Abstract
Background and purpose:
The dietary trace amines tyramine and β-phenylethylamine (β-PEA) can increase blood pressure. However, the mechanisms involved in the vascular effect of trace amines have not been fully established. The purpose of this study was to evaluate whether trace amine-dependent vasoconstriction was brought about by tyramine and β-PEA acting as indirect sympathomimetic agents, as previously assumed, or whether trace amine-dependent vasoconstriction could be mediated by recently discovered trace amine-associated (TAA) receptors.
Experimental approach:
The responses to p-tyramine and β-PEA were investigated in vitro in rings of the left anterior descending coronary arteries of pigs.
Key results:
p-Tyramine induced a concentration-dependent (0.1–3 mM) vasoconstriction. The maximum response and pD2 value for p-tyramine was unaffected by endothelium removal or pre-treatment with antagonists for adrenoceptors, histamine, dopamine or 5-HT receptors. β-PEA also produced a concentration-dependent (0.3–10 mM) vasoconstriction which was unaffected by endothelium removal, β-adrenoceptor or 5-HT receptor antagonists. A substantial, but reduced, response to β-PEA was obtained in the presence of prazosin (α1-adrenoceptor antagonist), haloperidol (D2/D3 dopamine receptor antagonist) or mepyramine (H1 histamine receptor antagonist). The pD2 value for β-PEA was unaffected by any of the antagonists tested.
Conclusions and implications:
Vasoconstriction induced by p-tyramine does not involve an indirect sympathomimetic effect, although vasoconstriction caused by β-PEA may occur, in part, by this mechanism. We therefore propose that trace amine-dependent vasoconstriction is mediated by phenylethylamine-specific receptors, which are closely related to or identical to TAA receptors. These receptors could provide a target for new antihypertensive therapies.
Keywords: p-Tyramine, β-phenylethylamine, coronary artery, trace amine, vasoconstriction, pressor response
Introduction
Until recently, the trace amines tyramine and β-phenylethylamine (β-PEA) were considered merely metabolic by-products of tyrosine and phenylalanine decarboxylation, respectively (Berry, 2004). Trace amines, such as tyramine and β-PEA occur widely in plants and animals and are metabolized by the enzyme MAO (Yang and Neff, 1973; Wu and Boulton, 1975; Philips and Boulton, 1979; Durden et al., 1988; Burchett and Hicks, 2006). High levels of tyramine and β-PEA are found in chocolate, alcoholic beverages and fermented foods such as cheese (Branchek and Blackburn, 2003). On consumption of trace amine-rich foods, tachycardia and hypertension can result (Zucchi et al., 2006), although these effects are typically observed at micromolar concentrations of tyramine and β-PEA. Micromolar trace amine concentrations typically occur when patients treated with MAO inhibitors consume foods that contain high concentrations of tyramine or β-PEA (Faraj et al., 1976; D'Andrea et al., 2003). The first signs of trace amine-associated hypertension were discovered by a neurologist who noticed that his wife, who was on MAO inhibitors, had severe headaches (caused by cerebral vasoconstriction followed by dilatation) when eating cheese (Blackwell, 1963). For this reason, the hypertensive crisis is still called the ‘cheese syndrome', even though other trace amine-rich foods cause the same problem.
Tyramine-induced hypertension is also known as the ‘tyramine pressor response'. Clinically, the tyramine pressor response is used as an experimental tool to study the mechanisms of noradrenaline release from sympathetic neurones (Raiteri et al., 1977; Knoll et al., 1996). The tyramine pressor test is defined as the amount of tyramine, by bolus i.v. injection, required to increase the systolic blood pressure by 30 mm Hg. Tyramine sensitivity is taken as an index of peripheral adrenergic function (Ghose, 1984).
Tyramine and β-PEA are found in nerve terminals containing classical biogenic amines, such as noradrenaline, dopamine and 5-HT (Premont et al., 2001). Tyramine and β-PEA also have chemical structures similar to noradrenaline, which led to the assumption that, in the pressor test, tyramine is taken into the pre-synaptic terminal by the same amine membrane transporter as noradrenaline, and releases this amine by displacing it from cytoplasmic pools of vesicles and causing it to diffuse into the synaptic cleft (Raiteri et al., 1977; Knoll et al., 1996). In other words, tyramine and other trace amines act as indirect sympathomimetic amines (Borowsky et al., 2001).
There has been some speculation, however, that the tyramine pressor test may be inadequate to assess noradrenaline release (Chalon et al., 2002). A dissociation between changes in blood pressure and plasma noradrenaline following injection of tyramine have demonstrated that the pressor effects of tyramine may be mediated in part by a noradrenaline-independent mechanism (Bianchetti et al., 1982; Chalon et al., 2002).
The role of trace amines in blood vessels, however, has been underestimated because there were no known dedicated synapses or receptors using trace amines (D'Andrea et al., 2003; Berry, 2004). The recent identification of a family of G-protein-coupled receptors, which bind and are activated by trace amines (known as trace amine-associated receptors, TAA receptors) (Borowsky et al., 2001) raises the possibility that these amines may be bona fide autacoids in the vasculature.
The observation that TAA receptors are expressed in the kidney supports a role for trace amines in blood pressure regulation (D'Andrea et al., 2003). Human leukocytes and rat heart also express transcripts for TAA receptors (D'Andrea et al., 2003; Zucchi et al., 2006) and platelets store and actively release tyramine, which may have an effect on vascular tone (D'Andrea et al., 2003).
In this study, we evaluated whether the pressor effects of the trace amines tyramine and β-PEA were mediated through a mechanism that was independent of the classical biogenic amines, such as noradrenaline. Despite the absence of antagonists for TAA receptors, we propose that tyramine and β-PEA could mediate pressor responses through TAA receptors. Owing to the limited availability of human vessels in which to test this hypothesis, porcine coronary vessels were used in this study. Porcine coronary vessels were chosen for their close anatomical and physiological similarity to human coronary vessels (Weaver et al., 1986; Cinca et al., 1989, 1992; Kamimura et al., 1996)
Materials and methods
Tissue preparation
Porcine hearts (either sex) were collected from the local slaughterhouse and rapidly transported at 4 °C. Gross dissection of the left anterior descending coronary artery was conducted upon arrival in the laboratory and the vessels placed in gassed (95% O2, 5% CO2) Krebs'-Henseleit solution (KHS; composition in mM: NaCl 120, KCl 4.76, NaHCO3 25, NaH2PO4H2O 1.18, CaCl22H2O 1.25, MgSO47H2O 1.18, glucose 5.5). After equilibration to room temperature, surrounding connective tissue was dissected free, and the arteries were cut into rings of approximately 5 mm in length. In studies requiring removal of the functional endothelium, arterial rings were denuded of endothelium by gentle rolling of the tissue segment after insertion of the tip of a fine pair of small forceps into the lumen. The arteries were then suspended between a stainless-steel hook and stationary support rod positioned in 20 ml organ baths filled with KHS maintained at 37±0.5 °C and continuously oxygenated with a 95% O2, 5% CO2 gas mixture. The rings were progressively stretched to a resting tension of 5 g. Isometric tension was measured by force transducers (Ormed, Welwyn Garden City, Hertfordshire, UK) coupled to a PowerLab/4SP computer system (AD Instruments, Charlgrove, Oxfordshire, UK) for data collection. Data were analysed using Chart v.4.1.1 software (AD Instruments).
