Abstract
A burgeoning body of evidence suggests that RhoA/Rho kinase (ROCK) signalling plays an important role in the pathogenesis of various experimental models of pulmonary hypertension (PH), including chronic hypoxia-, monocrotaline-, bleomycin-, shunt- and vascular endothelial growth factor receptor inhibition plus chronic hypoxia-induced PH. ROCK has been incriminated in pathophysiologic events ranging from mediation of sustained abnormal vasoconstriction to promotion of vascular inflammation and remodelling. In addition, the 3-hydoxy-3-methylglutaryl CoA reductase inhibitors, statins, which inhibit activation of RhoA by preventing post-translational isoprenylation of the protein and its translocation to the plasma membrane ameliorate PH in several different rat models, and may also be effective in PH patients. Also, phosphorylation of RhoA and prevention of its translocation to the plasma membrane are involved in the protective effect of the type 5-PDE inhibitor, sildenafil, against hypoxia- and bleomycin-induced PH. Collectively, these and other observations indicate that independent of the cause of PH, activation of the RhoA/ROCK pathway serves as a point of convergence of various signalling cascades in the pathogenesis of the disease. We propose that ROCK inhibitors and other drugs that inhibit this pathway might be useful in the treatment of various forms of PH.
Keywords: vasoconstriction, vascular remodelling, fasudil, Y-27632, Rac
Introduction
The complex pathophysiology of pulmonary hypertension (PH) includes vasoconstriction, vascular remodelling and in situ thrombosis. The cellular/molecular signalling pathways underlying these components of the pulmonary arteriopathy are not well understood. More importantly, current pharmacological treatment of patients with severe, progressive PH improves symptoms but does not prevent the untimely death due to right heart failure (Macchia et al., 2007), thus emphasizing the urgent need to identify more effective therapies. This overview summarizes current evidence that activation of the small GTPase RhoA and its downstream effector Rho-associated kinase (ROCK) is important in the pathogenesis of PH, and discusses the potential efficacy of RhoA/ROCK inhibitors in the treatment of PH. As most studies of the roles of RhoA/ROCK signalling in cardiovascular diseases have been in the systemic circulation, we briefly review that work before addressing evidence of its involvement in PH. Although not yet studied in PH as extensively as RhoA/ROCK, we also discuss the possible importance of another Rho family GTPase, Rac1.
RhoA/ROCK signalling
Rho (Ras homologous) GTP-binding proteins, which comprise multiple members of the Rho, Rac and Cdc42 subfamilies, and their downstream effectors interact with each other and with other intracellular signalling pathways to regulate numerous cellular processes. These include gene transcription, differentiation, proliferation, hypertrophy, apoptosis, phagocytosis, adhesion, migration and contraction (Riento and Ridley, 2003; Jaffe and Hall, 2005). The prototypical mechanism of RhoA GTPase signalling is that various environmental cues, acting through G-protein-coupled receptors or receptor-dependent and -independent TKs, activate guanine nucleotide exchange factors, which induce exchange of GDP for GTP binding and translocation of GTP-RhoA to the plasma membrane. Upon translocation to the plasma membrane, GTP-RhoA activates one or more of its effectors, including the two isoforms of ROCK, ROCK I (ROKβ) and ROCK II (ROKα). Negative regulators of RhoA activation include guanine nucleotide disassociation inhibitors, which oppose the exchange of GTP for GDP; GTPase-activating proteins, which catalyse dephosphorylation and inactivation of membrane-bound GTP-RhoA; statins, which inhibit isoprenylation of RhoA and thereby prevent translocation of GTP-RhoA to the cell membrane (Cordle et al., 2005; Rikitake and Liao, 2005) and PKA and PKG, which by phosphorylating RhoA also prevent membrane translocation of the GTP-bound protein (Sawada et al., 2001; Guilluy et al., 2005; Murthy, 2006). Following sustained activation, RhoA signalling can also be inhibited by transamidation, ubiquitylation and proteasomal degradation (Guilluy et al., 2007).
RhoA/ROCK-mediated vasoconstriction
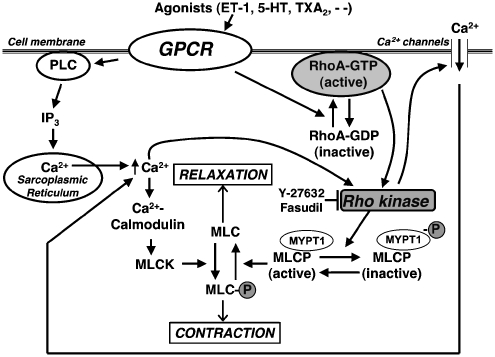
It is now clear that activation of RhoA/ROCK signalling is a major regulator of vascular tone (Figure 1) (Somlyo and Somlyo, 2003; Ratz et al., 2005). Smooth muscle cell (SMC) tension is determined primarily by phosphorylation (contraction) and dephosphorylation (relaxation) of the regulatory myosin light chain (MLC). Phosphorylation is catalysed by Ca2+/calmodulin-dependent MLC kinase (MLCK), and dephosphorylation by Ca2+-independent MLC phosphatase (MLCP) that is targeted to myosin by its regulatory myosin-binding subunit. Thus, the balance in activities of MLCK and MLCP regulates contraction. At a given level of cytosolic Ca2+, second messenger-mediated pathways can modulate the activity of both enzymes to modify MLC phosphorylation and force, that is, to modify the Ca2+ sensitivity of contraction. There are multiple mechanisms of Ca2+ sensitization, but two major pathways in vascular smooth muscle are inhibition of MLCP by ROCK-mediated phosphorylation of regulatory myosin-binding subunit, and PKC-mediated phosphorylation and activation of the MLCP-inhibitor protein CPI-17. ROCK can also phosphorylate CPI-17. Although RhoA/ROCK-mediated sustained vasoconstriction is generally attributable to inhibition of MLCP and Ca2+ sensitization, there are instances in which ROCK activity also induces Ca2+ signalling (Ghisdal et al., 2003; Luykenaar et al., 2004), and Ca2+ signals can activate RhoA/ROCK (Sakurada et al., 2003; Ratz et al., 2005; Wang et al., 2006). Thus, while considerable evidence now exists that regulating Ca2+ sensitivity is as important as regulating cytosolic [Ca2+] in the control of vascular tone, there can be agonist- and artery segment-dependent interplay between the two pathways (Ratz et al., 2005).
Figure 1.
Regulation of smooth muscle cell contraction. Vascular smooth muscle contraction is determined primarily by the balance in activities of Ca2+/calmodulin-dependent myosin light chain kinase (MLCK, contraction) and Ca2+-independent MLC phosphatase (MLCP, relaxation). G-protein-coupled receptor (GPCR) agonists (ET-1, endothelin-1; 5-HT, serotonin; TXA2, thromboxane A2; and so on) not only activate MLCK, but also inactivate MLCP by activation of RhoA/Rho kinase pathway and/or activation of the MLCP-inhibitor protein CPI-17 (not shown) to cause smooth muscle cell contraction. In some instances, Rho kinase activation can also induce Ca2+ signalling, and Ca2+ signals can activate RhoA/Rho kinase. IP3, inositol 1,4,5-triphosphate; PLC; MYPT1, regulatory myosin-binding subunit of MLCP (modified from Nagaoka et al. (2005) Am J Respir Crit Care Med 171: 494–499).
Ca2+ desensitization is also a mechanism of vasodilation. Besides inducing SMC relaxation by desensitizing receptors and decreasing cytosolic [Ca2+] and MLCK activity, the nitric oxide (NO)/soluble guanylate cyclase/cGMP/PKG pathway also decreases Ca2+ sensitivity by phosphorylating and inactivating RhoA or by directly phosphorylating MLCP, which increases MLCP activity (Sawada et al., 2001; Murthy, 2006). Similarly, vasodilation by stimuli that activate the adenylate kinase/cAMP/PKA pathway is also attributable partly to inhibition of RhoA/ROCK signalling (Murthy, 2006; Azam et al., 2007).
