Abstract
Context: Sex-steroid hormones amplify pulsatile GH secretion by unknown mechanisms. Ghrelin is the most potent natural GH secretagogue discovered to date. A plausible unifying postulate is that estradiol (E2) enhances hypothalamo-pituitary sensitivity to ghrelin (a physiological effect). The hypothesis is relevant to understanding the basis of hyposomatotropism in aging and other relatively hypogonadal states.
Objective: Our objective was to test the hypothesis that E2 supplementation potentiates ghrelin’s stimulation of pulsatile GH secretion.
Setting: The study was conducted at an academic medical center.
Subjects: Healthy postmenopausal women (n = 20) were included in the study.
Interventions: Separate-day iv infusions of saline vs. five graded doses of ghrelin were performed in volunteers prospectively randomly assigned to receive (n = 8) or not receive (n = 12) transdermal E2 for 21 d were performed.
Measures: GH secretion was estimated by deconvolution analysis and abdominal visceral fat mass determined by computerized axial tomography were calculated.
Results: E2 supplementation augmented ghrelin’s stimulation of basal (nonpulsatile) GH secretion by 3.6-fold (P = 0.022), increased GH responses to low-dose ghrelin by 2.9-fold (P = 0.035), did not alter ghrelin efficacy, and elicited more regular patterns of acylated ghrelin concentrations during saline infusion (P = 0.033). Abdominal visceral fat negatively determined responses to ghrelin (R = −0.346; P < 0.005).
Conclusions: Transdermal E2 supplementation potentiates GH secretion stimulated by physiological but not pharmacological concentrations of acylated ghrelin, and concomitantly regularizes patterns of bioactive ghrelin secretion in postmenopausal women. Accordingly, the estrogen milieu appears to control sensitivity of the hypothalamopituitary unit to acylated ghrelin.
Transdermal estradiol supplementation augments hypothalamo-pituitary sensitivity to physiological amounts of acylated ghrelin in postmenopausal women, a finding that may help devise novel secretagogues to obviate hyposomatotropism with aging and hypogonadal individuals.
Aging is associated with a progressive decline in GH and IGF-I availability (1,2,3). Healthy older individuals with relative hyposomatotropism and patients with pathological GH deficiency share a similar physical phenotype marked by osteopenia, sarcopenia, visceral adiposity, insulin resistance, hyperlipidemia, increased risk of cardiovascular disease, and diminished quality of life. Interventional studies indicate that GH-driven hepatic IGF-I production is preserved in aging humans (4). Indeed, administration of GH or its secretagogues in older adults for intervals of 1–6 months elevates GH and IGF-I concentrations, increases lean body mass, and decreases intra-abdominal adiposity (5,6,7). Nonetheless, the primary mechanistic bases of diminished GH secretion in aging and hypogonadal adults remain unknown.
Clinical investigations demonstrate that older individuals secrete less GH in each burst but exhibit an unchanged GH pulse frequency, half-life, and basal (nonpulsatile) secretion rate (8,9). The mass of GH secreted in each burst is determined by IGF-I, T4, glucocorticoids, free fatty acids, sex steroids, GHRH, somatostatin, and the GH-releasing peptide (GHRP), ghrelin (10,11,12,13,14,15). Estradiol (E2), GHRH, and ghrelin stimulate, whereas somatostatin inhibits, pulsatile GH secretion. Exposure to E2 increases the stimulatory potency of GHRH (16) and decreases the inhibitory potency of somatostatin (17) without affecting the efficacy of either peptide.
How sex steroids regulate the actions of ghrelin or synthetic GHRPs is controversial. Testosterone supplementation potentiated pharmacological GHRP stimulation in prepubertal boys but not older men (18,19), and E2 did so in both prepubertal girls and older women (18,20). E2 replacement in postmenopausal women amplified the effect of a single submaximal but not maximal ghrelin stimulus (21,22). In addition, gender in adults did not affect the GH response to a single maximally effective dose of ghrelin (23). Disparate outcomes might be harmonized under the postulate that different putative receptor subtypes mediate GH responses to synthetic GHRP’s vis-a-vis native ghrelin and/or that sex steroids control dose-varying effects of this class of secretagogues. In the latter context, physiological rather than pharmacological effects of ghrelin would be more relevant to explicating age-related decrements in pulsatile GH secretion. Accordingly, the present investigation tests the unifying hypothesis that E2 enhances hypothalamo-pituitary sensitivity to, but not the efficacy (maximal effect) of, graded ghrelin stimulation.