Experimental protocols
The viability of each porcine coronary arterial ring was determined by contraction with 60 mM KCl. KCl was removed by repeated changes of bath KHS and the presence of functional endothelium was confirmed by the demonstration of at least 70% relaxation in response to 10 μM bradykinin in rings pre-contracted with 5 nM U46619 (9,11-dideoxy-11α,9α-epoxymethano prostaglandin F2α). In denuded vascular rings, endothelium removal was confirmed by the absence of bradykinin-induced relaxation. Baseline tensions were re-established, following repeated changes in bath KHS to remove U46619 and bradykinin, and a cumulative concentration–response curve to either noradrenaline, p-tyramine, β-PEA, histamine, 5-HT or dopamine was constructed. Wherever necessary, antagonists were introduced into the baths 15 min before addition of agonist. Contraction produced by each concentration of agonist was measured after a steady state had been reached. Values are expressed as per cent KCl-induced contraction obtained during the viability test.
Statistical analysis
Data are represented as means±s.e.mean with n indicating the number of porcine hearts from which arterial rings were obtained. Maximal contractions were determined with the aid of a curve-fitting program (GraphPad, Prism). Comparison of the different concentration–response curves was performed by Student's t-test for unpaired data or two-way ANOVA (Concentration, treatment) using a computer statistical package (InStat3, Prism), followed by post hoc Dunnett's test applied to determine individual differences between multiple groups of data. A P-value of less than 0.05 was considered to indicate statistically significant differences. For some concentration–response curves, molar concentrations producing 50% of maximum effect (EC50) were calculated by fitting curves with the Boltzmann equation. pD2 values were then determined according to the equation pD2=−log (molar EC50) and compared using Student's t-test for unpaired data. A P-value <0.05 was considered statistically significant.
Drugs and chemicals
Tyramine hydrochloride (p-tyramine), 2-phenylethylamine hydrochloride (β-PEA), (±)-noradrenaline (+)-bitartrate, 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U46619), bradykinin triacetate, (±)-propranolol hydrochloride, prazosin hydrochloride, ketanserin tartrate, methiothepin mesylate, haloperidol, pyrilamine maleate (mepyramine), histamine, 5-HT and dopamine hydrochloride were obtained from Sigma (Sigma, Poole, Dorset, UK). All drugs were prepared daily as stock solutions in distilled water, with the exception of haloperidol, which was dissolved in hydrochloric acid before being neutralized to pH 7.4 with NaOH. All working solutions were obtained by dilution of stock solutions in KHS. All drug and molecular target nomenclature conforms to the British Journal of Pharmacology's ‘Guide to Receptors and Channels' (Alexander et al., 2008).
Results
Role of adrenoceptors in trace amine-induced vasoconstriction
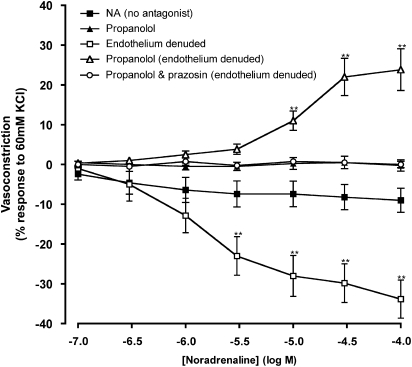
To ascertain the role of adrenoceptors in trace amine-induced vasoconstriction in the left anterior descending coronary artery, the effects of adrenoceptor antagonists against responses to noradrenaline were first evaluated. Cumulative addition of noradrenaline (0.1–100 μM) evoked a small relaxation in endothelium intact vessels, which was abolished in the presence of the non-selective β-adrenoceptor antagonist, propranolol (1 μM; Figure 1). The relaxation response to noradrenaline in the absence of antagonists was significantly enhanced upon removal of the endothelium (Figure 1 and Table 1). The opposite response occurred, however, in the presence of propranolol (1 μM) where vasoconstriction was observed to cumulative noradrenaline doses in the endothelium-denuded vessel. The vasoconstrictions were significantly greater at noradrenaline concentrations of 10–100 μM than the responses in the absence of propranolol in endothelium intact tissue (Figure 1 and Table 1). The noradrenaline-induced vasoconstriction observed in the presence of propranolol in denuded tissue was abolished by the α1-adrenoceptor antagonist, prazosin (1 μM; Figure 1).
Figure 1.
Contractile responses to noradrenaline (NA) in porcine isolated coronary arteries. Responses to noradrenaline were assessed in the absence and presence of endothelium, propranolol (1 μM) and prazosin (1 μM). Data are means±s.e.mean from four separate experiments, expressed as a percentage of the response to 60 mM KCl. **P<0.01 indicate significant differences from noradrenaline alone.
Table 1.