RhoA/ROCK in systemic vascular diseases
In addition to mediating sustained, abnormal vascular SMC contraction, RhoA/ROCK signalling has also been implicated in several other processes that contribute to systemic vascular diseases (Shimokawa and Takeshita, 2005; Budzyn et al., 2006; Loirand et al., 2006; Noma et al., 2006). These include SMC proliferation and migration, increased matrix protein production, endothelial cell (EC) contraction and increased permeability, oxidative stress, adhesion molecule expression and monocyte/macrophage infiltration, platelet activation and thrombosis, and inflammation. Given the regulation of the above cellular pathophysiological events by RhoA/ROCK, it is not surprising that the pathway has also been incriminated in important systemic vasculopathies. For example, several studies show that RhoA/ROCK signalling is involved in the pathogenesis of systemic hypertension, cerebral and coronary vasospasm, and arteriosclerosis and restenosis. Various inhibitors of RhoA/ROCK activity, such as TAT-C3, statins, rapamycin, Rad GTPase, dominant-negative ROCK, and the ROCK inhibitors fasudil (HA-1077) and Y-27632, have been found in animal models to suppress systemic vascular neointimal formation and constrictive remodelling in vein grafts (Yamanouchi et al., 2005; Furuyama et al., 2006), cardiac allograft vasculopathy (Hattori et al., 2004), balloon injury (Eto et al., 2000; Sawada et al., 2000; Shibata et al., 2003; Fu et al., 2005; Iso et al., 2006) and in-stent restenosis (Matsumoto et al., 2004; Guerin et al., 2005). Collectively, these studies indicate that inhibition of RhoA/ROCK signalling is associated with increased expression of p27Kip1 and suppression of neointimal cell proliferation, downregulation of bcl-2 and increased neointimal cell apoptosis, decreased expression of TGF-β1 and deposition of collagen, decreased expression of proinflammatory cytokines, such as monocyte chemoattractant protein-1, macrophage migration inhibitory factor and interferon-γ, and decreased vascular accumulation of macrophages and T cells. Similarly, ROCK inhibition also suppresses chronic NO synthase inhibitor- and angiotensin II-induced expression of connective tissue growth factor and monocyte chemoattractant protein-1, monocyte/macrophage infiltration, and medial thickening and perivascular fibrosis (Funakoshi et al., 2001; Kataoka et al., 2002; Kanda et al., 2005; Ruperez et al., 2005). Additional support for important links between RhoA/ROCK activation and vascular inflammation includes in vitro evidence that ROCK activity mediates, at least partly, adherence and transendothelial migration of monocytes and neutrophils (Ashida et al., 2001; Saito et al., 2002; Pizurki et al., 2003; Honing et al., 2004; Bolick et al., 2005), inflammatory mediator production by vascular SMCs, cardiomyocytes and monocytes (Ito et al., 2002; Segain et al., 2003; Horton et al., 2005), and T-cell activation (Bardi et al., 2003; Tharaux et al., 2003; Aihara et al., 2004). Thus, RhoA/ROCK signalling is a major player in the pathogenesis of systemic arterial inflammation and neointimal formation, and it seems likely this signalling pathway also contributes significantly to the arteriopathy of PH.
RhoA/ROCK in acute pulmonary vasoconstriction
As of 23 February 2008, the search terms ‘rho kinase and pulmonary hypertension' in Entrez PubMed captured 64 citations, 21 of them were reviews. The list did not include several additional reports on the function of ROCKs in acute pulmonary vasoconstriction. That the majority of these studies have been published in the past 2 years reflects the growing interest in the likelihood that RhoA/ROCK signalling plays important roles in mediating both vasoconstriction and vascular remodelling in PH, and that pharmacological inhibition of this signalling pathway might be efficacious in the treatment of the disease (Shimokawa and Takeshita, 2005; Fukumoto et al., 2007).
An early report of RhoA/ROCK signalling in regulation of pulmonary vascular tone was by Robertson et al. (2000) who observed in perfused rat lungs and isolated pulmonary arteries that ROCK-mediated Ca2+ sensitization was necessary for the sustained phase of acute hypoxic pulmonary vasoconstriction. They subsequently reported that an unidentified endothelium-derived factor was responsible for the hypoxic activation of RhoA/ROCK (Robertson et al., 2003). Similarly, Wang et al. (2001, 2003) observed activation of RhoA/ROCK in hypoxic pulmonary vasoconstriction, except in this case hypoxia directly activated RhoA in cultured pulmonary artery SMCs (PASMCs). Several studies have demonstrated the participation of ROCK in acute pulmonary vasoconstriction to various stimuli, including KCl (Robertson et al., 2000; Nagaoka et al., 2004), endothelin-1 (Weigand et al., 2006; Barman, 2007), thromboxane A2 (Janssen et al., 2001; Martin et al., 2004), prostaglandin F2α (Robertson et al., 2000), serotonin (Witzenrath et al., 2006), isoprostanes (Janssen et al., 2001), norepinephrine/phenylephrine (Janssen et al., 2001; Boer et al., 2002; Damron et al., 2002), platelet-activating factor (Martin et al., 2004), 20-hydroxyeicosatetraenoic acid (Yaghi and Sims, 2005), epoxyeicosatrienoic acids (Losapio et al., 2005), sphingosylphosphorylcholine (Thomas et al., 2005) and sphingosine-1-phosphate (Beutz et al., 2005). Whether the ROCK-dependent component of the vasoconstriction occurs parallel to or in series with increases in SMC cytosolic [Ca2+] seems to vary with agonist and pulmonary artery preparation.
RhoA/ROCK in rodent models of PH
Studies in chronically hypoxic, monocrotaline-injected and bleomycin-injected rats indicate that sustained ROCK-mediated vasoconstriction contributes substantially to the increased pulmonary vascular resistance in all three models based on the acute in vivo effects of selective ROCK inhibitors. Although Y-27632 and fasudil (or HA-1077), the two most frequently used ROCK inhibitors, are relatively selective for ROCK up to 10 μM (IC50 values of Y-27632 and fasudil for ROCK II are 0.162 and 0.158 μM, respectively), their higher concentrations also inhibit other kinases, such as PKC (IC50 values are 25.8 and 12.3 μM, respectively) and G (IC50 values are 3.27 and 1.65 μM, respectively) (Tamura et al., 2005). Nagaoka et al. (2004) found that acute administration of Y-27632 and fasudil, but not normoxic ventilation or the Ca2+ channel blocker nifedipine, to chronically hypoxic rats and perfused hypertensive lungs elicited considerable vasodilation and nearly normalized the high vascular resistance. Hyvelin et al. (2005) and McNamara et al. (2008) subsequently confirmed that sustained ROCK-mediated vasoconstriction played a central role in the PH of chronically hypoxic adult and neonatal rats, and similar observations have been made in rats with monocrotaline- (Nagaoka et al., 2005; Jiang et al., 2007) and bleomycin-induced PH (McNamara et al., 2008). These findings suggest that the PH in these models is due largely to sustained ROCK-mediated vasoconstriction, rather than simply to the pulmonary artery medial and adventitial thickening and arteriolar muscularization that accompanies the hypertension.
The above pharmacological observations agree with histological evidence in the vasodilated hypertensive lungs of hypoxic and monocrotaline-injected rats that the arterial wall thickening itself causes little inward remodelling (van Suylen et al., 1998; Howell et al., 2004). In this regard, Crossno et al. (2007) recently found that treatment of hypoxic rats with the PPARγ agonist rosiglitazone inhibited pulmonary vascular remodelling but not the development of PH. The hypertension was immediately reversed by acute i.v. fasudil. Interestingly, acute fasudil has also been observed to elicit substantial pulmonary vasodilation, albeit not to near-normal levels, in VEGF receptor blocker (Sugen 5416)-injected/chronic hypoxia-exposed rats (Oka et al., 2007a) and left-pneumonectomized plus monocrotaline-injected rats (M Oka and IF McMurtry, unpublished). In addition to increased pulmonary arterial muscularization, these latter two models also develop obstructive neointimal lesions in distal pulmonary arteries (Taraseviciene-Stewart et al., 2001; Nishimura et al., 2002). It is presumably these fixed neointimal lesions that prevent near-normalization of the high vascular resistance by acute inhibition of ROCK. Whether or not chronic treatment with ROCK inhibitors will reverse the neointimal lesions is unknown (see below).
