < 0.04 after controlling for BMI, age, and ethnicity) and HOMA index (5.6 vs. 2.9; adjusted P = 0.04). Similarly, the AUC for insulin during the OGTT was more than 2-fold greater in apneic than in non-apneic PCOS women (adjusted P = 0.06; Fig. 2).
Insulin resistance and glucose tolerance in PCOS: relationships to the severity of OSA
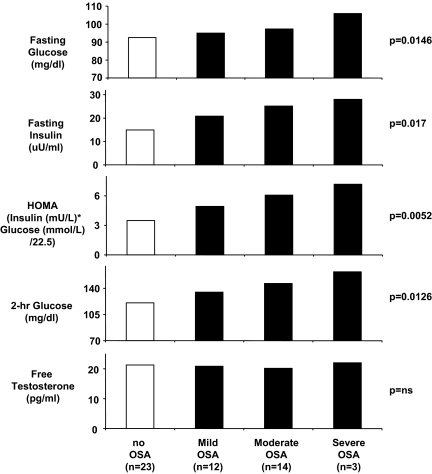
After controlling for age, BMI, and ethnicity, the severity of OSA (as assessed by the AHI value) was a highly significant predictor of the fasting concentrations of glucose and insulin as well as the 2-h glucose concentration and HOMA index. In contrast, no significant association between AHI and free testosterone levels was detected (Fig. 4). There was a strong correlation between the degree of sleep fragmentation as quantified by the microarousal index and the severity of OSA as assessed by the AHI (unadjusted r = 0.86; P = 0.0001). Therefore, when microarousal index was substituted for AHI in the analyses of severity of metabolic disturbances, results similar to those illustrated in Fig. 4 were found (adjusted P levels were 0.0127, 0.0170, 0.0055, and 0.0126 for fasting glucose, fasting insulin, HOMA index, and 2-h glucose, respectively). In contrast, minimum oxygen saturation was only moderately correlated with AHI (unadjusted r = −0.405; P = 0.0032) and was not a significant predictor of fasting glucose (P = 0.154), fasting insulin (P = 0.887), HOMA index (0.586), or 2-h glucose (P = 0.601). Thus, the degree of sleep fragmentation, rather than the severity of hypoxia, appeared to be related to the severity of insulin resistance and glucose intolerance in PCOS.
Figure 4.
Relationships between the severity of OSA (as assessed by the AHI) in women with PCOS and the fasting levels of glucose and insulin, HOMA index, 2-h glucose concentration after glucose challenge, and free testosterone concentrations. After controlling for age, BMI, and ethnicity, the severity of OSA (as assessed by the AHI value) was a highly significant predictor of the fasting concentrations of glucose and insulin as well as the 2-h glucose concentration and HOMA index. In contrast, no significant association between AHI and free testosterone levels was detected.
Discussion
We conducted a prospective cohort study in PCOS and control women to test the hypothesis that OSA is an important determinant of insulin resistance and glucose tolerance in PCOS. More than half of the 52 women with PCOS who were part of our cohort had OSA. Our analyses carefully controlled for the presence of OSA and for glucose tolerance status (normal vs. impaired). The results show that PCOS women without OSA who have maintained normal glucose tolerance are not more insulin resistant than control women. Fasting insulin levels, HOMA index, and insulin response to oral glucose were indeed all similar to those in control women. In contrast, PCOS women with OSA were clearly insulin resistant, even when they had normal glucose tolerance, and had markedly higher fasting insulin levels and HOMA indices. Thus, the major finding from our study is that insulin resistance in PCOS appears to be largely determined by the presence of OSA. It is generally accepted that insulin resistance is a consistent finding in PCOS (2,22). However, none of the previous studies of metabolic abnormalities in PCOS has controlled for the presence of OSA, which appears to affect at least 50% of PCOS women. Our results strongly suggest that PCOS is comprised of two sub-phenotypes: PCOS with OSA and PCOS without OSA, each with distinct metabolic alterations.
The prevalence of impaired glucose tolerance in PCOS women without OSA tended to be higher than in non-PCOS control women, suggesting that a defect in β-cell function, rather than in insulin action, is the main factor underlying the increased risk of diabetes in these non-apneic PCOS women. Consistent with epidemiological data in non-PCOS populations (8,23,24,25), the prevalence of impaired glucose tolerance in PCOS women with OSA was dependent on the severity of OSA.