Subjects and Methods
Subjects
The protocol was approved by the Mayo Institutional Review Board and reviewed by the U.S. Food and Drug Administration. The study was performed according the Helsinki declaration. Subjects were informed that information would be gathered for research purposes only.
Subjects were recruited through Institutional Review Board-approved newspaper ads, bulletins, institutional and community flyers and posters, and the Clinical Trials Center web page. Before participation, volunteers underwent medical screening as outpatients, including history and physical examination. Inclusion criteria were normal hepatic, renal, hematological, metabolic, and endocrine function [normal TSH, prolactin, and SHBG with an FSH concentration >30 IU/liter and E2 <10 pg/ml (36.7 pmol/liter)]. Reasons for exclusion were concurrent use of neuroactive medications or sex hormones, diabetes mellitus, untreated T4 deficiency, systemic disease, abnormal baseline biochemical data, drug or alcohol abuse, hemoglobin less than 11.6 g/dl, history of thrombotic arterial disease (stroke, transient ischemic attack, myocardial infarction, angina) or thrombophlebitis, history or suspicion of breast cancer, and unwillingness to provide written informed consent.
Protocol
Participants were admitted to the Clinical Research Unit (CRU) at 1730 h twice for overnight study at least 72 h apart. The design was a prospectively randomized double-masked placebo-controlled and cross-over infusion of ghrelin or saline. Volunteers were randomized to either receive (n = 8) or not receive (n = 12) E2 supplementation for 21 d. Cannulae were inserted in contralateral antecubital veins to allow combined sampling and peptide/saline infusions. Blood was sampled for 15.5 h beginning at 2200 h overnight for GH (every 10 min) and ghrelin (every 20 min) measurements. Saline was infused at a constant rate (20 ml/h) overnight for 8 h. At 0600 h, blood samples were withdrawn for later assay of E2, LH, FSH, IGF-I, IGF binding protein (IGFBP)-1, IGFBP-3, and SHBG. Immediately thereafter, saline or graded ghrelin doses were infused for the next 7.5 h. Subjects remained recumbent during each session except for lavatory visits. No daytime naps were allowed. Lunch was provided before discharge from the CRU.
Investigational drugs
E2 supplementation
A transdermal E2 repletion schedule was used to mimic E2 concentrations attained in the young-adult late follicular phase. The schedule comprised daily E2 patches at a starting dose of 0.05 mg (d 1), increased on d 6 to 0.1 mg, and on d 12 to 0.15 mg. The last dose was continued until d 21. Medroxyprogesterone was given orally (10 mg daily) for 12 d beginning on d 22.
Ghrelin infusion
The full-length acylated peptide was synthesized by PolyPeptide Laboratories, Inc. (Torrance, CA) or Actif Laboratories (Cincinnati, OH). Ghrelin was dissolved in 5% mannitol in sterile water at a concentration of 100 μg/ml in the Mayo Research Pharmacy, placed in sterile 5-ml glass vials, frozen and stored at −20 C. Aliquots were thawed once immediately before use. Infusion doses were saline 10 ml/h alone or combined with 0.15 μg/kg·h ghrelin, which was doubled every 90 min four times in succession to yield a five-step schedule and a final rate of 2.4 μg/kg·h.
Diet
To limit nutritional confounds, a standardized meal (8 kcal/kg 50% carbohydrate, 20% protein, and 30% fat) was served at 1800 h the night before infusions. Subjects then remained fasting, alcohol abstinent, and caffeine free until 1330 h the next day.
Body composition measurements
Volunteers had a single-slice computerized tomogram (CT) of the abdomen at L3–4 to quantify sc and visceral adipose tissue, as described earlier (24).