Additional evidence of involvement of RhoA/ROCK signalling in rat models of PH include reports that acute NO-induced vasodilation of hypoxic hypertensive pulmonary arteries is due to inhibition of RhoA/ROCK-mediated Ca2+ sensitization rather than to decreased cytosolic [Ca2+] (Jernigan et al., 2004), and acute ET-1-induced constriction is due largely to ROCK-mediated Ca2+ sensitization rather than to increased cytosolic [Ca2+] (Weigand et al., 2006). Furthermore, it has recently been found that chronic hypoxia-induced PH in rats is associated with induction of ROCK-dependent myogenic tone in small pulmonary arteries (Broughton et al., 2008). Also, treatment of chronically hypoxic rats with Y-27632 inhibits development of PH (McMurtry et al., 2003; Hyvelin et al., 2005), and treatment with the type PDE5 inhibitor sildenafil, which promotes phosphorylation of RhoA and thereby prevents translocation of GTP-RhoA to the PASMC plasma membrane, mimics the inhibition of hypoxia-induced PH by fasudil (Guilluy et al., 2005), and also attenuates bleomycin-induced activation of RhoA/ROCK and development of PH in mice (Hemnes et al., 2008). Acute administration of fasudil to Denver-raised fawn-hooded rats, which have lung dysplasia and severe PH, elicits marked pulmonary vasodilation and chronic treatment from birth to 10 weeks of age ameliorates the lung dysplasia and PH (Nagaoka et al., 2005, 2006). Similarly, treatment with fasudil inhibits and also reverses monocrotaline-induced PH (Abe et al., 2004). The latter inhibitory effects of fasudil were associated with improvement of endothelium-dependent relaxation and inhibition of vascular hypercontraction to serotonin, and with suppression of pulmonary artery macrophage infiltration, inhibition of vascular SMC proliferation and enhanced SMC apoptosis. In addition, Chapados et al. (2006) found in monocrotaline-injected rats that fasudil-induced inhibition of pulmonary artery medial thickening was associated with reduced expression of the matrix protein, tenascin-C. Finally, treatment with fasudil has been reported to inhibit activation of ROCK and development of shunt-induced PH in rats (Li et al., 2007a). The inhibition of pulmonary vascular remodelling by fasudil was associated with decreased proliferation and increased apoptosis of PASMCs. It is noteworthy that a recent report suggests transglutaminase-dependent activation of RhoA by serotonin (Walther et al., 2003) may be involved in chronic hypoxia-induced pulmonary artery remodelling and hypertension (Guilluy et al., 2007).
Studies in chronically hypoxic mice also implicate involvement of RhoA/ROCK signalling in the pathogenesis of PH. Fagan et al. (2004) observed that inhibition of PH by treatment of hypoxic mice with Y-27632 was associated with decreased muscularization of distal pulmonary arteries and an upregulation of lung tissue endothelial NOS (eNOS) expression. Similarly, Abe et al. (2006). reported that inhibition of hypoxic PH in wild-type mice by fasudil was accompanied by increased lung eNOS expression and Akt phosphorylation, and that the ROCK inhibitor was less effective in blunting the hypertension in eNOS-deficient mice.
The HMG-CoA reductase inhibitors, statins, can interfere with RhoA/ROCK signalling by blocking synthesis of mevalonate and its isoprenoid intermediate geranylgeranylpyrophosphate, which in turn prevents isoprenylation of RhoA and its translocation to the plasma membrane (Noma et al., 2006). Although not a universal finding (McMurtry et al., 2007), numerous studies report that treatment with statins attenuates, and in some cases reverses, PH in various rat models (Nishimura et al., 2002, 2003; Girgis et al., 2003, 2007; Lee et al., 2005; Murata et al., 2005; Guerard et al., 2006; Rakotoniaina et al., 2006; Taraseviciene-Stewart et al., 2006; Laudi et al., 2007). A recent study by Girgis et al. (2007) provides evidence that both attenuation and reversal of hypoxic PH by simvastatin are associated with decreases in lung tissue expression and activity of both ROCK I and II. Interestingly, dehydroepiandrosterone, another agent reported to block mevalonate synthesis and protein isoprenylation (Schulz and Nyce, 1991; Pascale et al., 1995), is a highly effective inhibitor of hypoxic PH in rats (Bonnet et al., 2003; Hampl et al., 2003; Oka et al., 2007b). A preliminary report indicates that dehydroepiandrosterone also inhibits formation of distal pulmonary artery neointimal lesions and development of PH in left-pneumonectomized/monocrotaline-injected rats (Homma et al., 2008). The inhibition of PH was associated with decreases in lung tissue RhoA, ROCK, and caspase-3 activities and decreased expression of cleaved ROCK I. The latter is noteworthy because cleaved ROCKs have lost their auto-inhibitory domain and are constitutively active, that is, are active independently of the status of RhoA (Sebbagh et al., 2001, 2005; Chang et al., 2006; Sapet et al., 2006).
RhoA/ROCK in human PH
Studies of RhoA/ROCK signalling in human PH are limited. A preliminary report by Hemnes et al. (2005) suggests that the blunted vasodilator responsiveness of hypertensive pulmonary arteries and perfused lung lobes isolated from patients undergoing lung transplantation for severe PH is associated with high RhoA/ROCK activity. The RhoA/ROCK activation was reduced by exposure of the isolated hypertensive pulmonary arteries to sildenafil. Also, low i.v. doses of fasudil acutely cause modest decreases in pulmonary vascular resistance in patients with PH (Fukumoto et al., 2005; Ishikura et al., 2006). Chronic simvastatin treatment has improved symptoms in patients with PH (Kao, 2005), but it is unknown if the effects are related to inhibition of RhoA/ROCK signalling (Xing et al., 2007).
Mechanisms of suppression of PH by ROCK inhibitors
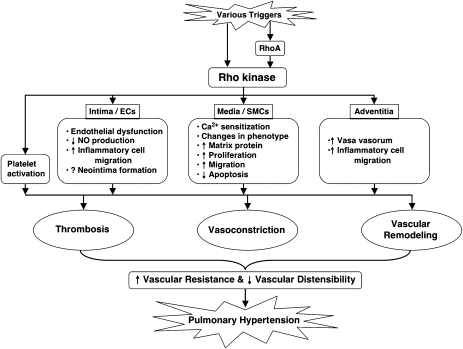
Collectively, the in vivo studies reviewed above (summarized in the Table 1) suggest that the activation of RhoA/ROCK signalling is significantly involved in the pathogenesis of PH in several different rodent models, and probably also in humans, and that this involvement ranges from mediation of sustained abnormal vasoconstriction to promotion of vascular inflammation and remodelling (illustrated in Figure 2). Some of these in vivo studies have found that suppression of PH by ROCK inhibitors is associated with decreased pulmonary artery expression of growth factors and of markers of cell proliferation, matrix protein production, and inflammatory cell infiltration, and with increased markers of apoptosis. However, it is difficult in these studies to interpret whether the antivascular remodelling effects of ROCK inhibitors are due to direct inhibition of molecular signalling pathways or to indirect consequences of vasodilation and lower pulmonary arterial pressure. There are a few in vitro studies supporting the possible direct involvement of RhoA/ROCK activation in mediating pulmonary vascular cell growth. For example, Liu et al. (2004) report that serotonin-induced proliferation of bovine PASMCs depends on multiple serotonin receptors and the serotonin transporter and involves several intracellular signalling pathways, including activation of RhoA and ROCK, and ROCK-mediated translocation of phosphorylated extracellular signal-regulated kinase to the nucleus (Liu and Fanburg, 2006). Similarly, inhibition of serotonin-induced PASMC proliferation and migration by atorvastatin has been attributed to prevention of RhoA membrane translocation and lack of activation of ROCK (Li et al., 2007b).