Effects of different biogenic amine antagonists on the maximum responses and pD2 values of p-tyramine, β-PEA, noradrenaline, histamine, dopamine and 5-HT in porcine coronary arteries
| Treatment | Maximum contraction (% KCl) | pD2 value | n |
|---|---|---|---|
| Noradrenaline | −8.1±19.6 | — | 4 |
| Propranolol 1 μM | 0.0±0.5 | — | 4 |
| Endothelium denuded | −40.2±5.9** | — | 4 |
| Propranolol 1 μM and endothelium denuded | 15.9±0.9** | 4.94±0.11 | 4 |
| Propranolol 1 μM and prazosin 1 μM & endothelium denuded | 0.2±0.3 | — | 4 |
| Histamine | 81.5±3.1 | 4.95±0.12 | 6 |
| Mepyramine 2 μM | 0.2±1.2*** | — | 6 |
| Dopamine | 9.5±0.5 | 1.40±0.05 | 4 |
| Haloperidol 0.7 nM | −0.1±0.4*** | — | 9 |
| 5-HT | 37.0±0.0 | 6.60±0.62 | 4 |
| Ketanserin 3 nM & methiothepin 50 nM | 1.0±0.6*** | — | 4 |
| p-Tyramine | 63.4±3.8 | 3.53±0.08 | 14 |
| Propranolol 1 μM | 92.3±16.8 | 3.33±0.26 | 6 |
| Prazosin 1 μM | 52.8±9.3 | 3.58±0.14 | 5 |
| Propranolol 1 μM and prazosin 1 μM | 72.3±6.2 | 3.31±0.10 | 5 |
| Endothelium denuded | 75.9±12.6 | 3.50±0.18 | 4 |
| Haloperidol 0.7 nM | 44.1±2.4 | 3.59±0.06 | 4 |
| Mepyramine 2 μM | 43.8±3.7 | 3.57±0.09 | 4 |
| Ketanserin 3 nM & methiothepin 50 nM | 38.0±2.2 | 3.48±0.05 | 4 |
| Propranolol 1 μM, prazosin 1 μM, haloperidol 0.7 nM, mepyramine 2 μM, ketanserin 3 nM & methiothepin 50 nM | 42.7±2.5 | 3.40±0.06 | 4 |
| β-PEA | 106.3±15.0 | 3.13±0.09 | 4 |
| Propranolol 1 μM | 108.0±16.2 | 3.52±0.08 | 4 |
| Prazosin 1 μM | 69.8±6.8 | 3.32±0.07 | 4 |
| Propranolol 1 μM & prazosin 1 μM | 65.7±5.2 | 3.10±0.07 | 4 |
| Endothelium denuded | 85.0±2.8 | 3.34±0.06 | 4 |
| Haloperidol 0.7 nM | 56.5±8.2* | 3.03±0.05 | 4 |
| Mepyramine 2 μM | 50.5±6.3* | 3.10±0.06 | 4 |
| Ketanserin 3 nM & methiothepin 50 nM | 66.5±4.2 | 3.07±0.05 | 4 |
| Propranolol 1 μM, prazosin 1 μM, haloperidol 0.7 nM, mepyramine 2 μM, ketanserin 3 nM & methiothepin 50 nM | 53.0±4.5* | 2.99±0.05 | 4 |
Data represent mean±s.e.mean, n=4–14 in each treatment group. *P<0.05, **P<0.01, ***P<0.0001 vs corresponding controls (ANOVA-Dunnett's or unpaired t-test-Welch corrected).
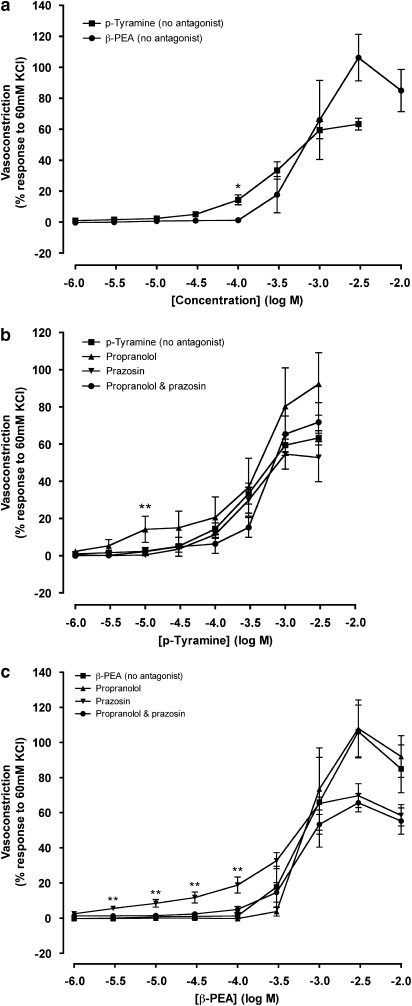
p-Tyramine and β-PEA produced concentration-dependent constrictions of endothelium intact porcine coronary arteries (Figures 2a and c and 3a). The vasoconstrictor responses to p-tyramine and β-PEA did not differ, with the exception of the dose of 0.1 mM at which p-tyramine produced a significantly greater constriction. The maximum response to β-PEA appeared greater than that of p-tyramine (Table 1), but this difference was not significant (Figures 2a and c and 3a). There was however a significant difference (P<0.05) between the pD2 values of p-tyramine and β-PEA, with the concentration–response curves to β-PEA to the right of p-tyramine.
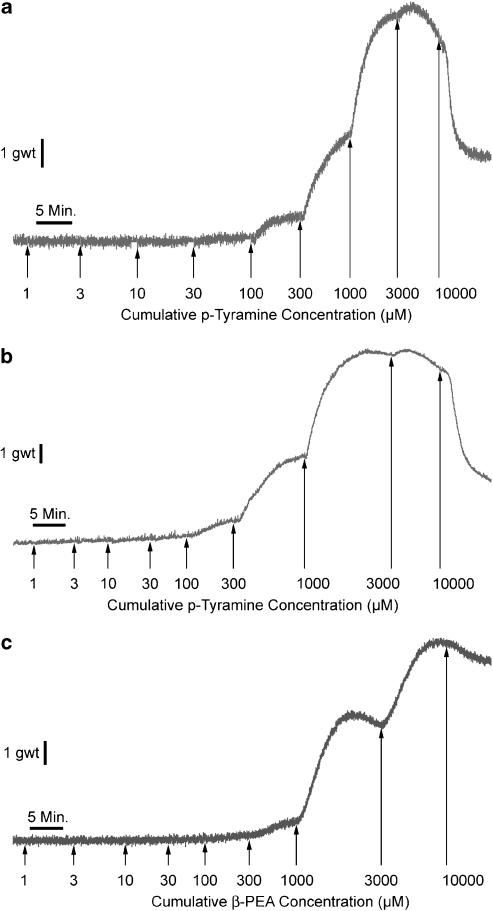
Figure 2.
Representative responses to cumulative concentrations of p-tyramine (a and b) and β-PEA (c) in the absence (a and c) or the presence (b) of all antagonists (propranolol, 1 μM; prazosin, 10 μM; ketanserin, 3 nM; methiothepin, 50 nM; haloperidol, 0.7 nM; mepyramine, 2 μM) in porcine coronary arterial rings with intact endothelium. The duration of each response is measured in minutes (min) and the magnitude of the response in gram (g).
Figure 3.
Concentration–response curves to p-tyramine (a and b) and β-phenylethylamine (β-PEA); (a and c) in the absence (no antagonist) and the presence of prazosin (1 μM) and propranolol (1 μM) constructed in porcine coronary arterial rings with intact endothelium. Data are means±s.e.mean from 5 to 14 separate experiments, expressed as a percentage of the response to 60 mM KCl. When no error bar is shown, the error is smaller than the symbol. *P<0.05 and **P<0.01 indicate significant differences from the control (p-tyramine or β-PEA in the absence of antagonist).
