Abstract
Background and purpose:
Isoform-specific ion channel blockers are useful for target validation in drug discovery and can provide the basis for new therapeutic agents and aid in determination of physiological functions of ion channels. The aim of this study was to generate a specific blocker of human TRPM3 channels as a tool to help investigations of this member of the TRP cationic channel family.
Experimental approach:
A polyclonal antibody (TM3E3) was made to a conserved peptide of the third extracellular (E3) loop of TRPM3 and tested for binding and functional effect. Studies of channel activity were made by whole-cell planar patch-clamp and fura-2 intracellular Ca2+ measurement.
Key results:
Ionic current mediated by TRPM3 was inhibited partially by TM3E3 over a period of 5–10 min. Ca2+ entry in TRPM3-expressing cells was also partially inhibited by TM3E3 in a peptide-specific manner and independently of the type of agonist used to activate TRPM3. TM3E3 had no effect on TRPC5, TRPV4, TRPM2 or an endogenous ATP response.
Conclusions and implications:
The data show the successful development of a specific TRPM3 inhibitor and give further confidence in E3 targeting as an approach to producing isoform-specific ion channel blockers.
Keywords: calcium channel, cation channel, transient receptor potential, antibody
Introduction
Ion channels have many important functions throughout biology, including human physiology and disease (Hille, 2001). They are not confined to the control of electrical excitability and dysfunction but also influence secretion, cell migration, gene transcription and other cellular parameters. Chemicals that inhibit or activate ion channels have proved beneficial in a wide range of human conditions and many more opportunities exist for the development of medicines targeted to ion channels. A common obstacle to the development of these new medicines is lack of a blocking or activating agent specific to the ion channel under consideration. When such an agent has existed, it has been of great value in predicting what drugs acting at the ion channel could be useful for or what problems might arise with their administration. Specific agents often play pivotal roles in revealing functions of an ion channel in different tissues and disease contexts. Acutely acting agents are useful because they do not allow time for compensatory effects, such as upregulation of a gene with a similar function. Unfortunately, agents that are specific for one ion channel (encoded by one gene) are rare. Therefore, methods that lead to the rational design of isoform-specific agents need to be developed.
One approach to the production of isoform-specific ion channel tools is the generation of E3-targeted antibodies (Zhou et al., 1998; Xu et al., 2005b). This approach is based on the finding that ion channels such as the Shaker and KV1 type of K+ channel are formed from arrangements of four identical or similar proteins, each with intracellular N and C termini, six transmembrane segments (S1–S6) and three extracellular loops—E1, E2 and E3 between S1 and S2, S3 and S4, and S5 and S6 respectively. The amino acids forming the ion permeation pathway lie between E3 and S6. The E3 region is also sometimes referred to as the turret because, in crystal and predicted structures, it is a discrete small tower at the upper (extracellular) end of S5. In some cases the turret has been considered only as a structural feature that maintains the position of the ion selectivity filter. However, there is also evidence that it is the binding site for toxins with effects on specific ion channels or families of channels, and physiological regulation via turrets has become apparent (Beech and Sukumar, 2008).
We reported previously on successes with E3 targeting as a strategy for making isoform-specific ion channel tools (Xu et al., 2005b). The amino acid sequence of E3 is quite long and variable between different channels and is often not post-translationally modified. Through targeting the E3 region, there is scope for producing isoform-specific agents that not only bind to the channel but also disrupt its functions. Many ion channels have structures that resemble that of Shaker channels and so the approach could have wide applicability. One of the Shaker-like ion channel types is the transient receptor potential (TRP) cationic channel, encoded by 28 genes in mammals (Nilius et al., 2007). The functions and therapeutic value of many of them is unknown and specific pharmacological agents are mostly lacking. We previously had success with E3 targeting in the generation of specific blockers of canonical transient receptor potential 1 (TRPC1) and TRPC5 (Xu and Beech, 2001; Xu et al., 2005b, 2006, 2008) and another research group reported success with vanilloid transient receptor potential type 1 (TRPV1) channels (Klionsky et al., 2006).