Table 1.
Summary of in vivo studies of RhoA/ROCK signaling and of acute and chronic effects of ROCK inhibitors in animal models and patients with PH
| PH models | RhoA/ROCK signaling | Treatments | Pulmonary effects | References |
|---|---|---|---|---|
| Chronic hypoxia | ||||
| Rat | ? | Acute Y-27632 | Vasodilation | (Nagaoka et al., 2004) |
| Rat | ? | Acute fasudil | Vasodilation | (Nagaoka et al., 2005) |
| Rat | ? | Acute fasudil | Vasodilation | (Crossno et al., 2007) |
| Rat | ? | Chronic Y-27632 | ↓ Hypertension and RVH | (McMurtry et al., 2003) |
| Rat | ↑ ROCK I and II expression | Acute and chronic Y-27632 | Vasodilation, ↓ hypertension, wall thickness and RVH | (Hyvelin et al., 2005) |
| Rat | ↑ RhoA and ROCK activity | Chronic sildenafil and fasudil | ↓ Hypertension, wall thickness and RVH | (Guilluy et al., 2005) |
| Rat | ↑ ROCK activity | Chronic simvastatin | ↓ Hypertension, wall thickness and RVH | (Girgis et al., 2007) |
| Neonatal rat | ↑ ROCK expression and activity | Acute Y-27632 and fasudil | Vasodilation | (McNamara et al., 2008) |
| FHR | ? | Acute fasudil | Vasodilation | (Nagaoka et al., 2005) |
| Neonatal FHR | ↑ RhoA activity and ROCK I expression | Chronic fasudil | ↓ Hypertension, wall thickness and RVH | (Nagaoka et al., 2006) |
| Mouse | ↑ ROCK activity | Chronic Y-27632 | ↓ Hypertension, neomuscularization and RVH ↑eNOS | (Fagan et al., 2004) |
| Mouse | ? | Chronic fasudil | ↓ Hypertension and RVH ↑eNOS | (Abe et al., 2006) |
| Monocrotaline | ||||
| Rat | ? | Acute fasudil | Vasodilation | (Nagaoka et al., 2005) |
| Rat | ↑ ROCK activity | Acute fasudil | Vasodilation | (Jiang et al., 2007) |
| Rat | ↑ ROCK activity | Chronic fasudil | ↓ Hypertension, wall thickness and RVH | (Abe et al., 2004) |
| Rat | ? | Chronic fasudil | ↓ Wall thickness and TN-C expression | (Chapados et al., 2006) |
| Rat | ? | Chronic fasudil+beraprost | ↓ Hypertension, wall thickness and RVH | (Tawara et al., 2007) |
| SU5416/hypoxia | ||||
| Rat | ↑ ROCK activity | Acute fasudil | Vasodilation | (Oka et al., 2007a) |
| Shunt | ||||
| Rat | ↑ RhoA and ROCK activity | Chronic fasudil | ↓ Hypertension, wall thickness and RVH | (Li et al., 2007a) |
| Bleomycin | ||||
| Neonatal rat | ↑ ROCK expression and activity | Acute Y-27632 and fasudil | Vasodilation | (McNamara et al., 2008) |
| Mouse | ↑ RhoA and ROCK activity | Chronic sildenafil | ↓ Hypertension, wall thickness and RVH | (Hemnes et al., 2008) |
| Human PH | ||||
| PH patients | ? | Acute fasudil | Vasodilation | (Fukumoto et al., 2005) |
| PH patients | ? | Acute fasudil | Vasodilation | (Ishikura et al., 2006) |
Abbreviations: eNOS, endothelial NOS; FHR, foetal heart rate; PH, pulmonary hypertension; ROCK, Rho-associated kinase; RVH, right ventricular hypertrophy.
Figure 2.
Schematic illustration of possible pathways and effects of RhoA/Rho kinase signalling in the pathogenesis of pulmonary hypertension (PH) (modified from Fukumoto et al. (2007) Tohoku J Exp Med 211: 309–320).
Chapados et al. (2006) have observed that Y-27632-induced inhibition of ROCK not only prevents stress fiber formation and the spreading of vascular SMC cultured on denatured collagen but also reduces nuclear extracellular signal-regulated kinase and tenascin-C expression. Importantly, they propose a scheme whereby signals arising from changes in medial collagen biochemistry can activate SMC RhoA/ROCK signalling, presumably through integrins, to mediate both sustained vasoconstriction and vascular remodelling. RhoA activation of downstream effectors such as ROCK, PKN and diaphanous-related formins 1 and 2 is important in regulating myocardin/serum response factor-dependent transcription of contractile proteins and thereby maintaining SMC contractile phenotype (Deaton et al., 2005; Hinson et al., 2007; Staus et al., 2007), and what remains to be sorted out is exactly how activation of RhoA/ROCK signalling can mediate cell contraction on the one hand and proliferation, migration and matrix protein synthesis on the other. Perhaps RhoA/ROCK signalling is differentially regulated among subsets of SMCs within a given segment of pulmonary artery (Bailly et al., 2004). RhoA/ROCK signalling is apparently activated in at least some SMCs of the hypertensive pulmonary arteries of various rodent models, but we do not yet know the status of this signalling cascade in the ECs and adventitial fibroblasts or in the cells of pulmonary artery neointimal lesions.
Negative results with ROCK inhibitors in rodent models of PH
In contrast to the considerable evidence for important involvement of RhoA/ROCK signalling in the pathophysiology of PH, there have been some negative results. For example, we observed that while administration of the ROCK inhibitor Y-27632 by subcutaneous osmotic minipump to chronically hypoxic mice (30 mg kg−1 day−1) and rats (40 mg kg−1 day−1) attenuated the PH (McMurtry et al., 2003; Fagan et al., 2004), treatment with fasudil in the drinking water (30 and 40 mg kg−1 day−1, respectively) did not (unpublished). On the other hand, fasudil administered to mice in the drinking water at a higher dose (100 mg kg−1 day−1) (Abe et al., 2006), and to rats by gavage (30 mg kg−1 day−1) (Guilluy et al., 2005), did inhibit development of hypoxic PH. Also, Shimokawa's group originally observed marked inhibition of monocrotaline-induced PH in rats by 30-mg kg−1 day−1 fasudil in the drinking water (Abe et al., 2004), but more recently found only modest effects of this dose when administered by i.p. minipump (Tawara et al., 2007). Finally, we observed in SU5416-injected/hypoxia-exposed rats that fasudil in the drinking water at 30 mg kg−1 day−1 attenuated the initial lung inflammation and development of PH, but administration of this dose twice daily by gavage for 3 weeks did not reverse the pathology once it had become established (unpublished). Although the gavage administration of fasudil acutely caused pulmonary vasodilation in these rats, the twice-daily dosing protocol apparently did not lead to persistent effects. It remains to be determined if higher or more frequent dosing will be effective.
Thus, not surprisingly, the effectiveness of ROCK inhibitors, and especially of fasudil, against PH seems to depend on dose, route of administration and animal model. We speculate that if given at sufficiently high doses, ROCK inhibitors will unmask some degree of vasoconstriction in most forms of PH, including severe PH in humans. However, ROCK activity contributes to basal systemic vascular tone (Bussemaker et al., 2007), and such high doses would likely have to be given through inhalation to avoid systemic hypotension (Nagaoka et al., 2005). Fasudil has been reported to have beneficial effects in patients with systemic hypertension, stroke, vasospastic angina, stable effort angina and chronic heart failure (Liao et al., 2007; Shimokawa and Rashid, 2007), but whether long-term oral treatment with the drug at doses that do not cause systemic hypotension will reverse severe, established PH remains to be determined. We will hopefully know this soon, as clinical trials with oral fasudil in patients with PH are currently being planned in Japan (Fukumoto et al., 2007). As is seemingly the case with the currently approved PH therapeutic agents, it may be more efficacious to use fasudil or some other ROCK inhibitor, in combination with another type of drug (Tawara et al., 2007). Fasudil is currently the only ROCK inhibitor approved for human use, but several biotechnology and pharmaceutical companies are investigating numerous other ROCK isoform-selective and -nonselective compounds (Liao et al., 2007; Shimokawa and Rashid, 2007).