The presence of prazosin (1 μM), alone or in combination with propranolol (1 μM), had no significant effect on the p-tyramine concentration–response curve in endothelium intact tissue. Propranolol was found to increase significantly the constriction obtained with p-tyramine at 10 μM, but it did not affect the maximum contraction induced by p-tyramine (Figure 3b and Table 1). The pD2 values for p-tyramine concentration–response curves were unaffected by either propranolol or prazosin, alone or in combination (Table 1).
Propranolol (1 μM) had no effect on the β-PEA-dependent vasoconstriction. In contrast, the presence of prazosin, alone or in combination with propranolol, resulted in a fall in the maximum vasoconstriction caused by β-PEA, however this was not significant (Table 1 and Figure 3c). As with p-tyramine, the pD2 value for the β-PEA vasoconstriction was unaffected by propranolol or prazosin (Table 1).
The concentration–response curves to both p-tyramine and β-PEA, in the presence and absence of adrenoceptor antagonists, lie to the right of the noradrenaline (1 μM propranolol and endothelium denuded) concentration–response curve (Table 1). The maximum vasoconstrictions to p-tyramine (63.4±3.8%) and β-PEA (106.3±15.0%) were also significantly greater than the maximum response to noradrenaline (1 μM propranolol and endothelium denuded; 15.9±0.9%). The maximum response to noradrenaline (1 μM propranolol and endothelium denuded) was achieved at 0.1 mM. p-Tyramine and β-PEA in the absence of antagonist were able to achieve a comparable vasoconstriction (∼15% of the 60 mM KCl vasoconstriction) at concentrations of 0.1–0.3 mM.
Trace amine-dependent vasoconstriction in endothelium denuded coronary artery
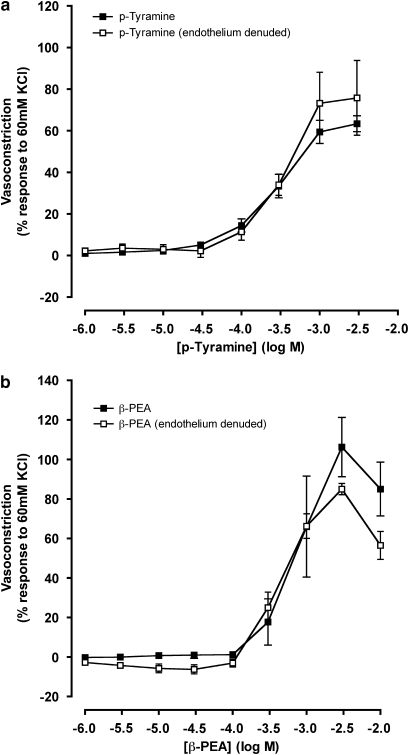
Vasoconstriction by p-tyramine and β-PEA was evaluated in endothelium intact and endothelium denuded tissue (Figure 4). Vasorelaxation to 10 μM bradykinin (mean, 28.9±1.1% response to U46619) in arterial rings pre-contracted with 5 nM U46619 confirmed that the endothelium was intact. No change in the U46619-induced contraction upon addition of 10 μM bradykinin (mean, 102.9±1.3% response to U46619) confirmed that the endothelium had been successfully removed from the tissue. No differences were observed between concentration–response curves to trace amines in the presence or absence of endothelium. Both maximum responses (Table 1 and Figure 4) for endothelium intact and endothelium denuded tissue remained unchanged, and no shift in the concentration–response curves was observed (Table 1).
Figure 4.
Concentration–response curves to p-tyramine (a) and β-phenylethylamine (β-PEA; b) in the absence and the presence of endothelium in porcine coronary arterial rings. Data are means±s.e.mean from four separate experiments, expressed as a percentage of the response to 60 mM KCl. When no error bars are shown, the error is smaller than the symbol. There were no significant differences between the responses at any of the concentrations tested.
Role of histamine, 5-HT and dopamine receptors in trace amine-induced vasoconstriction
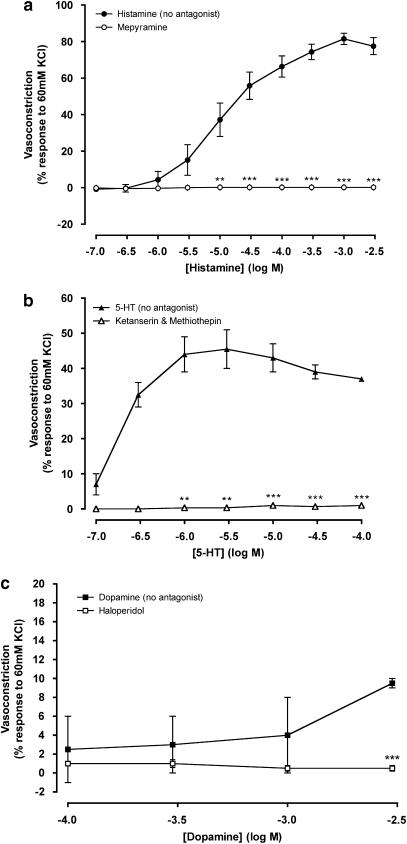
The trace amine-induced coronary constriction was not eliminated by antagonists of adrenoceptors, therefore the roles of other biogenic amine receptors were evaluated. The magnitude of the responses to histamine, 5-HT and dopamine were established from cumulative concentration–response curves (Figure 5 and Table 1). Histamine brought about a maximum constriction in the coronary artery five times the magnitude of that produced by noradrenaline (1 μM propranolol and endothelium denuded; Table 1). Histamine-dependent vasoconstriction, up to a concentration of 3 mM, was abolished by the H1 receptor antagonist mepyramine (2 μM; Figure 5a and Table 1). Maximum vasoconstriction by 5-HT was approximately twice that caused by noradrenaline (1 μM propranolol and endothelium denuded; Table 1). A combination of 3 nM ketanserin (5-HT2A receptor antagonist) and 50 nM methiothepin (5-HT1D antagonist) abolished 5-HT-dependent vasoconstriction up to 0.1 mM 5-HT (Figure 5b and Table 1). Vasoconstriction induced by dopamine could only be attained at concentrations that would far exceed those found endogenously (>1 mM). Dopamine at the highest concentration tested (3 mM) gave a constriction of 9.5±0.5% (Figure 5c and Table 1), which was eliminated by 0.7 nM haloperidol (D2 and D3 receptor antagonist).
Figure 5.
Concentration–response curves to histamine (a), 5-HT (b) and dopamine (c) in the absence and presence of mepyramine (a), ketanserin and methiothepin (b) or haloperidol (c) constructed in arterial rings with intact endothelium. Data are means±s.e.mean from four to nine separate experiments, expressed as a percentage of the response to 60 mM KCl. When no error bar is shown, the error is smaller than the symbol. **P<0.01 and ***P<0.001 indicate significant differences between histamine, 5-HT or dopamine in the presence and absence of their respective antagonists.