Melastatin transient receptor potential type 3 (TRPM3) is a member of the M (melastatin) subtype of the TRP channel family (Grimm et al., 2003; Lee et al., 2003; Oberwinkler and Phillipp, 2007). It is not only expressed in the brain and kidney, but also in other tissues. In overexpression studies, it forms Ca2+-permeable, non-selective, cation channels and its activity is regulated by several different factors including sphingosine and pregnenolone sulphate (Grimm et al., 2003, 2005; Lee et al., 2003; Wagner et al., 2006). It is blocked by the chemical 2-aminoethoxydiphenylborate (Xu et al., 2005a) but this substance is not specific for TRPM3. Specific blockers and cellular functions of TRPM3 are unknown. Here we describe success in generating a TRPM3 blocker by targeting the E3 region of this protein.
Methods
Antibody preparation and use
Anti-TRPM3 antiserum (TM3E3) was custom-made in rabbit to the peptide CLFPNEEPSWKLAKN (Sigma-Genosys, Haverhill, UK), which is completely conserved in several species including human, mouse and rat. For functional assays TM3E3 was either used directly at 1:4000 dilution or after dialysis at 1:500 (or as specified in Figure 3b). Dialysis was performed in phosphate-buffered saline, exchanged 3 times, for 48 h at 4 °C, with gentle stirring. Anti-TRPC1 antiserum (T1E3) has been described (Xu and Beech, 2001). For immunoflourescence labelling, cells were allowed to adhere to coverslips and fixed in 2% paraformaldehyde for 5 min. Incubation in primary antibody at 1:1000 dilution was done overnight at 4 °C and in secondary antibody (goat anti-rabbit IgG-FITC) for 2 h at room temperature (21±2 °C). For control experiments, primary antibody was omitted or pre-absorbed to its antigenic peptide (10 μM). Images were sampled using a Zeiss confocal microscope controlled with Zeiss LSM imaging software.
Figure 3.
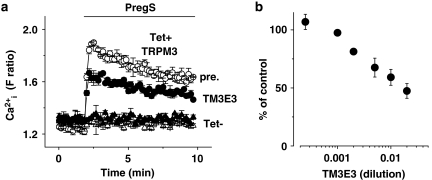
Inhibition of Ca2+ entry. Intracellular Ca2+ measurements from HEK 293 cells with (Tet+) and without (Tet−) induced expression of TRPM3. Responses to 25 μM pregnenolone sulphate (PregS) are shown. (a) Typical FlexStation experiment showing comparison of the effects of pre-incubation with dialysed TM3E3 or its pre-immune (pre.) serum (1:500 dilution). Typical of n/N=3/18. (b) Mean data showing block of the PregS response by a range of dilutions of dialysed TM3E3 antiserum, as a percentage of responses in dialysed pre-immune serum (n/N=3/18).
Cells expressing TRP channels
Human embryonic kidney (HEK) 293 cells were maintained in Dulbecco's modified Eagle's medium-F12+Glutamax-1 (Gibco, http://www.invitrogen.com) supplemented with 10% foetal calf serum and penicillin–streptomycin at 37 °C in a 5% CO2 incubator. Experiments described in Figure 4 were performed on HEK 293 cells transiently transfected with cDNA encoding human TRPM3-yellow fluorescent protein (YFP) or YFP using FuGENE 6 Transfection Reagent (Roche, Welwyn Garden City, UK). Functional studies were carried out 48 h after transfection and on successfully transfected cells, as identified by their YFP fluorescence. For the stable expression of human TRPM3 (LTRPC3; accession number AJ505026), TRPC5 (Zeng et al., 2004) or TRPM2 (McHugh et al., 2003), the T-REx expression system (Invitrogen, http://www.invitrogen.com) was employed. Tetracycline-inducible cell lines were maintained in the presence of 400 μg mL−1 zeocin and 5 μg mL−1 blasticidin S. To induce expression, cells were incubated with 1 μg mL−1 tetracycline (Sigma, Poole, UK) for 24–72 h prior to experiments. Non-induced cells without addition of tetracycline (Tet−) were used as control. Chinese hamster ovary (CHO) K1 cells stably expressing human TRPV4 were maintained in Ham's F12 (Gibco) in the presence of 1 mg mL−1 G418 (Sigma). Wild-type CHO cells were used as a control. Channel nomenclature in this article conforms to recommended guidelines (Alexander et al., 2008).
Figure 4.