Rac in pathogenesis of PH
Rac is another ubiquitously expressed small GTPase that contributes to regulation of a wide variety of cellular functions. With respect to vascular diseases, Rac activation has most often been linked to increased oxidative stress by its role in assembly and activation of the NADPH oxidase complex and resultant increase in production of superoxide anion (Hordijk, 2006). Mice engineered to overexpress a constitutively active mutant of Rac1 in SMCs develop moderate systemic hypertension associated with increased vascular levels of superoxide and peroxynitrite (Hassanain et al., 2007). Black and his colleagues found in lambs with a surgically created left to right shunt that PH was associated with increased superoxide-dependent oxidative stress in hypertensive pulmonary arteries, and the increased superoxide production was attributed to combined effects of activation of NADPH oxidase and an uncoupling of eNOS (Grobe et al., 2006). The same group also previously reported that serum-induced proliferation of lamb PASMCs is dependent on activation of Rac1 and NADPH-mediated ROS production (Patil et al., 2004). This is similar to the observations of Fanburg's laboratory that serotonin-induced growth of bovine PASMCs involves Rac1/NADPH oxidase/ROS-dependent activation of extracellular signal-regulated kinase and stimulation of c-fos and cyclin D1 expression (Lee et al., 2001; Simon et al., 2005). Carlin et al. (2007) recently observed that hypoxia-induced activation of p38 mitogen-activated protein kinase and proliferation of rat pulmonary artery adventitial fibroblasts were inhibited by statins and the Rac1 inhibitor NSC23766, but not by the ROCK-inhibitor hydroxyfasudil. In contrast, ECs cultured from chronically hypoxic piglets exhibited decreased activities of Rac1 and its downstream effector p21 activated kinase and increased activity of RhoA (Wojciak-Stothard et al., 2006).
Conclusion
Although numerous studies collectively suggest that RhoA/ROCK signalling is involved significantly in the pathogenesis of experimental as well as human PH, and that ROCK inhibitors show promise in the treatment of PH, several questions regarding the roles and mechanisms of RhoA/ROCK signalling in the development and progression of the disease remain unanswered. For example, it remains unknown what upstream signals are responsible for the activation of RhoA/ROCK signalling. The relative roles of various GPCRs, reactive oxygen species, extracellular matrix proteins and integrins, RhoA-independent signals, cleaved and constitutively active ROCKs, and increased transmural pressure per se in different segments of the pulmonary arterial tree in different forms of PH are unclear. The relative roles of ROCK I and II are also uncertain. Although ROCKs are undoubtedly involved, do any of the other downstream effectors or RhoA, including diaphanous-related formins 1 and 2, PKN and citron kinase, play important roles in the pathogenesis of PH? ROCK-mediated constriction of pulmonary resistance arteries clearly contributes to increased pulmonary vascular resistance in several experimental models of PH, but whether this acts parallel to (Ca2+ sensitization) or in series with Ca2+ signalling has not been established. Is the high vascular tone due only to increased ROCK-dependent MLC phosphorylation or is increased actin polymerization through RhoA-, ROCK- or c-Abl-dependent pathways (Anfinogenova et al., 2007; Zhang and Gunst, 2008) also involved? Similarly, it would be interesting to know if SMC contraction, by either MLC phosphorylation or actin polymerization, contributes to the decreased distensibility of hypertensive conduit pulmonary arteries. The hallmark of severe progressive PH in humans is the formation of obstructive neointimal lesions in small pulmonary arteries and a poor pulmonary vascular response to acute vasodilator testing. Thus, it will be informative to test if ROCK-mediated vasoconstriction contributes to the increased pulmonary vascular resistance in patients with severe PH, even in those unresponsive to conventional vasodilators such as inhaled NO, inhaled iloprost and i.v. epoprostenol, and if chronic administration of ROCK inhibitors effectively reverses the obstructive neointimal lesions. In this regard, there is a need to define the differential effects on pulmonary vascular function and structure of activation RhoA/ROCK (and Rac1) in SMCs, ECs, fibroblasts and perivascular inflammatory cells. Finally, several recent studies implicate ROCK activity in the left ventricular fibrosis and contractile dysfunction (Balakumar and Singh, 2006; Brown et al., 2006; Chang et al., 2006; Peters and Michel, 2007; Shi et al., 2008), and it will be important to define the roles of Rac1, RhoA and ROCKs in the right ventricular failure of severe PH.
Acknowledgments
The writing of this review was supported by grants from the National Institute of Health (HL 14985 and HL 07171) and the American Heart Association (SDG 0335208N and BGIA 0765477Z), and by the University of South Alabama salary support for IF McMurtry. We thank Asahi Kasei Corporation (Tokyo, Japan) for providing fasudil and MN Gillespie for helpful editorial comments.
Abbreviations
- EC
endothelial cell
- eNOS
endothelial NOS
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- NO
nitric oxide
- PASMCs
pulmonary artery smooth muscle cells
- PH
pulmonary hypertension
- ROCK
Rho-associated kinase
- SMC
smooth muscle cell
Conflict of interest
The authors state no conflict of interest.
References
- Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- Abe K, Tawara S, Oi K, Hizume T, Uwatoku T, Fukumoto Y, et al. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol. 2006;48:280–285. doi: 10.1097/01.fjc.0000248244.64430.4a. [DOI] [PubMed] [Google Scholar]
- Aihara M, Dobashi K, Iizuka K, Nakazawa T, Mori M. Effect of Y-27632 on release of cytokines from peripheral T cells in asthmatic patients and normal subjects. Int Immunopharmacol. 2004;4:557–561. doi: 10.1016/j.intimp.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Anfinogenova Y, Wang R, Li Q-F, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res. 2007;101:420–428. doi: 10.1161/CIRCRESAHA.107.156463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida N, Arai H, Yamasaki M, Kita T. Distinct signaling pathways for MCP-1-dependent integrin activation and chemotaxis. J Biol Chem. 2001;276:16555–16560. doi: 10.1074/jbc.M009068200. [DOI] [PubMed] [Google Scholar]
- Azam MA, Yoshioka K, Ohkura S, Takuwa N, Sugimoto N, Sato K, et al. Ca2+-independent, inhibitory effects of cyclic adenosine 5′-monophosphate on Ca2+ regulation of phosphoinositide 3-kinase C2alpha, Rho, and myosin phosphatase in vascular smooth muscle. J Pharmacol Exp Ther. 2007;320:907–916. doi: 10.1124/jpet.106.111443. [DOI] [PubMed] [Google Scholar]
- Bailly K, Ridley AJ, Hall SM, Haworth SG. RhoA activation by hypoxia in pulmonary arterial smooth muscle cells is age and site specific. Circ Res. 2004;94:1383–1391. doi: 10.1161/01.RES.0000128405.83582.2e. [DOI] [PubMed] [Google Scholar]
- Balakumar P, Singh M. Differential role of rho-kinase in pathological and physiological cardiac hypertrophy in rats. Pharmacology. 2006;78:91–97. doi: 10.1159/000095784. [DOI] [PubMed] [Google Scholar]
- Bardi G, Niggli V, Loetscher P. Rho kinase is required for CCR7-mediated polarization and chemotaxis of T lymphocytes. FEBS Lett. 2003;542:79–83. doi: 10.1016/s0014-5793(03)00351-x. [DOI] [PubMed] [Google Scholar]
- Barman SA. Vasoconstrictor effect of endothelin-1 on hypertensive pulmonary arterial smooth muscle involves Rho-kinase and protein kinase C. Am J Physiol Lung Cell Mol Physiol. 2007;293:L472–L479. doi: 10.1152/ajplung.00101.2006. [DOI] [PubMed] [Google Scholar]
- Beutz MA, Nagaoka T, Oka M, McMurtry I F. Sphingosine-1-phosphate constricts fawn-hooded rat pulmonary arteries. Proc Am Thoracic Soc. 2005;2:A706. [Google Scholar]