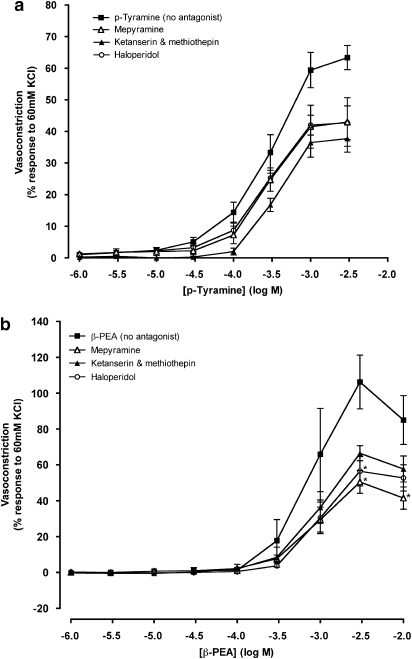
p-Tyramine-dependent vasoconstriction (Figure 6a) was unaffected by antagonists of histamine (2 μM mepyramine), dopamine (0.7 nM haloperidol) or 5-HT (3 nM ketanserin and 3 nM methiothepin). Both the maximum response and pD2 value for p-tyramine remained unaltered (Table 1). β-PEA-dependent vasoconstrictions were reduced by mepyramine (2 μM) at the highest concentrations studied (3–10 mM). Haloperidol (0.7 nM) also significantly reduced the maximum contraction to β-PEA (Figure 6b and Table 1). pD2 values for β-PEA were unaffected by antagonists of histamine, 5-HT and dopamine (Table 1).
Figure 6.
Concentration–response curves to p-tyramine (a) and β-phenylethylamine (β-PEA; b) in the absence (no antagonist) and the presence of ketanserin (3 nM) and methiothepin (50 nM), haloperidol (0.7 nM) or mepyramine (2 μM) constructed in porcine coronary arterial rings with intact endothelium. Data are means±s.e.mean from 5 to 14 separate experiments, expressed as a percentage of the response to 60 mM KCl. When no error bar is shown, the error is smaller than the symbol. *P<0.05 indicate significant differences from the control (p-tyramine or β-PEA in the absence of antagonist).
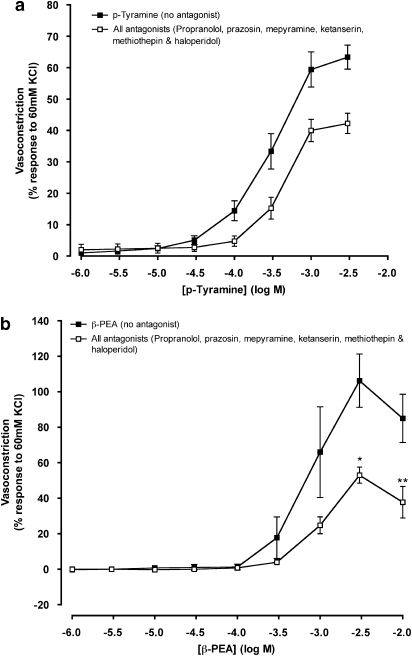
To eliminate the effects that trace amines might have on a range of amine receptors, a cocktail of antagonists for β- and α1-adrenoceptors (1 μM propranolol and 1 μM prazosin), histamine receptor H1 (2 μM mepyramine), 5-HT receptors 5-HT1D and 5-HT2A (3 nM ketanserin and 30 nM methiothepin) and dopamine receptors D2 and D3 (0.7 nM haloperidol), was added to the tissue before construction of concentration–response curves to trace amines. p-Tyramine-induced constriction appeared slightly reduced, compared with responses in the absence of antagonists, but this difference was not significant (Figure 7a). Typical responses are shown in Figures 2a and b. There was also no significant difference in the maximum contraction to p-tyramine (Table 1). The cocktail of antagonists affected β-PEA-induced vasoconstriction to a greater degree than vasoconstriction caused by p-tyramine (Figure 7b). The maximum contraction to β-PEA was almost halved from 106.3±15.0% in the absence of antagonists to 53.0±4.5% in their presence (Figure 7b and Table 1). In the presence of a cocktail of antagonists, there was no significant difference between the maximum responses obtained for p-tyramine and β-PEA.
Figure 7.
Concentration–response curves to p-tyramine (a) and β-phenylethylamine (β-PEA; b) in the absence (no antagonist) and the presence of all antagonists (propranolol, 1 μM; prazosin, 10 μM; ketanserin, 3 nM; methiothepin, 50 nM; haloperidol, 0.7 nM and mepyramine, 2 μM) constructed in porcine coronary arterial rings with intact endothelium. Data are means±s.e.mean from 4 to 14 separate experiments, expressed as a percentage of the response to 60 mM KCl. When no error bar is shown, the error is smaller than the symbol. *P<0.05 and **P<0.01 indicate significant differences from the control (p-tyramine or β-PEA in the absence of antagonist).
The noradrenaline (1 μM propranolol and endothelium denuded) concentration–response curve lay to the left of all the p-tyramine and β-PEA concentration–response curves, regardless of the antagonists added to the coronary artery (Table 1). The pD2 values for p-tyramine and β-PEA in the presence of the cocktail of antagonists did not differ significantly from the values in the absence of antagonist (Table 1).
Discussion and conclusions
The left anterior branch of the coronary artery contains a high proportion of β2-adrenoceptors and relatively few α1-adrenoceptors (Corr and Burnstock, 1991). In this study, noradrenaline in the absence of adrenoceptor antagonists was unable to cause vasoconstriction in endothelium intact tissue, and in fact caused a small concentration-dependent relaxation. It is likely that this vasorelaxation, caused by the activation of large numbers of β2-adrenoceptors, outweighed any vasoconstriction caused by the activation of α1-adrenoceptors. α2-Adrenoceptors on the endothelium also mediate vasorelaxation (Thorin et al., 1998). Therefore, a vasoconstrictor response to noradrenaline was only achieved after the β2-adrenoceptor-dependent vasorelaxation was eliminated using propranolol (Enocksson et al., 1995), and the α2-adrenoceptor-dependent vasodilatation eliminated by removing the endothelium (Thorin et al., 1998). Constriction of the coronary artery by noradrenaline occurred by smooth muscle α1-adrenoceptors and was eliminated with prazosin, an α1-adrenoceptor antagonist (Honner and Docherty, 1999).