Hormone assays
GH concentrations were determined in duplicate by two-site immunoenzymatic chemiluminescence assay performed on the DxI automated system (Beckman Instruments, Chaska, MN), as described (22). E2 was measured by liquid-chromatography tandem mass spectrometry.
IGFBP-1, IGFBP-3, and total IGF-I were measured by immunoradiometric assays (Diagnostic Systems Laboratories, Webster, TX) and acylated ghrelin by competitive RIA (LINCO Research, Inc./Millipore Research, St. Charles, MO), as reported (22,25).
Calculations and statistics
Deconvolution analysis
The entire GH concentration time series (total 15.5 h) collected at night (2200–0600 h) and the next morning (0600–1330 h) was analyzed together using a recently developed deconvolution method (26). The automated MATLAB program (The MathWorks, Inc., Natick, MA) first detrends the data and normalizes concentrations to the unit interval (zero, one) (27). Second, successive potential pulse-time sets, each containing one fewer burst, are created by a smoothing process (a nonlinear adaptation of the heat-diffusion equation). Third, a maximum-likelihood expectation deconvolution method estimates all secretion and elimination rates simultaneously for each candidate pulse-time set. The parameters (and units) are frequency (number of bursts per 24 h, λ of Weibull distribution), regularity of interpulse intervals (unitless γ of Weibull), fast and slow half-lives (min), basal and pulsatile secretion rates (concentration units per 24 h), mass secreted per burst (concentration units), and waveform [time delay to maximal secretion after burst onset mode of waveform (min)] (26,27).
GH secretion rates during ghrelin and saline infusions (0600–1330 h) were also analyzed via PULSE, a waveform-independent deconvolution method. This nonparametric technique requires a priori specification of hormone half-life, which was first estimated via the MATLAB deconvolution program.
Approximate entropy (ApEn) analysis
ApEn (1, 20%) was used as a scale- and model-independent regularity statistic to quantify the orderliness of GH release (28). Higher ApEn denotes greater disorderliness of the secretion process.
Statistics
Analyses were performed using Systat software (version 11; Systat Software, Inc., San Jose, CA).
Baseline hormone concentrations in the study groups receiving (n = 8) and not receiving (n = 12) E2 supplementation were compared using an unpaired Student’s t test. Two-way ANOVA in a repeated-measures design was applied to test the effects of E2 and ghrelin on GH concentrations. Means were contrasted post hoc using Tukey’s honestly significantly different test (29). Significance was construed for P < 0.05. Data are presented as the mean ± sem.
Linear regression analysis was used to relate the dependent variable, GH secretion rate (mean over 90-min segments for each of the five ghrelin infusion rates), to the independent variable, abdominal visceral fat. P values for each 90-min segment were transformed to their natural logarithms, the sum taken and multiplied by −2. The result is a χ2 statistic of N-1 df.
Results
There were 20 healthy postmenopausal women (body mass index 25.4 ± 0.9 kg/m2 and age 60.6 ± 2.0 yr) enrolled. Body mass index and age in the two groups did not differ (12 not receiving E2 and eight receiving E2 supplementation). Abdominal visceral fat cross-sectional areas on CT also were not significantly different. Baseline screening hormone concentrations were similar (Table 1).
Table 1.
Outpatient baseline screening hormone measurements
| Hormone | Randomization
|
|
|---|---|---|
| No E2 (n = 12) | E2 (n = 8) | |
| E2 (pg/ml) | <10 | <10 |
| SHBG (nmol/liter) | 55 ± 7.2 | 50 ± 9.1 |
| LH (IU/liter) | 32 ± 3.8 | 36 ± 5.3 |
| FSH (IU/liter) | 55 ± 7.2 | 50 ± 9.1 |
| Prolactin (μg/liter) | 7.4 ± 0.81 | 9.3 ± 0.92 |
| IGF-I (μg/liter) | 133 ± 13 | 127 ± 1.5 |
Samples were obtained at screening before any E2 supplementation. There were no significant baseline hormone differences (0.17 < P < 0.86) by unpaired Student’s t tests. Data are the mean ± sem.