- Boer C, van der Linden PJ, Scheffer GJ, Westerhof N, de Lange JJ, Sipkema P. RhoA/Rho kinase and nitric oxide modulate the agonist-induced pulmonary artery diameter response time. Am J Physiol Heart Circ Physiol. 2002;282:H990–H998. doi: 10.1152/ajpheart.00093.2001. [DOI] [PubMed] [Google Scholar]
- Bolick DT, Orr AW, Whetzel A, Srinivasan S, Hatley ME, Schwartz MA, et al. 12/15-lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2005;25:2301–2307. doi: 10.1161/01.ATV.0000186181.19909.a6. [DOI] [PubMed] [Google Scholar]
- Bonnet S, Dumas-de-La-Roque E, Begueret H, Marthan R, Fayon M, Dos Santos P, et al. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA. 2003;100:9488–9493. doi: 10.1073/pnas.1633724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008. [DOI] [PubMed]
- Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006;27:97–104. doi: 10.1016/j.tips.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bussemaker E, Pistrosch F, Forster S, Herbrig K, Gross P, Passauer J, et al. Rho kinase contributes to basal vascular tone in humans: role of endothelium-derived nitric oxide. Am J Physiol Heart Circ Physiol. 2007;293:H541–H547. doi: 10.1152/ajpheart.00770.2006. [DOI] [PubMed] [Google Scholar]
- Carlin CM, Peacock AJ, Welsh DJ. Fluvastatin inhibits hypoxic proliferation and p38 MAPK activity in pulmonary artery fibroblasts. Am J Respir Cell Mol Biol. 2007;37:447–456. doi: 10.1165/rcmb.2007-0012OC. [DOI] [PubMed] [Google Scholar]
- Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci USA. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapados R, Abe K, Ihida-Stansbury K, McKean D, Gates AT, Kern M, et al. ROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodeling. Circ Res. 2006;99:837–844. doi: 10.1161/01.RES.0000246172.77441.f1. [DOI] [PubMed] [Google Scholar]
- Cordle A, Koenigsknecht-Talboo J, Wilkinson B, Limpert A, Landreth G. Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem. 2005;280:34202–34209. doi: 10.1074/jbc.M505268200. [DOI] [PubMed] [Google Scholar]
- Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, et al. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L885–L897. doi: 10.1152/ajplung.00258.2006. [DOI] [PubMed] [Google Scholar]
- Damron DS, Kanaya N, Homma Y, Kim SO, Murray PA. Role of PKC, tyrosine kinases, and Rho kinase in alpha-adrenoreceptor-mediated PASM contraction. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1051–L1064. doi: 10.1152/ajplung.00345.2001. [DOI] [PubMed] [Google Scholar]
- Deaton RA, Su C, Valencia TG, Grant SR. Transforming growth factor-{beta}1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J Biol Chem. 2005;280:31172–31181. doi: 10.1074/jbc.M504774200. [DOI] [PubMed] [Google Scholar]
- Eto Y, Shimokawa H, Hiroki J, Morishige K, Kandabashi T, Matsumoto Y, et al. Gene transfer of dominant negative Rho kinase suppresses neointimal formation after balloon injury in pigs. Am J Physiol Heart Circ Physiol. 2000;278:H1744–H1750. doi: 10.1152/ajpheart.2000.278.6.H1744. [DOI] [PubMed] [Google Scholar]
- Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- Fu M, Zhang J, Tseng YH, Cui T, Zhu X, Xiao Y, et al. Rad GTPase attenuates vascular lesion formation by inhibition of vascular smooth muscle cell migration. Circulation. 2005;111:1071–1077. doi: 10.1161/01.CIR.0000156439.55349.AD. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91:391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto Y, Tawara S, Shimokawa H. Recent progress in the treatment of pulmonary arterial hypertension: expectation for rho-kinase inhibitors. Tohoku J Exp Med. 2007;211:309–320. doi: 10.1620/tjem.211.309. [DOI] [PubMed] [Google Scholar]
- Funakoshi Y, Ichiki T, Shimokawa H, Egashira K, Takeda K, Kaibuchi K, et al. Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension. 2001;38:100–104. doi: 10.1161/01.hyp.38.1.100. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Komori K, Shimokawa H, Matsumoto Y, Uwatoku T, Hirano K, et al. Long-term inhibition of Rho kinase suppresses intimal thickening in autologous vein grafts in rabbits. J Vasc Surg. 2006;43:1249–1256. doi: 10.1016/j.jvs.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Ghisdal P, Vandenberg G, Morel N. Rho-dependent kinase is involved in agonist-activated calcium entry in rat arteries. J Physiol. 2003;551:855–867. doi: 10.1113/jphysiol.2003.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RE, Li D, Zhan X, Garcia JG, Tuder RM, Hassoun PM, et al. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol. 2003;285:H938–H945. doi: 10.1152/ajpheart.01097.2002. [DOI] [PubMed] [Google Scholar]
- Girgis RE, Mozammel S, Champion HC, Li D, Peng X, Shimod L, et al. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1105–L1110. doi: 10.1152/ajplung.00411.2006. [DOI] [PubMed] [Google Scholar]
- Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, et al. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- Guerard P, Rakotoniaina Z, Goirand F, Rochette L, Dumas M, Lirussi F, et al. The HMG-CoA reductase inhibitor, pravastatin, prevents the development of monocrotaline-induced pulmonary hypertension in the rat through reduction of endothelial cell apoptosis and overexpression of eNOS. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:401–414. doi: 10.1007/s00210-006-0082-1. [DOI] [PubMed] [Google Scholar]
- Guerin P, Sauzeau V, Rolli-Derkinderen M, Al Habbash O, Scalbert E, Crochet D, et al. Stent implantation activates RhoA in human arteries: inhibitory effect of rapamycin. J Vasc Res. 2005;42:21–28. doi: 10.1159/000082873. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Rolli-Derkinderen M, Tharaux P-L, Melino G, Pacaud P, Loirand G. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J Biol Chem. 2007;282:2918–2928. doi: 10.1074/jbc.M604195200. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guerin P, Sagan C, Pacaud P, et al. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol. 2005;146:1010–1018. doi: 10.1038/sj.bjp.0706408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl V, Bibova J, Povysilova V, Herget J. Dehydroepiandrosterone sulphate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J. 2003;21:862–865. doi: 10.1183/09031936.03.00084503. [DOI] [PubMed] [Google Scholar]
- Hassanain HH, Gregg D, Marcelo ML, Zweier JL, Souza HP, Selvakumar B, et al. Hypertension caused by transgenic overexpression of Rac1. Antioxid Redox Signal. 2007;9:91–100. doi: 10.1089/ars.2007.9.91. [DOI] [PubMed] [Google Scholar]
- Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Kaibuchi K, et al. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- Hemnes AR, Wigley F, Rodrigues FW, Girgis RE, Yang SC, Conte JV, et al. Pulmonary hypertension is associated with increased expression and activity of phosphodiesterase type 5A. Circulation. 2005;112:II-221–II-222. [Google Scholar]
- Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L24–L33. doi: 10.1152/ajplung.00245.2007. [DOI] [PubMed] [Google Scholar]
- Hinson JS, Medlin MD, Lockman K, Taylor JM, Mack CP. Smooth muscle cell-specific transcription is regulated by nuclear localization of the myocardin-related transcription factors. Am J Physiol Heart Circ Physiol. 2007;292:H1170–H1180. doi: 10.1152/ajpheart.00864.2006. [DOI] [PubMed] [Google Scholar]
- Homma N, Nagaoka T, Karoor V, Imamura M, Taraseviciene-Stewart L, et al. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone Am J Physiol Lung Cell Mol Physiol 2008. in press [DOI] [PMC free article] [PubMed]
- Honing H, van den Berg TK, van der Pol SM, Dijkstra CD, van der Kammen RA, Collard JG, et al. RhoA activation promotes transendothelial migration of monocytes via ROCK. J Leukoc Biol. 2004;75:523–528. doi: 10.1189/jlb.0203054. [DOI] [PubMed] [Google Scholar]
- Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- Horton JW, Maass DL, Ballard-Croft C. Rho-associated kinase modulates myocardial inflammatory cytokine responses. Shock. 2005;24:53–58. doi: 10.1097/01.shk.0000167109.96000.7f. [DOI] [PubMed] [Google Scholar]