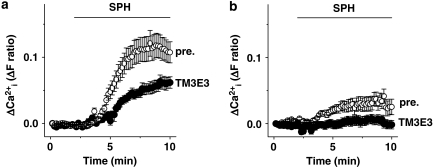
Inhibition of TRPM3 activated by a different agonist. Microscope-based intracellular Ca2+ measurements showing responses to 20 μM sphingosine (SPH). Comparisons are shown of the effects of pre-incubation with TM3E3 or its pre-immune (pre.) serum (1:4000 dilution) in HEK 293 cells transiently expressing (a) TRPM3-yellow fluorescent protein (YFP) (pre n/N=3/29, TM3E3 n/N=3/22), or (b) YFP alone (pre n/N=3/29, TM3E3 n/N=3/39).
Planar patch-clamp recording
Recordings were made at room temperature using the Patchliner planar patch-clamp system (Nanion, München, Germany) in whole-cell mode. Prior to recordings cells were detached from culture flasks with 0.05% trypsin/EDTA and resuspended at a density of 1 × 106–5 × 107 cells per mL. The bath (external) solution contained (mM): 145 NaCl, 3 KCl, 10 CsCl, 2 CaCl2, 2 MgCl2 10 HEPES and 10 glucose; titrated to pH 7.4 with NaOH. The intracellular recording solution contained (mM): 135 CsCl, 1 EGTA, 2 MgCl2 10 HEPES, 5 NaATP and 0.1 Na2GTP; titrated to pH 7.2 with CsOH. Corrections were not made for liquid–liquid junction potentials. Voltage ramps were applied from −100 to +100 mV for 1 s every 10 s from a holding potential of 0 mV. Currents were filtered at 1 kHz and sampled at 3 kHz.
Intracellular Ca2+ measurement
Cells were pre-incubated with fura-2 AM for 1 h at 37 °C followed by a 0.5 h wash at room temperature. Measurements were made at room temperature on a fluorescence microscope or a 96-well fluorescence plate reader (FlexStation II384, Molecular Devices, Sunnyvale, CA, USA). The inverted microscope (Zeiss, Welwyn Garden City, UK) was equipped with a 40 × Fluar oil-immersion objective (NA 1.3). Fura-2 dye was excited by light of 340 and 380 nm from a xenon arc lamp, the wavelength of which was selected by a monochromator (Till Photonics, Gräfelfing, Germany). Emitted light at 510 nm was collected via an emission filter and images captured every 10 s by an Orca-ER digital camera (Hamamatsu, Shizuoka, Japan). The same excitation wavelengths were used in the FlexStation, where wells within columns of the 96-well plate were loaded alternately for test and control conditions. For either method, the change (Δ) in intracellular calcium (Ca2+i) concentration is indicated as the ratio of fura-2 fluorescence (F) emission intensities for 340 and 380 nm (F ratio). The recording solution contained (mM): 130 NaCl, 5 KCl, 8 D-glucose, 10 HEPES, 1.5 CaCl2 and 1.2 MgCl2, titrated to pH 7.4 with NaOH.
Data analysis
Averaged data are presented as mean±s.e.mean. Data produced in pairs (test and control) were compared using t-tests, where statistical significance is indicated by *P<0.05 and no significant difference by NS. When there were more than two groups, data sets were first compared using ANOVA. For conventional Ca2+ imaging using the microscope-based system, n/N is the number of coverslips/total number of individual cells. For FlexStation experiments, n/N is the number of independent experiments/the number of individual wells of the 96-well plate.
Reagents
General salts, gadolinium chloride (Gd3+), hydrogen peroxide, 2-aminoethoxydiphenyl borate, pregnenolone sulphate and ATP were purchased from Sigma. D-erythro-sphingosine C18 was from Sigma or Matreya (Pleasant Gap, PA, USA). Fura-2 AM was from Invitrogen and 4α-phorbol-12,13-didecanoate (4αPDD) from Calbiochem (Gibbstown, NJ, USA). When necessary for solubility, stock solutions were prepared in 100% dimethyl sulphoxide or ethanol (sphingosine only) and diluted 1:1000 into the recording solution.