Our finding that over half (56%) of women with PCOS had OSA is consistent with findings from three previous studies involving a total of nearly 100 patients (12,13,14). The increased risk of OSA in our cohort of women with PCOS was about 7-fold greater than in controls even after controlling for age, BMI, and ethnicity. This high prevalence of OSA is particularly striking for young premenopausal women because male gender, together with obesity, is a major risk factor. The prevalence of OSA in PCOS may be in fact comparable to that found in obese men (26,27). OSA is more likely to be unrecognized in women than in men, partly because women with OSA have more complaints of insomnia and depression, and fewer complaints of snoring and respiratory symptoms, than men. Young women with PCOS are unlikely to be diagnosed and treated for OSA (28) because the community of clinicians who treat PCOS is not yet aware of the high risk of OSA in these patients. OSA is a risk factor for hypertension, stroke, cardiovascular disease (29,30,31), and insulin resistance (6), and treatment of OSA has beneficial effects on these comorbidities.
Because sex steroids have been proposed to play a role in the pathogenesis of OSA, one possibility for the high prevalence of OSA in PCOS could be androgen excess, a defining feature of PCOS (12,32,33). However, our results do not support this hypothesis, because testosterone levels were virtually identical in PCOS women with and without OSA. Furthermore, there were no significant associations between androgen levels and the severity of OSA.
A substantial number (42%) of women with PCOS had impaired glucose tolerance, a proportion that is consistent with prior studies (3,4). Previously, we as well as others had not recognized the potential impact of OSA on this finding. Indeed, the prevalence of impaired glucose tolerance is approximately 2-fold higher (55 vs. 23%) in women with PCOS who have OSA compared with those without OSA. The mechanisms underlying the development of impaired glucose tolerance in PCOS may thus differ according to the presence or absence of OSA.
Our study does not directly address the mechanisms underlying the association between OSA, insulin resistance, and glucose intolerance. Although the direction of causality has not been definitely proven, studies that have assessed insulin resistance and glucose tolerance after continuous positive airway pressure (CPAP) treatment of OSA (34) have provided some evidence suggesting that OSA may cause insulin resistance. Intermittent hypoxia, high sympathetic output, sleep fragmentation and sleep loss, dysregulation of the hypothalamo-pituitary axis, endothelial dysfunction, and alterations in cytokine and adipokine release have all been proposed as potential mechanisms linking OSA to insulin resistance and glucose intolerance in non-PCOS populations (6,7). We found that the degree of sleep fragmentation, rather than hypoxia related to OSA, was an independent predictor of insulin resistance and glucose responses to an oral glucose challenge in PCOS women. This observation is consistent with our recent studies in young healthy adults in which experimental induction of sleep fragmentation, similar to that seen in OSA, resulted in deterioration of insulin sensitivity and glucose tolerance (35).
In summary, OSA is highly prevalent in women with PCOS and is a major determinant of insulin resistance and glucose intolerance among these women. Our findings support the existence of two sub-phenotypes of women with PCOS, those with and those without OSA, in which the presence of OSA is associated with a stronger risk for development of type 2 diabetes. Because the vast majority of PCOS women in our study were obese, our findings will need replication in a sample of PCOS women with normal body weight. In addition, the results of this study stress the importance of systematic identification and treatment of OSA in the management of PCOS patients.
Footnotes
This work was supported by grants from the National Institutes of Health (M01-RR-00055, PO1-AG11412, RO1-HL-075079, P50-HD057796, and P60-DK20595) and a gift from the Blum-Kovler Foundation. ClinicalTrials.gov identifier is NCT00203996.
Disclosure statement: E.T., E.V.C., L.H., and D.A.E. having nothing to declare.
First Published Online July 22, 2008
Abbreviations: AHI, Apnea-hypopnea index; AUC, area under the curve; BMI, body mass index; CV, coefficient of variation; HOMA, homeostasis model assessment; OGTT, oral glucose tolerance test; OSA, obstructive sleep apnea; PCOS, polycystic ovary syndrome.