- Howell K, Ooi H, Preston R, McLoughlin P. Structural basis of hypoxic pulmonary hypertension: the modifying effect of chronic hypercapnia. Exp Physiol. 2004;89:66–72. doi: 10.1113/expphysiol.2003.026765. [DOI] [PubMed] [Google Scholar]
- Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res. 2005;97:185–191. doi: 10.1161/01.RES.0000174287.17953.83. [DOI] [PubMed] [Google Scholar]
- Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, et al. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- Iso Y, Suzuki H, Sato T, Shoji M, Shimizu N, Shibata M, et al. Rho-kinase inhibitor suppressed restenosis in porcine coronary balloon angioplasty. Int J Cardiol. 2006;106:103–110. doi: 10.1016/j.ijcard.2004.12.079. [DOI] [PubMed] [Google Scholar]
- Ito T, Ikeda U, Shimpo M, Ohki R, Takahashi M, Yamamoto K, et al. HMG-CoA reductase inhibitors reduce interleukin-6 synthesis in human vascular smooth muscle cells. Cardiovasc Drugs Ther. 2002;16:121–126. doi: 10.1023/a:1015701415588. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Premji M, Netherton S, Coruzzi J, Lu-Chao H, Cox PG. Vasoconstrictor actions of isoprostanes via tyrosine kinase and Rho kinase in human and canine pulmonary vascular smooth muscles. Br J Pharmacol. 2001;132:127–134. doi: 10.1038/sj.bjp.0703784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan NL, Walker BR, Resta TC. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1220–L1229. doi: 10.1152/ajplung.00196.2004. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Tawara S, Abe K, Takaki A, Fukumoto Y, Shimokawa H. Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;49:85–89. doi: 10.1097/FJC.0b013e31802df112. [DOI] [PubMed] [Google Scholar]
- Kanda T, Hayashi K, Wakino S, Homma K, Yoshioka K, Hasegawa K, et al. Role of Rho-kinase and p27 in angiotensin II-induced vascular injury. Hypertension. 2005;45:724–729. doi: 10.1161/01.HYP.0000153316.59262.79. [DOI] [PubMed] [Google Scholar]
- Kao PN. Simvastatin treatment of pulmonary hypertension: an observational case series. Chest. 2005;127:1446–1452. doi: 10.1378/chest.127.4.1446. [DOI] [PubMed] [Google Scholar]
- Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39:245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- Laudi S, Trump S, Schmitz V, West J, McMurtry IF, Mutlak H, et al. Serotonin transporter protein in pulmonary hypertensive rats treated with atorvastatin. Am J Physiol Lung Cell Mol Physiol. 2007;293:L630–L638. doi: 10.1152/ajplung.00110.2006. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee DS, Kim EK, Choe KH, Oh YM, Shim TS, et al. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med. 2005;172:987–993. doi: 10.1164/rccm.200501-041OC. [DOI] [PubMed] [Google Scholar]
- Lee SL, Simon AR, Wang WW, Fanburg BL. H2O2 signals 5-HT-induced ERK MAP kinase activation and mitogenesis of smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L646–L652. doi: 10.1152/ajplung.2001.281.3.L646. [DOI] [PubMed] [Google Scholar]
- Li F, Xia W, Li A, Zhao C, Sun R. Long-term inhibition of Rho kinase with fasudil attenuates high flow induced pulmonary artery remodeling in rats. Pharmacol Res. 2007a;55:64–71. doi: 10.1016/j.phrs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Li M, Liu Y, Dutt P, Fanburg BL, Toksoz D. Inhibition of serotonin-induced mitogenesis, migration, and ERK MAPK nuclear translocation in vascular smooth muscle cells by atorvastatin. Am J Physiol Lung Cell Mol Physiol. 2007b;293:L463–L471. doi: 10.1152/ajplung.00133.2007. [DOI] [PubMed] [Google Scholar]
- Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fanburg BL. Serotonin-induced growth of pulmonary artery smooth muscle requires activation of phosphatidylinositol 3-kinase/serine-threonine protein kinase B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am J Respir Cell Mol Biol. 2006;34:182–191. doi: 10.1165/rcmb.2005-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res. 2004;95:579–586. doi: 10.1161/01.RES.0000141428.53262.a4. [DOI] [PubMed] [Google Scholar]
- Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- Losapio JL, Sprague RS, Lonigro AJ, Stephenson AH. 5,6-EET-induced contraction of intralobar pulmonary arteries depends on the activation of Rho-kinase. J Appl Physiol. 2005;99:1391–1396. doi: 10.1152/japplphysiol.00473.2005. [DOI] [PubMed] [Google Scholar]
- Luykenaar KD, Brett SE, Wu BN, Wiehler WB, Welsh DG. Pyrimidine nucleotides suppress KDR currents and depolarize rat cerebral arteries by activating Rho kinase. Am J Physiol Heart Circ Physiol. 2004;286:H1088–H1100. doi: 10.1152/ajpheart.00903.2003. [DOI] [PubMed] [Google Scholar]
- Macchia A, Marchioli R, Marfisi R, Scarano M, Levantesi G, Tavazzi L, et al. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J. 2007;153:1037–1047. doi: 10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Martin C, Goggel R, Ressmeyer AR, Uhlig S. Pressor responses to platelet-activating factor and thromboxane are mediated by Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L250–L257. doi: 10.1152/ajplung.00420.2003. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Uwatoku T, Oi K, Abe K, Hattori T, Morishige K, et al. Long-term inhibition of Rho-kinase suppresses neointimal formation after stent implantation in porcine coronary arteries: involvement of multiple mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:181–186. doi: 10.1161/01.ATV.0000105053.46994.5B. [DOI] [PubMed] [Google Scholar]
- McMurtry IF, Bauer NR, Fagan KA, Nagaoka T, Gebb SA, Oka M. Hypoxia and Rho/Rho-kinase signaling. Lung development versus hypoxic pulmonary hypertension. Adv Exp Med Biol. 2003;543:127–137. [PubMed] [Google Scholar]
- McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin (RATS) study. Am J Physiol Lung Cell Mol Physiol. 2007;293:L933–L940. doi: 10.1152/ajplung.00310.2006. [DOI] [PubMed] [Google Scholar]
- McNamara PJ, Murthy P, Kantores C, Teixeira L, Engelberts D, van Vliet T, et al. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2008;294:L205–L213. doi: 10.1152/ajplung.00234.2007. [DOI] [PubMed] [Google Scholar]
- Murata T, Kinoshita K, Hori M, Kuwahara M, Tsubone H, Karaki H, et al. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2005;25:2335–2342. doi: 10.1161/01.ATV.0000186184.33537.48. [DOI] [PubMed] [Google Scholar]
- Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, et al. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:494–499. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Gebb SA, Karoor V, Homma N, Morris KG, McMurtry IF, et al. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol. 2006;100:996–1002. doi: 10.1152/japplphysiol.01028.2005. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, et al. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287:L665–L672. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Faul JL, Berry GJ, Vaszar LT, Qiu D, Pearl RG, et al. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med. 2002;166:1403–1408. doi: 10.1164/rccm.200203-268OC. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108:1640–1645. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–C668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, et al. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007a;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, et al. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res. 2007b;74:377–387. doi: 10.1016/j.cardiores.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale RM, Simile MM, De Miglio MR, Nufris A, Seddaiu MA, Muroni MR, et al. Inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase activity and gene expression by dehydroepiandrosterone in preneoplastic liver nodules. Carcinogenesis. 1995;16:1537–1542. doi: 10.1093/carcin/16.7.1537. [DOI] [PubMed] [Google Scholar]