Trace amines increase total peripheral resistance, which contributes to the pressor response, and it has long been believed that this is due to p-tyramine and β-PEA displacing classical biogenic amines, such as noradrenaline, from their storage vesicles (Raiteri et al., 1977; Knoll et al., 1996). This has led to their classification as indirectly acting sympathomimetic amines (Raiteri et al., 1977; Knoll et al., 1996). The evidence presented in this paper, however, suggests that this may not be the case, at least in the coronary artery. Trace amines are capable of producing constriction in the coronary artery that highly exceeds that caused by noradrenaline. α1-Adrenoceptors play no role in vascular constriction to p-tyramine, and play only a small role in constriction to β-PEA, because their vasoconstrictor responses were not eliminated by prazosin. Endothelial α2-adrenoceptors also have no role in the trace amine response in coronary artery, as removal of the endothelium (and hence α2-adrenoceptors) again had no influence on trace amine response.
If α1-adrenoceptors, and therefore an indirect sympathomimetic action, do not account for the vasoconstrictor responses to p-tyramine and β-PEA, then the next question was whether other local autacoids or their receptors might mediate their responses. In this study, we selected three vasoactive compounds histamine, dopamine and 5-HT. Concentration–response curves for these three agonists were generated in the presence or absence of selective antagonists.
Histamine produced a substantial vasoconstriction, which was abolished by the H1 receptor antagonist, mepyramine, confirming that it was due entirely to this receptor subtype (Haley, 1983). The response to p-tyramine was not significantly reduced by mepyramine, indicating that histamine H1 receptors are not involved in the vasoconstriction. The maximum response but not the pD2 for β-PEA, however, was reduced by mepyramine, although unlike histamine, the response was not abolished. Whether this is because β-PEA has some histaminergic properties, whether it is a nonspecific effect of mepyramine or whether mepyramine also exerts some inhibitory effect on the receptor that mediates the vasoconstriction by β-PEA cannot be determined by these results.
5-HT also exerted a substantial vasoconstriction of similar magnitude to that of p-tyramine and β-PEA. This response was abolished by the combination of 5-HT2A and 5-HT1D antagonists, ketanserin and methiothepin (Schoeffter et al., 1996; Nilsson et al., 1999; Frishman and Grewall, 2000). This confirms the role of these receptor subtypes in the vasoconstriction of coronary arteries by 5-HT (Nilsson et al., 1999). However, this combination of antagonists failed to inhibit significantly the vasoconstriction induced by p-tyramine or β-PEA, indicating that these 5-HT receptor subtypes were not involved in the vasoconstriction caused by these trace amines. Next, we examined the responses to dopamine, which produced only a small vasoconstriction at the highest concentration added (3 mM). This is the first time that responses to this amine appear to have been evaluated in the left anterior descending coronary artery. The response was abolished by the dopamine D2 and D3 antagonist, haloperidol (Creese et al., 1975). Vasodilatation is the usual response to dopamine in vascular beds, such as the renal and mesenteric vasculature, a response mediated by dopamine D1 receptors (Martin and Broadley, 1995). Vasoconstriction by dopamine is usually mediated by postjunctional α1-adrenoceptors (Duval et al., 1985). The fact that dopamine exerted a minor effect compared with the α1-adrenoceptor-mediated vasoconstriction by noradrenaline (endothelium removed and β-adrenoceptors blocked), however, suggests that α1-adrenoceptors were not involved here. Furthermore, the vasoconstriction was abolished by haloperidol, indicating that D2 receptors were probably involved. Haloperidol did not inhibit the vasoconstriction by p-tyramine but did reduce the maximum response to β-PEA. These results suggest that dopamine receptors do not mediate the response to these trace amines but that haloperidol has some nonspecific or non-selective effect on the responsiveness of the porcine coronary artery. When antagonists for the receptors for the vasoconstrictors noradrenaline, histamine, 5-HT and dopamine were combined, a substantial vasoconstrictor response to p-tyramine and β-PEA remained. As all antagonists had abolished the responses to their respective agonists, it can be assumed that the residual response was not mediated by any of these receptors.
The present results are consistent with previous results from these laboratories using other indirectly acting sympathomimetic amines. We recently showed that the amphetamines, 3,4-methylenedioxymethamphetamine (ecstasy) and cathinone, the active constituent of Khat leaves, caused vasoconstriction of pig coronary arteries, which was not due to an indirect sympathomimetic action (Baker et al., 2007). We have also shown that cathinone exerts coronary vasoconstriction in guinea-pig isolated hearts and constricts guinea-pig aorta, responses which are not antagonized by prazosin, and therefore not due to indirect sympathomimetic activity (Al-Motarreb et al., 2002; Al-Motarreb and Broadley, 2003).
The question therefore is raised as to the nature of the receptor that mediates this coronary vasoconstriction. In guinea-pig aortae, we have shown that the vasoconstriction by 3,4-methylenedioxymethamphetamine is not inhibited by antagonists of endothelin ETA/ETB, angiotensin AT1, 5-HT2, thromboxane TP receptors or α1-adrenoceptors (Baker and Broadley, 2003). We suggest that the same would apply for the structurally related trace amines, p-tyramine and β-PEA, in pig coronary arteries. Thus, the known receptors for vasoconstrictor biogenic amines do not appear to mediate the coronary vasoconstriction by these trace amines. Possible candidate target receptors are the TAA receptors. This extensive family of G-protein-coupled receptors, was discovered fairly recently and includes human and rat TAA1 receptor, which bind p-tyramine and β-PEA, and rat TAA4 receptor for which β-PEA is a very weak agonist but not p-tyramine (Borowsky et al., 2001). p-Tyramine is the most potent agonist known for human and rat TAA1 receptor expressed in COS-7 (African green monkey kidney fibroblast) and HEK293 cells (human embryonic kidney cell line) transfected with the receptor. β-PEA is also an agonist for TAA1 receptor in transfected cells, but to a lesser degree (Borowsky et al., 2001; Lindemann et al., 2005).
The amount of cAMP produced by transfected cells expressing rat and human TAA1 receptor in response to stimulation by p-tyramine and β-PEA was used to ascertain the potencies of trace amines at this receptor. The potency orders of p-tyramine and β-PEA is consistent with this study, which found that p-tyramine-dependent vasoconstriction curves lay to the left of those for β-PEA suggesting that should the two trace amines be acting on the same receptor, p-tyramine is a more potent agonist of the receptor than β-PEA. Should the vasoconstriction observed in this study be the result of TAA receptor activation, it is likely that the response would be due to TAA1 receptors rather than TAA4 receptors.