Results
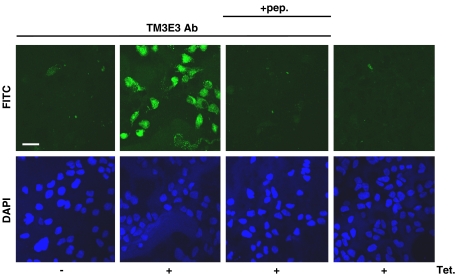
Human TRPM3 cDNA was stably incorporated in HEK 293 cells under a tetracycline-inducible promoter so that addition of tetracycline (Tet+) to the culture medium induced expression of exogenous TRPM3. The Tet+ cells were labelled positively with TM3E3, the anti-TRPM3 polyclonal antiserum (Figure 1). Without tetracycline induction (Tet−) there was little or no labelling of cells, as also found on omission of the antiserum from the labelling protocol or pre-adsorption of the anti-TRPM3 antibodies of the antiserum to the antigenic peptide (Figure 1). The data suggest tetracycline induction of TRPM3 expression was successful and that the antiserum bound the exogenous TRPM3.
Figure 1.
Overexpression of melastatin transient receptor potential 3 (TRPM3) and binding of antibody. Fluorescence images of human embryonic kidney (HEK) 293 cells, with (Tet+) or without (Tet−) induction of TRPM3 expression. The upper panel shows green fluorescence (from FITC conjugated to the secondary antibody) and the lower panel blue fluorescence (from DAPI, which labels cell nuclei). Inclusion of anti-TRPM3 antibody (TM3E3 Ab) in the staining protocol is specified, where ‘+pep.' indicates pre-adsorption of TM3E3 to its antigenic peptide (10 μM). Scale bar=20 μm. Images are representative of three independent experiments.
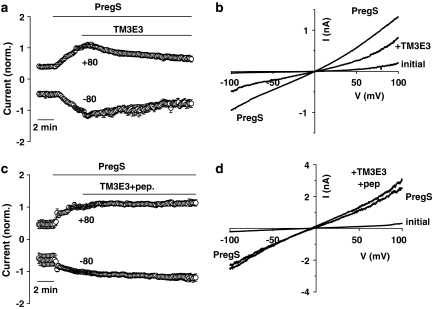
To determine whether TM3E3 has a functional effect on TRPM3 channels we performed micro-fluidic whole-cell patch-clamp recordings on tetracycline-induced TRPM3 HEK 293 cells. TRPM3 activity was evoked by pregnenolone sulphate and then dialysed TM3E3 (1:500) was applied (Figure 2a). TM3E3 caused partial reduction in current over a period of 5–10 min (Figure 2a). The current–voltage relationship (I–V) of the TM3E3-blocked current was similar to that of the pregnenolone sulphate-evoked current, consistent with the same ion channel underlying both events (Figure 2b). Application of TM3E3 pre-adsorbed to its antigenic peptide had no effect (Figures 2c and d).
Figure 2.
Inhibition of ionic current. Currents were measured under voltage-clamp using whole-cell planar patch-clamp recording. The holding potential was 0 mV and 0.2-s ramp changes in voltage from −100 to +100 mV were applied every 10 s. All cells were HEK 293 cells induced to express TRPM3. (a and c) Mean currents sampled at +80 and −80 mV, each normalized to the amplitude immediately before bath-application of antiserum (TM3E3) without (a, n=8) or with (c, n=4) pre-adsorption to 10 μM antigenic peptide. Pregnenolone sulphate (PregS) was bath applied at 25 μM. TM3E3 was used at 1:500 dilution. (b and d) Typical current-voltage relationships (I–Vs) from the experiments underlying (a) and (c).
Functional studies were also performed by measuring intracellular Ca2+ and pre-incubating with TM3E3 because this type of experimental approach gives additional capabilities over electrophysiology and wider applicability. In these experiments, TM3E3 was simply added to the cell culture medium during the pre-incubation period. It was not present in the medium during recordings but remained bound to TRPM3, as confirmed by immunocytochemistry (Figure 1). Pre-incubation protocols have an advantage of minimizing the amount of antibody used per experiment. Dialysed TM3E3 (1:500) partially inhibited Ca2+ entry evoked by pregnenolone sulphate (Figure 3a), whereas antiserum targeted to another TRP channel (T1E3 targeted to TRPC1) (Xu and Beech, 2001) had no effect (1:500 dilution, P>0.05, n/N=3/18; data not shown). More concentrated TM3E3 led to greater, but not complete, block (Figure 3b).