Preinfusion hormone measurements
Table 2 summarizes mean preinfusion concentrations of E2, LH, FSH, total IGF-I, IGFBP-1, IGFBP-3, and SHBG obtained at 0600 h in the CRU after 18–21 d E2 or no E2 administration. E2 and SHBG concentrations were significantly higher, and FSH and LH concentrations lower, in women receiving E2.
Table 2.
Inpatient preinfusion hormone measurements
| Hormone | Randomization
|
P value E2vs. No E2 | |
|---|---|---|---|
| No E2 (n = 12) | E2 (n = 8) | ||
| E2 (pg/ml) | 16 ± 1.8 | 215 ± 60 | <0.001 |
| SHBG (nmol/liter) | 47 ± 6.1 | 85 ± 19 | 0.043 |
| LH (IU/liter) | 26 ± 4.0 | 13 ± 2.1 | 0.017 |
| FSH (IU/liter) | 74 ± 5.7 | 30 ± 8.4 | 0.007 |
| IGF-I (μg/liter) | 110 ± 6.4 | 90 ± 16 | NS |
| IGFBP-1 (μg/liter) | 34 ± 5.1 | 23 ± 2.7 | NS |
| IGFBP-3 (mg/liter) | 4.1 ± 0.2 | 3.8 ± 0.34 | NS |
Samples were obtained before ghrelin or saline infusion after 3 wk E2 or no E2exposure. P values were determined by an unpaired Student’s t test. Data are the mean ± sem. NS, P > 0.05.
Overnight unstimulated GH profiles
Overnight saline-infused GH concentration time series are shown in Fig. 1. Means were 0.67 ± 0.10 μg/liter and 0.98 ± 0.18 μg/liter in the no E2 and E2 groups, respectively (P < 0.001 for treatment and time effects).
Figure 1.
Mean (± sem) nocturnal serum GH-concentration time series in postmenopausal women receiving (-•- n = 8) and not receiving (-○- n = 12) transdermal E2 supplementation for 18–21 d. Data reflect blood sampling every 10 min for 8 h fasting beginning at 2200 h. Statistical estimates are by two-way ANOVA with repeated measures. MN, Midnight.
Effect of graded ghrelin infusion on acylated ghrelin concentrations and GH secretion
Ghrelin infusions increased plasma acylated ghrelin concentrations (mean over 90 min) linearly and comparably in the two study groups (each P < 0.001). Maximal acylated ghrelin concentrations reached 1451 ± 244 (no E2) and 1560 ± 277 pg/ml (E2) (P > 0.6). Values remained low and similar in the two cohorts during saline infusion viz., 97 ± 12 (no E2) vs. 121 ± 26 pg/ml (E2).
Ghrelin infusions significantly increased GH concentrations (Fig. 2) and GH secretion (Fig. 3A) in both cohorts (P < 0.001 effect for each). In the absence of supplemental E2, ghrelin stimulated basal, pulsatile, and total GH secretion by 2.6-fold (P < 0.05), 3.3-fold (P < 0.001), and 3.2-fold (P < 0.001), respectively. During E2 supplementation, ghrelin elevated GH secretion over no E2/saline control values by 6.7-fold (basal, P = 0.008), 2-fold (pulsatile, P = 0.029), and 2.5-fold (total, P = 0.008). E2 selectively enhanced ghrelin-driven basal GH secretion by 3.6-fold (P = 0.022) (Fig. 3B).
Figure 2.
GH concentration profiles obtained by sampling blood every 10 min for 7.5 h during iv infusion of saline (circles) or ghrelin (triangles) in 20 healthy postmenopausal women. Saline or ghrelin infusions were started at 0600 h at a dose of 0.15 μg/kg·h. The dose was doubled every 90 min (arrows and times) a total of four times (five doses in total, highest dose 2.4 μg/kg·h). Subjects received (n = 8) or did not receive E2 (n = 12) for 18–21 d. Data are the mean ± sem.