The basal concentration range of circulating trace amines differs greatly between patients of different ages and sex (Boulton, 1976; Faraj et al., 1976; Ghose, 1984; Reimann et al., 1992; Huebert et al., 1994; D'Andrea et al., 2003). Concentrations comparable to those observed in this study could be achieved by a number of means. Pressor responses are typically associated with a high dietary intake of tyramine (Branchek and Blackburn, 2003; Zucchi et al., 2006), especially in those taking MAO inhibitors to prevent its degradation in the intestine before reaching the vasculature (Faraj et al., 1976; D'Andrea et al., 2003). Elevated trace amine levels could also be attained in cigarette smokers, who have 30–40% lower monoamine oxidase B, that degrades β-PEA and p-tyramine, and 20–30% lower monoamine oxidase A that degrades tyramine (Berlin and Anthenelli, 2001). Tyramine is also a natural constituent of tobacco leaves, and is added to tobacco as a component of cocoa, which is used as a flavouring agent; thus the daily intake of tyramine from cigarettes is higher than tyramine intake from other sources such as chocolate or wine (Rambali et al., 2002).
Other individuals that may experience a trace amine-associated hypertensive crisis, as a consequence of the trace amine pressor response, are women taking isoflavones. Isoflavones are a popular alternative to hormone replacement therapy for treating symptoms associated with the menopause (Hutchins et al., 2005). Isoflavones, such as daidzin or daidzein, or their metabolites (equol) are capable of inhibiting MAO, consequently when isoflavones are taken in association with a trace amine-rich diet, hypertensive crises have been reported (Hutchins et al., 2005).
In the light of the evidence presented in this study, further research needs to be done into the role of trace amine receptors in vasoconstriction, as, at least in the left anterior coronary artery, the response to trace amines is not caused by these amines acting as indirectly acting sympathomimetics as previously assumed (Borowsky et al., 2001). Regardless of the TAA receptor subtype by which the vasoconstriction could be brought about, further research into the possible role of these receptors in the cardiovascular system will be hindered by the lack of antagonists for these receptors. Furthermore, without the genetic sequence of the porcine TAA receptor, other strategies such as small interfering RNA are not possible. For the same reason, it has also not been possible to undertake reverse transcription-PCR studies to identify the mRNA for TAA receptors in the porcine coronary artery. Although the human sequence is known, there appear to be no conserved regions in the pig, which allow preparation of suitable primers. It appears that trace amines have a direct effect on coronary artery smooth muscle, possibly through TAA receptors. These receptors could be possible targets for new antihypertensive medications. The magnitude of the vasoconstriction in the coronary artery has important implications for smokers, which should be emphasized in any antismoking counselling. Further research also needs to be done into isoflavone supplements and dietary trace amine interactions, so that practitioners can advise that elevated blood pressure could be a potential side effect of this medication if used concurrently with a tyramine- or β-PEA-rich diet.
In conclusion, this study has shown that the dietary trace amines, p-tyramine and β-PEA, produced coronary vasoconstriction that far exceeds that of noradrenaline alone. The vasoconstriction by p-tyramine was not, despite accepted dogma, caused by an indirect sympathomimetic action through adrenoceptors. p-Tyramine and β-PEA caused vasoconstriction independent of histamine, dopamine and 5-HT receptors, and adrenoceptors which may be through a novel phenylethylaminergic receptor that is identical, or closely related, to the recently identified TAA receptors.
Acknowledgments
This work was funded by a project grant from the British Heart Foundation. We are grateful to Dr Will Ford for his helpful advice.
Abbreviations
- β-PEA
β-phenylethylamine
- KHS
Krebs'-Henseleit solution
- 5-HT
5-hydroxytryptamine
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Motarreb A, Baker K, Broadley KJ. Khat: pharmacological and medical aspects and its social use in Yemen. Phytother Res. 2002;16:403–413. doi: 10.1002/ptr.1106. [DOI] [PubMed] [Google Scholar]
- Al-Motarreb AL, Broadley KJ. Coronary and aortic vasoconstriction by cathinone, the active constituent of khat. Auton Autacoid Pharmacol. 2003;23:319–326. doi: 10.1111/j.1474-8673.2004.00303.x. [DOI] [PubMed] [Google Scholar]
- Baker K, Broadley KJ. Vascular actions of ecstasy: roles of adrenergic neurones, endothelin, thromboxane, 5-HT, angiotensin and alpha1-receptors. Br J Pharmacol. 2003;138:166P. [Google Scholar]
- Baker KE, Herbert AA, Broadley KJ. Vasoconstriction of porcine left anterior descending coronary artery by ecstasy and cathinone is not an indirect sympathomimetic effect. Vascul Pharmacol. 2007;47:10–17. doi: 10.1016/j.vph.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol. 2001;4:33–42. doi: 10.1017/S1461145701002188. [DOI] [PubMed] [Google Scholar]
- Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- Bianchetti MG, Minder I, Beretta-Piccoli C, Meier A, Weidmann P. Effects of tyramine on blood pressure and plasma catecholamines in normal and hypertensive subjects. J Mol Med. 1982;60:465–470. doi: 10.1007/BF01720361. [DOI] [PubMed] [Google Scholar]
- Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2:849–850. doi: 10.1016/s0140-6736(63)92743-0. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AA. Identification, distribution, metabolism, and function of meta and para tyramine, phenylethylamine and tryptamine in brain. Adv Biochem Psychopharmacol. 1976;15:57–67. [PubMed] [Google Scholar]
- Branchek TA, Blackburn TP. Trace amine receptors as targets for novel therapeutics: legend, myth and fact. Curr Opin Pharmacol. 2003;3:90–97. doi: 10.1016/s1471-4892(02)00028-0. [DOI] [PubMed] [Google Scholar]
- Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006;79:223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Chalon S, Bieck PR, Goldstein DJ, Detke MJ, Bymaster FP, Potter WZ, et al. The tyramine pressor test may have limited sensitivity, especially in the presence of dual serotonin/norepinephrine uptake inhibition Neuropsychopharmacology 200226698–699.author reply 700–691 [DOI] [PubMed] [Google Scholar]
- Cinca J, Bardaji A, Salas-Caudevilla A. Ventricular arrhythmias and local electrograms after chronic regional denervation of the ischemic area in the pig heart. J Am Coll Cardiol. 1989;14:225–232. doi: 10.1016/0735-1097(89)90078-8. [DOI] [PubMed] [Google Scholar]