To determine whether the effect of TM3E3 related specifically to pregnenolone sulphate or the TRPM3 channel more generally we used a different TRPM3 activator. Sphingosine is an activator but with a weaker effect than pregnenolone sulphate. Responses were only reliably observed after transient transfection of HEK 293 cells with TRPM3 cDNA. In these experiments, YFP-tagged TRPM3 was used to enable successfully transfected cells to be identified by fluorescence microscopy. Cells pre-incubated with TM3E3 antiserum (1:4000) exhibited reduced sphingosine responses compared with control cells pre-incubated with pre-immune serum from the same rabbit (Figure 4a). Therefore, TM3E3 inhibited TRPM3 activated by an agonist other than pregnenolone sulphate. It was notable that control cells (overexpressing only YFP) had a response to sphingosine, which was small but suppressed by TM3E3 (Figure 4b). Although the TRPM3 protein signal was unconvincing in immunocytochemistry experiments (Figure 1), mRNA encoding TRPM3 was detected in wild-type HEK 293 cells (Supplementary Figure 1).
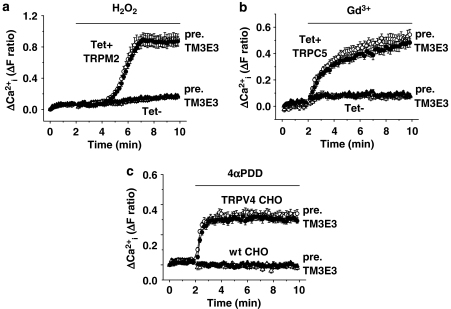
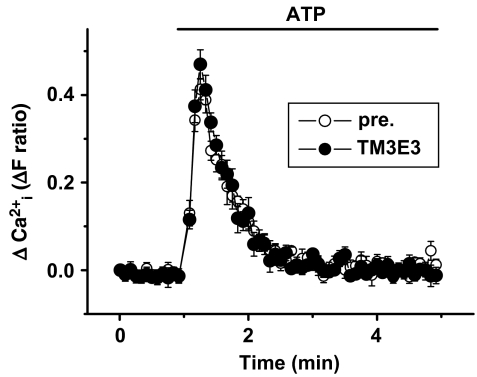
Functional specificity of TM3E3 was investigated using the same pre-incubation protocol as employed for studies of TRPM3 but with other, closely related, ion channel proteins. The TRPM2 channel (McHugh et al., 2003) was expressed under the tetracycline-inducible system and activated by hydrogen peroxide. It was unaffected by 1:4000 TM3E3 antiserum (Figure 5a). The TRPC5 channel (Zeng et al., 2004) was also expressed in the tetracycline-inducible system but activated by gadolinium (Gd3+), a lanthanide ion. TRPC5 was unaffected by TM3E3 (Figure 5b). To investigate the other major TRP channel subtype, TRPV, we carried out studies on Chinese hamster ovary cells stably expressing TRPV4 (Watanabe et al., 2002). TRPV4, activated by 4α-phorbol-12,13-didecanoate, was unaffected by TM3E3 (Figure 5c). We also investigated endogenous ATP-evoked Ca2+ signals in HEK 293 cells, which are mediated by purinoceptors and mostly reflect Ca2+ release. Again, TM3E3 had no effect (Figure 6). Therefore, the data suggest TM3E3 has specificity for TRPM3, lacking effect on closely related ion channels or agonist-evoked Ca2+ release.
Figure 5.
Functional specificity within the TRP family. Intracellular Ca2+ measurements from HEK 293 cells with (Tet+) and without (Tet−) induced expression of (a) TRPM2 or (b) canonical transient receptor potential 5 (TRPC5). (c) Chinese hamster ovary (CHO) cells without (wt, wild type) or with stable expression of vanilloid transient receptor potential 4 (TRPV4). The different TRP channels were activated by hydrogen peroxide (a, 1 mM H2O2), gadolinium (b, 0.1 mM Gd3+) and 4α-phorbol-12,13-didecanoate (c, 10 μM 4αPDD). Cells were pre-incubated with TM3E3 or its pre-immune (pre.) serum (1:4000 dilution): typical of n=3 (N=27–34) in each case.