Figure 3.
A, Basal, pulsatile, and total GH secretion during 7.5 h graded ghrelin vs. saline infusion in postmenopausal women given or not given E2 as indicated. P values were determined via Tukey’s post hoc test after two-way ANOVA. B, Impact of ghrelin vs. saline infusion on basal (nonpulsatile) GH secretion in individual postmenopausal women not receiving (n = 12, left graph) and receiving (n = 8, right graph) E2 supplementation. P values reflect the paired Student’s t test.
Mean GH-concentration plots over the first 90 min of saline and lowest-dose ghrelin (0.15 μg/kg·h) infusions are shown in Fig. 4A. By two-way ANOVA and post hoc Tukey’s test, ghrelin elevated GH concentrations 1.9-fold more in the E2-supplemented than E2-deficient group at 50, 60, and 70 min (0.05 > P > 0.017). Similarly, total 90-min GH secretion was 2.0-fold higher in women receiving than not receiving E2, viz., 19 ± 3.3 vs. 9.7 ± 2.1 μg/liter·90 min (P = 0.026). In contrast, E2 did not augment ghrelin efficacy because maximal GH concentrations attained at the highest ghrelin infusion rate did not differ in the two cohorts (Fig. 4B).
Figure 4.
A, Mean GH concentration time series in postmenopausal women receiving E2 (-•- saline and -▾- ghrelin, n = 8) and not receiving E2 (-○- saline and –▿- ghrelin, n = 12). Data reflect 90-min infusions of saline vs. ghrelin 0.15 μg/kg·h beginning at 0600 h to test hypothalamo-pituitary sensitivity to ghrelin. *P < 0.02 for E2/ghrelin vs. no E2/ghrelin, and #P < 0.006 for E2/ghrelin vs. E2 /saline by Tukey’s post hoc test after two-way ANOVA. B, Comparable data obtained during 90-min infusions of saline vs. ghrelin 2.4 μg/kg·h to test the influence of E2 on ghrelin efficacy. Capital letters (A, B, and C) denote P < 0.01 via two-way ANOVA.
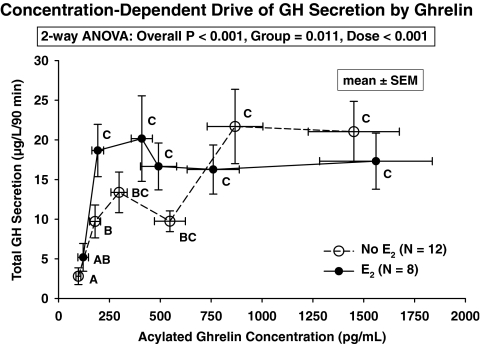
To compare hypothalamo-pituitary sensitivity with ghrelin, GH concentrations (dependent variable) were regressed on time (0–60 min, independent variable) during infusion of the lowest dose of ghrelin (0.15 μg/kg·h). E2 compared with no E2 exposure elevated the slope of this regression by 2.9-fold (viz., E2 0.359 ± 0.10 vs. no E2 0.125 ± 0.05 slope units; P = 0.035). To verify estrogen’s augmentation of ghrelin-stimulated GH secretion, the latter was related to acylated ghrelin concentrations (90-min means of each) (Fig. 5). Two-way ANOVA yielded strong effects of both E2 treatment (P = 0.011) and acylated ghrelin concentrations (P < 0.001) on GH secretion. Estrogenic potentiation was verified at low but not high concentrations of acyl ghrelin.
Figure 5.
Relationship between total (pulsatile plus basal) GH secretion and (90-min mean) plasma acylated ghrelin concentrations without (placebo) and with estrogen supplementation (E2). Two-way ANOVA revealed strong overall effects of time (P < 0.001) and E2 status (P = 0.011). E2 potentiated GH secretion under low but not pharmacological acylated ghrelin concentrations. Data are the mean ± sem. Unshared alphabetical superscripts denote significantly different means.