References
- Ehrmann DA 2005 Medical progress: polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal K, Futterweit W, Dobrjansky A 1989 Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38:1165–1174 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J 1999 Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22:141–146 [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A 1999 Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- Sanders M 2005 Sleep breathing disorders. In: Kryger M, Roth T, Dement WC, eds. Principles and practice of sleep medicine. Philadelphia: WB Saunders; 969–1157 [Google Scholar]
- Tasali E, Ip MS 2008 Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc 5:207–217 [DOI] [PubMed] [Google Scholar]
- Tasali E, Mokhlesi B, Van Cauter E 2008 Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 133:496–506 [DOI] [PubMed] [Google Scholar]
- Seicean S, Kirchner HL, Gottlieb DJ, Punjabi NM, Resnick H, Sanders M, Budhiraja R, Singer M, Redline S 2008 Sleep disordered breathing and impaired glucose metabolism in normal weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care 31:1001–1006 [DOI] [PubMed] [Google Scholar]
- Schahin S, Nechanitzky T, Dittel C, Fuchs FS, Hahn EG, Konturek PC, Ficker JH, Harsch IA 2008 Long-term improvement of insulin sensitivity during CPAP therapy in the obstructive sleep apnoea syndrome. Med Sci Monit 14:CR117–CR121 [PubMed] [Google Scholar]
- West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR 2007 The effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 62:969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsch IA, Schahin SP, Radespiel-Tröger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, Ficker JH 2004 Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 169:156–162 [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP 2001 Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 86:1175–1180 [DOI] [PubMed] [Google Scholar]
- Gopal M, Duntley S, Uhles M, Attarian H 2002 The role of obesity in the increased prevalence of obstructive sleep apnea syndrome in patients with polycystic ovarian syndrome. Sleep Med 3:401–404 [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Legro R, Bixler EO, Grayev A, Kales A, Chrousos GP 2001 Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab: 517–520 [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A 1968 A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute [Google Scholar]
- 1992 EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15:173–184 [PubMed] [Google Scholar]
- 1999 Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–689 [PubMed] [Google Scholar]
- 1997 Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- VanHelder T, Symons JD, Radomski MW 1993 Effects of sleep deprivation and exercise on glucose tolerance. Aviat Space Environ Med 64:487–492 [PubMed] [Google Scholar]
- Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN 2005 Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:66–71 [DOI] [PubMed] [Google Scholar]
- Dunaif A 1997 Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE 2004 Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 160:521–530 [DOI] [PubMed] [Google Scholar]
- Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL 2002 Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165:677–682 [DOI] [PubMed] [Google Scholar]
- Sulit L, Storfer-Isser A, Kirchner HL, Redline S 2006 Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep 29:777–783 [DOI] [PubMed] [Google Scholar]
- Daltro C, Gregorio PB, Alves E, Abreu M, Bomfim D, Chicourel MH, Araújo L, Cotrim HP 2007 Prevalence and severity of sleep apnea in a group of morbidly obese patients. Obes Surg 17:809–814 [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A 1994 Sleep apnea and sleep disruption in obese patients. Arch Intern Med 154:1705–1711 [PubMed] [Google Scholar]
- Subramanian S, Desai A, Joshipura M, Surani S 2007 Practice patterns of screening for sleep apnea in physicians treating PCOS patients. Sleep Breath 11:233–237 [DOI] [PubMed] [Google Scholar]
- Marin JM, Carrizo SJ, Vicente E, Agusti AG 2005 Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053 [DOI] [PubMed] [Google Scholar]
- Peppard P, Young T, Palta M, Dempsey J, Skatrud J 2000 Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 284:3015–3021 [DOI] [PubMed] [Google Scholar]
- Yaggi HK, Araujo AB, McKinlay JB 2006 Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 29:657–661 [DOI] [PubMed] [Google Scholar]
- Liu K, Yee B, Philips C, Grunstein RR 2007 Sleep apnea and neuroendocrine function. Sleep Med Clin 2:225–236 [Google Scholar]
- Tasali E, Van Cauter E, Ehrmann D 2008 Polycystic ovary syndrome and obstructive sleep apnea. Sleep Med Clin 3:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T 2005 Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med 165:447–452 [DOI] [PubMed] [Google Scholar]
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E 2008 Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 105:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]