Figure 6.
Lack of effect of TM3E3 on agonist-evoked Ca2+ release. Intracellular Ca2+ measurements from non-induced (Tet−) HEK 293 cells showing an endogenous response to extracellular application of 10 μM ATP. Cells were pre-incubated with TM3E3 or its pre-immune (pre.) serum (1:500 dilution): typical of n/N=3/18.
Discussion
The data suggest that targeting E3 has enabled generation of an antibody that binds to and specifically blocks TRPM3 cationic channels. The blocking effect was independent of the agonist used to activate TRPM3 and evident in electrophysiological and Ca2+-measurement studies. An acute blocking effect was observed or the antibody could be used in a short pre-incubation protocol that minimizes the amount of antibody dedicated to each experiment. Block of TRPM3 activity was partial but, based on experience with similar agents (Xu and Beech, 2001; Xu et al., 2005b, 2006, 2008), this degree of blockade should be sufficient to confer value in assays designed to reveal functions of TRPM3 in native systems.
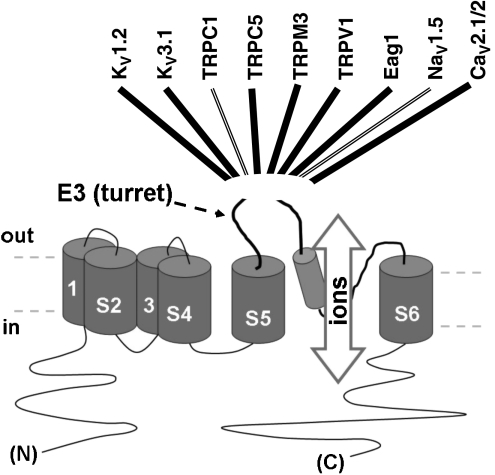
E3-targeted blocking antibodies have now been generated to nine ion channels of the six transmembrane type (Figure 7). Each appears to be specific. Intriguingly, all except the anti-TRPV1 antibodies produce only about 50% blockade of channel function. Each also acts over a similar time course. The similarities suggest a common underlying mechanism, but the mechanism is unknown; possibilities include partial occlusion of ion permeation, allosteric modulation via the turret or internalization of the entire channel protein.
Figure 7.
Summary of E3-targeted antibody blockers. The illustration in the lower half of the figure is the general topology of a six membrane-spanning ion channel subunit. NaV1.5 and CaV2.1/2 are concatamers of four such subunits. Names listed across the top are ion channels to which E3-targeted blocking antibodies have been successfully generated. There are double lines to TRPC1 and NaV1.5 because two different E3-targeted antibodies have been generated, each to different peptides within E3. References are: Zhou et al., 1998; Xu and Beech, 2001; Rosado et al., 2002; Chioni et al., 2005; Xu et al., 2005b; Klionsky et al., 2006; Gomez-Varela et al., 2007; Liao et al., 2008.
Other E3-targeted blocking antibodies have proved useful in studies of native channels, including TRPC1, TRPC5, NaV1.5, Eag1 and CaV2.1/2 (Xu and Beech, 2001; Xu et al., 2005b, 2006, 2008; Brackenbury et al., 2007; Gomez-Varela et al., 2007; Liao et al., 2008). These studies have involved experiments on cells in culture as well as experiments on excised whole tissues and in vivo. We expect TM3E3 will be similarly useful. Complete conservation of the TM3E3 antigenic peptide sequence in human, rat and mouse will make it useful in animal model systems, as well as in human cell and tissue studies. In addition to using the antibodies to inhibit channel function, they may also be useful to specifically deliver a chemical to an active site, for example by conjugating the chemical to the antibody. In the case of Eag1, a block occurred with a monoclonal antibody, which is an important step towards use of this strategy for therapeutics. Unfortunately, monoclonal antibodies to E3 of TRPV1 lacked functional effects, even though polyclonal antibodies were highly effective (Klionsky et al., 2006). In this latter case, there must have been at least one antibody in the polyclonal selection that had a functional effect, but reproduction of its effect with a monoclonal antibody is most likely to require screening of large numbers of antibodies.