ApEn analysis corroborated the capability of E2 to increase irregularity (decrease orderliness) of GH concentrations during saline infusion (higher ApEn than after placebo transdermal patch; P = 0.015). In contrast, E2 decreased irregularity of acylated ghrelin profiles during saline infusion, denoting a more orderly release process (P = 0.033) (Fig. 6).
Figure 6.
E2 increases irregularity (higher ApEn) of GH secretion patterns (left) but decreases irregularity (enhances orderliness, lower ApEn) of acylated ghrelin release during saline infusion (right). Data are the mean ± sem. Contrasts were evaluated by an unpaired two-tailed Student’s t test.
Linear regression analysis
Linear regression analysis in the combined cohorts (n = 20 subjects) was used to relate acylated ghrelin-stimulated GH-secretion rates to abdominal visceral fat mass estimated by CT. Regression was performed independently at each ghrelin dose to obviate multiple colinearity. Individual P values were aggregated into a χ2 statistic of 19.5 (5 df) from −2 ln Pi (i = 1, 6; P < 0.005). The median R value was −0.346.
Side effects of ghrelin infusion were limited to flushing and facial warmth in one woman.
Discussion
The present clinical investigation used multistep ghrelin infusions to unveil that estrogen supplementation enhances hypothalamo-pituitary sensitivity to ghrelin without altering ghrelin efficacy, and imposes more regular patterns of acylated ghrelin release. The distinction between estrogenic potentiation of physiological (sensitivity) but not pharmacological (efficacy) actions of ghrelin may help explain numerous earlier discrepant reports, which used single maximal doses of ghrelin and/or made no gender comparisons when using several ghrelin doses (21,22,30). One study that used a single maximally stimulatory amount of ghrelin (1 μg/kg bolus) identified no sex difference in peak GH responses (21). This result would be expected if E2 facilitates submaximal rather than maximal effects of this GH secretagogue, as inferred here. Because E2 can also amplify submaximal stimulation of GH secretion by GHRH (16) and mute submaximal inhibition by somatostatin (17), we postulate that estrogen may sensitize hypothalamo-pituitary responses to ghrelin via these effects. Whether E2 also augments somatotrope synthesis is not known.
The capability of E2 to enforce more regular (orderly) patterns of acylated ghrelin secretion was unexpected. In contrast, E2 is known to reduce regularity of GH secretion patterns, putatively by impairing negative feedback on GH production (9). The opposite response of ghrelin and GH patterns to E2 suggests that estrogen enhances rather than reduces feedback regulation of ghrelin release. How age and other modes of estrogen replacement influence the effect on ghrelin secretion has not been defined (31,32). The mechanism by which feedback occurs is not known but could involve vagal inputs to the stomach (33).
Infusion of ghrelin stimulated basal (nonpulsatile) GH secretion, and E2 enhanced this effect. The mechanisms that control nonpulsatile (constitutive) GH release are not known (10). However, an important component of ghrelin-receptor signaling is constitutive. Gene mutations that impair such signaling have been associated with short stature (15). Our finding that E2 supplementation elevates ghrelin-induced nonpulsatile GH secretion would be consistent with up-regulation of constitutive ghrelin-receptor activity.
Regression analysis revealed that GH secretion under ghrelin drive correlated negatively with abdominal visceral fat across the five infusion rates. Median R = −0.346 (R2 = 0.12) indicates that additional factors also determine the variability in stimulated GH secretion. Potential factors include age, physical fitness, free fatty acid availability, and nonestrogenic steroids (11). From a mechanistic vantage, estrogen’s enhancement of GH secretion in the face of unchanged total IGF-I, IGFBP-1, and IGFBP-3 concentrations suggests sensitization of hypothalamo-pituitary responses to ghrelin at fixed IGF-I availability (3,9,34).
In summary, transdermal E2 supplementation augments hypothalamo-pituitary sensitivity to physiological amounts of acylated ghrelin in postmenopausal women. Estrogen may enhance physiological ghrelin action by influencing comodulators like GHRH and somatostatin, and/or by up-regulating basal ghrelin-receptor gene expression. Insights gained here may aid in devising novel secretagogue-based strategies to obviate hyposomatotropism in aging and hypogonadal individuals.