- Patil S, Bunderson M, Wilham J, Black SM. Important role for Rac1 in regulating reactive oxygen species generation and pulmonary arterial smooth muscle cell growth. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1314–L1322. doi: 10.1152/ajplung.00383.2003. [DOI] [PubMed] [Google Scholar]
- Peters SL, Michel MC. The RhoA/Rho kinase pathway in the myocardium. Cardiovasc Res. 2007;75:3–4. doi: 10.1016/j.cardiores.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003;139:329–336. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotoniaina Z, Guerard P, Lirussi F, Goirand F, Rochette L, Dumas M, et al. The protective effect of HMG-CoA reductase inhibitors against monocrotaline-induced pulmonary hypertension in the rat might not be a class effect: comparison of pravastatin and atorvastatin. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:195–206. doi: 10.1007/s00210-006-0112-z. [DOI] [PubMed] [Google Scholar]
- Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005;288:C769–C783. doi: 10.1152/ajpcell.00529.2004. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson TP, Aaronson PI, Ward JP. Ca2+ sensitization during sustained hypoxic pulmonary vasoconstriction is endothelium dependent. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1121–L1126. doi: 10.1152/ajplung.00422.2002. [DOI] [PubMed] [Google Scholar]
- Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000;131:5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruperez M, Sanchez-Lopez E, Blanco-Colio LM, Esteban V, Rodriguez-Vita J, Plaza JJ, et al. The Rho-kinase pathway regulates angiotensin II-induced renal damage. Kidney Int. 2005;68 Suppl 99:S39–S45. doi: 10.1111/j.1523-1755.2005.09908.x. [DOI] [PubMed] [Google Scholar]
- Saito H, Minamiya Y, Saito S, Ogawa J. Endothelial Rho and Rho kinase regulate neutrophil migration via endothelial myosin light chain phosphorylation. J Leukoc Biol. 2002;72:829–836. [PubMed] [Google Scholar]
- Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, et al. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003;93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–1876. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, et al. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- Sawada N, Itoh H, Yamashita J, Doi K, Inoue M, Masatsugu K, et al. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem Biophys Res Commun. 2001;280:798–805. doi: 10.1006/bbrc.2000.4194. [DOI] [PubMed] [Google Scholar]
- Schulz S, Nyce JW. Inhibition of protein isoprenylation and p21ras membrane association by dehydroepiandrosterone in human colonic adenocarcinoma cells in vitro. Cancer Res. 1991;51:6563–6567. [PubMed] [Google Scholar]
- Sebbagh M, Hamelin J, Bertoglio J, Solary E, Breard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med. 2005;201:465–471. doi: 10.1084/jem.20031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Segain JP, Raingeard de la Bletiere D, Sauzeau V, Bourreille A, Hilaret G, Cario-Toumaniantz C, et al. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn's disease and experimental colitis. Gastroenterology. 2003;124:1180–1187. doi: 10.1016/s0016-5085(03)00283-x. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhang YW, Summers LJ, Dorn GW, II, Wei L. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. J Mol Cell Cardiol. 2008;44:551–560. doi: 10.1016/j.yjmcc.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Kai H, Seki Y, Kusaba K, Takemiya K, Koga M, et al. Rho-kinase inhibition reduces neointima formation after vascular injury by enhancing Bax expression and apoptosis. J Cardiovasc Pharmacol. 2003;42 Suppl 1:S43–S47. doi: 10.1097/00005344-200312001-00011. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- Simon AR, Severgnini M, Takahashi S, Rozo L, Andrahbi B, Agyeman A, et al. 5-HT induction of c-fos gene expression requires reactive oxygen species and Rac1 and Ras GTPases. Cell Biochem Biophys. 2005;42:263–276. doi: 10.1385/CBB:42:3:263. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Staus DP, Blaker AL, Taylor JM, Mack CP. Diaphanous 1 and 2 regulate smooth muscle cell differentiation by activating the myocardin-related transcription factors. Arterioscler Thromb Vasc Biol. 2007;27:478–486. doi: 10.1161/01.ATV.0000255559.77687.c1. [DOI] [PubMed] [Google Scholar]
- Tamura M, Nakao H, Yoshizaki H, Shiratsuchi M, Shigyo H, Yamada H, et al. Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta. 2005;1754:245–252. doi: 10.1016/j.bbapap.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, Tuder RM, et al. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L668–L676. doi: 10.1152/ajplung.00491.2005. [DOI] [PubMed] [Google Scholar]
- Tawara S, Fukumoto Y, Shimokawa H. Effects of combined therapy with a Rho-kinase inhibitor and prostacyclin on monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;50:195–200. doi: 10.1097/FJC.0b013e31806befe6. [DOI] [PubMed] [Google Scholar]
- Tharaux PL, Bukoski RC, Rocha PN, Crowley SD, Ruiz P, Nataraj C, et al. Rho kinase promotes alloimmune responses by regulating the proliferation and structure of T cells. J Immunol. 2003;171:96–105. doi: 10.4049/jimmunol.171.1.96. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Snetkov VA, Patel R, Leach RM, Aaronson PI, Ward JP. Sphingosylphosphorylcholine-induced vasoconstriction of pulmonary artery: activation of non-store-operated Ca2+ entry. Cardiovasc Res. 2005;68:56–64. doi: 10.1016/j.cardiores.2005.05.013. [DOI] [PubMed] [Google Scholar]
- van Suylen RJ, Smits JF, Daemen MJ. Pulmonary artery remodeling differs in hypoxia- and monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med. 1998;157:1423–1428. doi: 10.1164/ajrccm.157.5.9709050. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yoshioka K, Azam MA, Takuwa N, Sakurada S, Kayaba Y, et al. Class II phosphoinositide 3-kinase alpha-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle. Biochem J. 2006;394:581–592. doi: 10.1042/BJ20051471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2001;25:628–635. doi: 10.1165/ajrcmb.25.5.4461. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lanner MC, Jin N, Swartz D, Li L, Rhoades RA. Hypoxia inhibits myosin phosphatase in pulmonary arterial smooth muscle cells: role of Rho-kinase. Am J Respir Cell Mol Biol. 2003;29:465–471. doi: 10.1165/rcmb.2002-0157OC. [DOI] [PubMed] [Google Scholar]
- Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2006;290:L284–L290. doi: 10.1152/ajplung.00449.2004. [DOI] [PubMed] [Google Scholar]
- Witzenrath M, Ahrens B, Kube SM, Hocke AC, Rosseau S, Hamelmann E, et al. Allergic lung inflammation induces pulmonary vascular hyperresponsiveness. Eur Respir J. 2006;28:370–377. doi: 10.1183/09031936.06.00080105. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Tsang LYF, Paleolog E, Hall SM, Haworth SG. Rac1 and RhoA as regulators of endothelial phenotype and barrier function in hypoxia-induced neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1173–L1182. doi: 10.1152/ajplung.00309.2005. [DOI] [PubMed] [Google Scholar]
- Xing XQ, Gan Y, Wu SJ, Chen P, Zhou R, Xiang XD. Statins may ameliorate pulmonary hypertension via RhoA/Rho-kinase signaling pathway. Med Hypotheses. 2007;68:1108–1113. doi: 10.1016/j.mehy.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Yaghi A, Sims SM. Constrictor-induced translocation of NFAT3 in human and rat pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1061–L1074. doi: 10.1152/ajplung.00096.2005. [DOI] [PubMed] [Google Scholar]
- Yamanouchi D, Banno H, Nakayama M, Sugimoto M, Fujita H, Kobayashi M, et al. Hydrophilic statin suppresses vein graft intimal hyperplasia via endothelial cell-tropic Rho-kinase inhibition. J Vasc Surg. 2005;42:757–764. doi: 10.1016/j.jvs.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Zhang W, Gunst SJ. Interactions of airway smooth muscle cells with their tissue matrix: Implications for contraction. Proc Am Thorac Soc. 2008;5:32–39. doi: 10.1513/pats.200704-048VS. [DOI] [PMC free article] [PubMed] [Google Scholar]