In conclusion, we report the discovery of an isoform-specific blocker of TRPM3 cationic channels, which can be used by simple addition to the extracellular medium. Furthermore, it provides an additional example of the success of E3 targeting as a method for producing such isoform-specific agents and thus should increase confidence in this approach to developing ion channel pharmacology.
Supplementary Material
Acknowledgments
The work was supported by Wellcome Trust and British Heart Foundation grants to DJB. JN was funded by a BBSRC Collaborative PhD Studentship with Astra-Zeneca. The TRPM3 clone was a kind gift from C Harteneck (Berlin).
Abbreviations
- HEK 293 cells
human embryonic kidney cells
- TRPC
canonical transient receptor potential
- TRPM
melastatin transient receptor potential
- TRPV
vanilloid transient receptor potential
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Alexander SPH, Mathie A, Peters JA.Guide to Receptors & Channels (GRAC) Br J Pharmacol 2008153Suppl 2S1–209.3rd edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Sukumar P. Channel regulation by extracellular redox protein. Channels. 2008;1:400–403. doi: 10.4161/chan.1.6.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Chioni AM, Diss JK, Djamgoz MB. The neonatal splice variant of NaV1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2007;101:149–160. doi: 10.1007/s10549-006-9281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chioni AM, Fraser SP, Pani F, Foran P, Wilkin GP, Diss JK, et al. A novel polyclonal antibody specific for the NaV1.5 voltage-gated Na+ channel ‘neonatal' splice form. J Neurosci Methods. 2005;147:88–98. doi: 10.1016/j.jneumeth.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Gomez-Varela D, Zwick-Wallasch E, Knotgen H, Sanchez A, Hettmann T, Ossipov D, et al. Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res. 2007;67:7343–7349. doi: 10.1158/0008-5472.CAN-07-0107. [DOI] [PubMed] [Google Scholar]
- Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem. 2003;278:21493–21501. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- Grimm C, Kraft R, Schultz G, Harteneck C. Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine. Mol Pharmacol. 2005;67:798–805. doi: 10.1124/mol.104.006734. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sinauer Associates Inc.: Sunderland, Massachusetts, USA; 2001. [Google Scholar]
- Klionsky L, Tamir R, Holzinger B, Bi X, Talvenheimo J, Kim H, et al. A polyclonal antibody to the prepore loop of transient receptor potential vanilloid type 1 blocks channel activation. J Pharmacol Exp Ther. 2006;319:192–198. doi: 10.1124/jpet.106.108092. [DOI] [PubMed] [Google Scholar]
- Lee N, Chen J, Sun L, Wu S, Gray KR, Rich A, et al. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3) J Biol Chem. 2003;278:20890–20897. doi: 10.1074/jbc.M211232200. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Safa P, Chen YR, Sobel RA, Boyden ES, Tsien RW. Anti-Ca2+ channel antibody attenuates Ca2+ currents and mimics cerebellar ataxia in vivo. Proc Natl Acad Sci USA. 2008;105:2705–2710. doi: 10.1073/pnas.0710771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem. 2003;278:11002–11006. doi: 10.1074/jbc.M210810200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Oberwinkler J, Phillipp SE. TRPM3. Handb Exp Pharmacol. 2007;179:253–267. doi: 10.1007/978-3-540-34891-7_15. [DOI] [PubMed] [Google Scholar]
- Rosado JA, Brownlow SL, Sage SO. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J Biol Chem. 2002;277:42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- Wagner TFJ, Loch S, Straub I, Drews A, Lambert S, Flockerzi V, et al. Characterization of TRPM3-like steroid receptors in rodent endocrine cells Acta Physiologica Congress 2006189P16-L5-13 [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Boulay G, Flemming R, Beech DJ. E3-targeted anti-TRPC5 antibody inhibits store-operated calcium entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol. 2006;291:H2653–H2659. doi: 10.1152/ajpheart.00495.2006. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, et al. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005a;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Lei M, Li J, Gao B, Xiong C, et al. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol. 2005b;23:1289–1293. doi: 10.1038/nbt1148. [DOI] [PubMed] [Google Scholar]
- Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, et al. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559:739–750. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BY, Ma W, Huang XY. Specific antibodies to the external vestibule of voltage-gated potassium channels block current. J Gen Physiol. 1998;111:555–563. doi: 10.1085/jgp.111.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.