- Cinca J, Worner F, Carreno A, Bardaji A, Salas-Caudevilla A, Soler-Soler J. Reperfusion arrhythmias after chronic regional denervation of the ischaemic myocardium in pigs. Cardiovasc Res. 1992;26:962–967. doi: 10.1093/cvr/26.10.962. [DOI] [PubMed] [Google Scholar]
- Corr L, Burnstock G. Vasodilator response of coronary smooth muscle to the sympathetic co-transmitters noradrenaline and adenosine 5′-triphosphate. Br J Pharmacol. 1991;104:337–342. doi: 10.1111/j.1476-5381.1991.tb12432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding: differentiation of agonist and antagonist states with 3H-dopamine and 3H-haloperidol. Life Sci. 1975;17:933–1001. doi: 10.1016/0024-3205(75)90454-3. [DOI] [PubMed] [Google Scholar]
- D'Andrea G, Terrazzino S, Fortin D, Farruggio A, Rinaldi L, Leon A. HPLC electrochemical detection of trace amines in human plasma and platelets and expression of mRNA transcripts of trace amine receptors in circulating leukocytes. Neurosci Lett. 2003;346:89–92. doi: 10.1016/s0304-3940(03)00573-1. [DOI] [PubMed] [Google Scholar]
- Durden DA, Nguyen TV, Boulton AA. Kinetics of intraventricularly injected trace amines and their deuterated isotopomers. Neurochem Res. 1988;13:943–950. doi: 10.1007/BF00970766. [DOI] [PubMed] [Google Scholar]
- Duval N, Hicks PE, Langer SZ. Dopamine preferentially stimulates postsynaptic alpha 2-adrenoceptors in the femoral vascular bed, but alpha 1-adrenoceptors in the renal vascular bed of the anaesthetised dog. Eur J Pharmacol. 1985;108:265–272. doi: 10.1016/0014-2999(85)90448-0. [DOI] [PubMed] [Google Scholar]
- Enocksson S, Shimizu M, Lonnqvist F, Nordenstrom J, Arner P. Demonstration of an in vivo functional beta 3-adrenoceptor in man. J Clin Invest. 1995;95:2239–2245. doi: 10.1172/JCI117914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj BA, Bowen PA, Isaacs JW, Rudman D. Hypertyraminemia in cirrhotic patients. N Engl J Med. 1976;294:1360–1364. doi: 10.1056/NEJM197606172942502. [DOI] [PubMed] [Google Scholar]
- Frishman WH, Grewall P. Serotonin and the heart. Ann Med. 2000;32:195–209. doi: 10.3109/07853890008998827. [DOI] [PubMed] [Google Scholar]
- Ghose K. Tyramine pressor test: implications and limitations. Methods Find Exp Clin Pharmacol. 1984;6:455–464. [PubMed] [Google Scholar]
- Haley TJ. Physical and biological properties of pyrilamine. J Pharm Sci. 1983;72:3–12. doi: 10.1002/jps.2600720103. [DOI] [PubMed] [Google Scholar]
- Honner V, Docherty JR. Investigation of the subtypes of alpha1-adrenoceptor mediating contractions of rat vas deferens. Br J Pharmacol. 1999;128:1323–1331. doi: 10.1038/sj.bjp.0702913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebert ND, Dulery BD, Schoun J, Schwach V, Hinze C, Haegele KD. Kinetics and metabolism of p-tyramine during monoamine oxidase inhibition by mofegiline. Clin Pharmacol Ther. 1994;56:537–542. doi: 10.1038/clpt.1994.175. [DOI] [PubMed] [Google Scholar]
- Hutchins AM, McIver IE, Johnston CS. Hypertensive crisis associated with high dose soya isoflavone supplementation in a post-menopausal woman: a case report [ISRCTN98074661] BMC Women's Health. 2005;5:1–5. doi: 10.1186/1472-6874-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura R, Suzuki S, Nozaki S, Sakamoto H, Maruno H, Kawaida H. Branching patterns in coronary artery and ischemic areas induced by coronary arterial occlusion in the CLAWN miniature pig. Exp Anim. 1996;45:149–153. doi: 10.1538/expanim.45.149. [DOI] [PubMed] [Google Scholar]
- Knoll J, Miklya I, Knoll B, Marko R, Racz D. Phenylethylamine and tyramine are mixed-acting sympathomimetic amines in the brain. Life Sci. 1996;58:2101–2114. doi: 10.1016/0024-3205(96)00204-4. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Martin SW, Broadley KJ. Atypical antagonism of D1-receptor-mediated vasodilator response in the perfused kidney by SCH23390. Pharmacol Res. 1995;31:289–297. doi: 10.1016/1043-6618(95)80034-4. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Longmore J, Shaw D, Pantev E, Bard JA, Branchek T, et al. Characterisation of 5-HT receptors in human coronary arteries by molecular and pharmacological techniques. Eur J Pharmacol. 1999;372:49–56. doi: 10.1016/s0014-2999(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Philips SR, Boulton AA. The effect of monoamine oxidase inhibitors on some arylalkylamines in rate striatum. J Neurochem. 1979;33:159–167. doi: 10.1111/j.1471-4159.1979.tb11718.x. [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR, Caron MG. Following the trace of elusive amines. Proc Natl Acad Sci USA. 2001;98:9474–9475. doi: 10.1073/pnas.181356198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri M, Del Carmine R, Bertollini A, Levi G. Effect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur J Pharmacol. 1977;41:133–143. doi: 10.1016/0014-2999(77)90202-3. [DOI] [PubMed] [Google Scholar]
- Rambali B, Van Andel I, Schenk E, Wolterink G, van der Werken G, Stevenson H, et al. The contribution of cocoa additive to cigarette smoking addiction RIVM report 650270002/2002 20021–207.The Netherlands National Institute for Public Health and the Environment: Bilthoven, The Netherlands, pp
- Reimann IW, Firkusny L, Antonin KH, Bieck PR. Intravenous amine pressor tests in healthy volunteers. Within- and between subject variances and sex differences. Eur J Clin Pharmacol. 1992;42:137–141. doi: 10.1007/BF00278472. [DOI] [PubMed] [Google Scholar]
- Schoeffter P, Ullmer C, Bobirnac I, Gabbiani G, Lubbert H. Functional, endogenously expressed 5-hydroxytryptamine 5-ht7 receptors in human vascular smooth muscle cells. Br J Pharmacol. 1996;117:993–994. doi: 10.1111/j.1476-5381.1996.tb16687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorin E, Huang PL, Fishman MC, Bevan JA. Nitric oxide inhibits alpha2-adrenoceptor-mediated endothelium-dependent vasodilation. Circ Res. 1998;82:1323–1329. doi: 10.1161/01.res.82.12.1323. [DOI] [PubMed] [Google Scholar]
- Weaver ME, Pantely GA, Bristow JD, Ladley HD. A quantitative study of the anatomy and distribution of coronary arteries in swine in comparison with other animals and man. Cardiovasc Res. 1986;20:907–917. doi: 10.1093/cvr/20.12.907. [DOI] [PubMed] [Google Scholar]
- Wu PH, Boulton AA. Metabolism distribution, and disappearance of injected beta-phenylethylamine in the rat. Can J Biochem. 1975;53:42–50. doi: 10.1139/o75-007. [DOI] [PubMed] [Google Scholar]
- Yang HY, Neff NH. Beta-phenylethylamine: a specific substrate for type B monoamine oxidase of brain. J Pharmacol Exp Ther. 1973;187:365–371. [PubMed] [Google Scholar]
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]