Acknowledgments
We thank Kay Nevinger and Donna Scott for support of manuscript preparation, Ashley Bryant for graphical presentations, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
Footnotes
This work was supported in part via the Clinical Translational Research-Center Grant UL 1 RR024150 to Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD), R01 AG29362 and R01 AG19695 from the National Institutes of Health (Bethesda, MD), and The Netherlands Organization for Scientific Research (The Hague, The Netherlands).
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 8, 2008
Abbreviations: ApEn, Approximate entropy; CRU, Clinical Research Unit; CT, computerized tomogram; E2, estradiol; GHRP, GH-releasing peptide; IGFBP, IGF binding protein.
References
- Finkelstein JW, Roffwarg HP, Boyar RM, Kream J, Hellman L 1972 Age-related change in the twenty-four-hour spontaneous secretion of growth hormone. J Clin Endocrinol Metab 35:665–670 [DOI] [PubMed] [Google Scholar]
- Weltman A, Weltman JY, Hartman ML, Abbott RD, Rogol AD, Evans WS, Veldhuis JD 1994 Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. J Clin Endocrinol Metab 78:543–548 [DOI] [PubMed] [Google Scholar]
- Lieman HJ, Adel TE, Forst C, von Hagen S, Santoro N 2001 Effects of aging and estradiol supplementation on GH axis dynamics in women. J Clin Endocrinol Metab 86:3918–3923 [DOI] [PubMed] [Google Scholar]
- Lissett CA, Shalet SM 2003 The insulin-like growth factor-I generation test: peripheral responsiveness to growth hormone is not decreased with ageing. Clin Endocrinol (Oxf) 58:238–245 [DOI] [PubMed] [Google Scholar]
- Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis JD 2004 Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. J Clin Endocrinol Metab 89:2290–2300 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Patrie J, Frick K, Weltman JY, Weltman AL 2005 Administration of recombinant human GHRH-1,44-amide for three months reduces abdominal visceral fat mass and increases physical-performance measures in postmenopausal women. Eur J Endocrinol 153:669–677 [DOI] [PubMed] [Google Scholar]
- Harman SM, Blackman MR 2003 The effects of growth hormone and sex steroid on lean body mass, fat mass, muscle strength, cardiovascular endurance and adverse events in healthy elderly women and men. Horm Res 60:121–124 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Liem AY, South S, Weltman A, Weltman J, Clemmons DA, Abbott R, Mulligan T, Johnson ML, Pincus SM, Straume M, Iranmanesh A 1995 Differential impact of age, sex-steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab 80:3209–3222 [DOI] [PubMed] [Google Scholar]
- Shah N, Evans WS, Veldhuis JD 1999 Actions of estrogen on the pulsatile, nyctohemeral, and entropic modes of growth hormone secretion. Am J Physiol 276(5 Pt 2):R1351–R1358 [DOI] [PubMed] [Google Scholar]
- Giustina A, Veldhuis JD 1998 Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY 2006 Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27:101–140 [DOI] [PubMed] [Google Scholar]
- Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M 2001 Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest 107:1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I 2002 Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest 109:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE 1993 GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet 4:227–232 [DOI] [PubMed] [Google Scholar]
- Pantel J, Legendre M, Cabrol S, Hilal L, Hajaji Y, Morisset S, Nivot S, Vie-Luton MP, Grouselle D, de Kerdanet M, Kadiri A, Epelbaum J, Le Bouc Y, Amselem S 2006 Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest 116:760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Evans WS, Bowers CY 2003 Estradiol supplementation enhances submaximal feedforward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. J Clin Endocrinol Metab 88:5484–5489 [DOI] [PubMed] [Google Scholar]
- Bray MJ, Vick TM, Shah N, Anderson SM, Rice LW, Iranmanesh A, Evans WS, Veldhuis JD 2001 Short-term estradiol replacement in postmenopausal women selectively mutes somatostatin’s dose-dependent inhibition of fasting growth hormone secretion. J Clin Endocrinol Metab 86:3143–3149 [DOI] [PubMed] [Google Scholar]
- Loche S, Colao A, Cappa M, Bellone J, Aimaretti G, Farello G, Faedda A, Lombardi G, Deghenghi R, Ghigo E 1997 The growth hormone response to hexarelin in children: reproducibility and effect of sex steroids. J Clin Endocrinol Metab 82:861–864 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Mielke K, Miles JM, Bowers CY 2005 Testosterone supplementation in healthy older men drives GH and IGF-I secretion without potentiating peptidyl secretagogue efficacy. Eur J Endocrinol 153:577–586 [DOI] [PubMed] [Google Scholar]
- Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD 2001 Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab 86:551–560 [DOI] [PubMed] [Google Scholar]
- Arvat E, Gianotti L, Broglio F, Maccagno B, Bertagna A, Deghenghi R, Camanni F, Ghigo E 1997 Oestrogen replacement does not restore the reduced GH-releasing activity of Hexarelin, a synthetic hexapeptide, in post-menopausal women. Eur J Endocrinol 136:483–487 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Iranmanesh A, Mielke K, Miles JM, Bowers CY 2006 Estradiol potentiates ghrelin-stimulated pulsatile growth hormone secretion in postmenopausal women. J Clin Endocrinol Metab 91:3559–3565 [DOI] [PubMed] [Google Scholar]
- Broglio F, Benso A, Castiglioni C, Gottero C, Prodam F, Destefanis S, Gauna C, van der Lely AJ, Deghenghi R, Bo M, Arvat E, Ghigo E 2003 The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab 88:1537–1542 [DOI] [PubMed] [Google Scholar]
- Erickson D, Keenan DM, Farhy LS, Mielke K, Bowers CY, Veldhuis JD 2005 Determinants of dual secretagogue drive of burst-like growth hormone secretion in premenopausal women studied under a selective estradiol clamp. J Clin Endocrinol Metab 90:1741–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Mielke K, Miles JM, Carpenter PC, Bowers CY 2006 Ghrelin potentiates growth hormone secretion driven by putative somatostatin withdrawal and resists inhibition by human corticotropin-releasing hormone. J Clin Endocrinol Metab 91:2441–2446 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD 2003 Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol Regul Integr Comp Physiol 285:R664–R673 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Chattopadhyay S, Veldhuis JD 2005 Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol 236:242–255 [DOI] [PubMed] [Google Scholar]
- Pincus SM 2000 Irregularity and asynchrony in biologic network signals. Methods Enzymol 321:149–182 [DOI] [PubMed] [Google Scholar]
- Zar JH 1996 Biostatistical analysis. 3rd ed. Upper Saddle River, NJ: Prentice Hall [Google Scholar]
- Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E 2001 Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab 86:1169–1174 [DOI] [PubMed] [Google Scholar]
- Kellokoski E, Poykko SM, Karjalainen AH, Ukkola O, Heikkinen J, Kesaniemi YA, Horkko S 2005 Estrogen replacement therapy increases plasma ghrelin levels. J Clin Endocrinol Metab 90:2954–2963 [DOI] [PubMed] [Google Scholar]
- Akamizu T, Murayama T, Teramukai S, Miura K, Bando I, Irako T, Iwakura H, Ariyasu H, Hosoda H, Tada H, Matsuyama A, Kojima S, Wada T, Wakatsuki Y, Matsubayashi K, Kawakita T, Shimizu A, Fukushima M, Yokode M, Kangawa K 2006 Plasma ghrelin levels in healthy elderly volunteers: the levels of acylated ghrelin in elderly females correlate positively with serum IGF-I levels and bowel movement frequency and negatively with systolic blood pressure. J Endocrinol 188:333–344 [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K 2005 Ghrelin: structure and function. Physiol Rev 85:495–522 [DOI] [PubMed] [Google Scholar]
- Lissett CA, Shalet SM 2003 The impact of dose and route of estrogen administration on the somatotropic axis in normal women. J Clin Endocrinol Metab 88:4668–4672 [DOI] [PubMed] [Google Scholar]