Abstract
Background and purpose:
Sensory nerves regulate central and local reflexes such as airway plasma protein leakage, bronchoconstriction and cough. Sensory nerve activity may be enhanced during inflammation such that these protective effects become exacerbated and deleterious. Cannabinoids are known to inhibit airway sensory nerve function. However, there is still controversy surrounding which receptor is involved in eliciting these effects.
Experimental approach:
We have adopted a pharmacological approach, including using a novel, more selective CB2 receptor agonist, GW 833972A (1000-fold selective CB2/CB1), and receptor selective antagonists to investigate the inhibitory activity of cannabinoids on sensory nerve activity in vitro and in vivo in guinea-pig models of cough and plasma extravasation.
Key results:
GW 833972A inhibited capsaicin-induced depolarization of the human and guinea-pig and prostaglandin E2 (PGE2) and hypertonic saline-induced depolarization of the guinea-pig isolated vagus nerve in vitro. GW 833972A also inhibited citric acid-induced cough but not plasma extravasation in the guinea-pig and this effect was blocked by a CB2 receptor antagonist.
Conclusions and implications:
This confirms and extends previous studies highlighting the role of CB2 receptors in the modulation of sensory nerve activity elicited both by the exogenous ligands capsaicin and hypertonic saline but also by endogenous modulators such as PGE2 and low pH stimuli. These data establish the CB2 receptor as an interesting target for the treatment of chronic cough.
Keywords: cannabinoids; airway plasma protein extravasation; human, sensory nerves; neurogenic inflammation; asthma/chronic obstructive pulmonary disease
Introduction
Sensory nerves in the airways regulate central and local reflex events such as bronchoconstriction, airway plasma protein leakage and cough. Sensory nerve activity may be enhanced during inflammation such that these protective reflexes become exacerbated and deleterious (Barnes, 2001; Belvisi, 2002). Sensory nerve reflexes are under the control of different classes of sensory fibres, including the myelinated, rapidly adapting receptors and the non-myelinated, chemosensitive C-fibres with bronchial or pulmonary endings that are activated by mechanical and chemical stimuli (Sant'Ambrogio et al., 1978; Coleridge and Coleridge, 1984; Canning et al., 2004). In the airways, activation of rapidly adapting receptors (irritant receptors) and C-fibres elicits cough, bronchoconstriction, plasma protein exudation and mucus secretion via an afferent central reflex pathway (Widdicombe, 1954; Karlsson et al., 1988; Karlsson, 1993; Coleridge and Coleridge, 1994; Lalloo et al., 1995; Belvisi, 2003). Activation of C-fibres in the guinea-pig and rodent airways also mediates efferent excitatory non-adrenergic non-cholinergic responses such as bronchoconstriction, mucus secretion, plasma protein exudation and vasodilatation via the peripheral release of neuropeptides, a phenomenon known as ‘neurogenic inflammation' (Barnes et al., 1991).
Currently, there is renewed interest in the therapeutic potential of cannabinoids, including the major active principle of marijuana, Δ9-tetrahydrocannabinol. Non-selective cannabinoids have been shown to have wide therapeutic applications for a number of important medical conditions, including pain, anxiety, glaucoma, nausea, emesis, muscle spasms and wasting diseases. However, associated side effects such as sedation, cognitive dysfunction, tachycardia and psychotropic effects have hampered the use of these compounds in treatment protocols (Porter and Felder, 2001). Cannabinoids mediate their effects via at least two specific G protein-coupled receptors, termed the CB1 and CB2 receptor (Matsuda et al., 1990; Munro et al., 1993). CB1 receptors are predominantly distributed throughout the brain and spinal cord and are also expressed at low levels in several peripheral tissues. In contrast, CB2 receptors are not commonly expressed in the CNS (Munro et al., 1993; Griffin et al., 1997; Buckley et al., 2000), but primarily on immune tissues such as the spleen, tonsils and lymphocytes (Galiegue et al., 1995). Studies suggest that cannabinoids have diverse effects on sensory nerve function. Activation of spinal CB1 receptors inhibits nociceptive transmission (Harris et al., 2000), hyperalgesia and neuropeptide release from central primary afferent fibres (Richardson et al., 1998a). The endocannabinoid anandamide has also been shown to reduce carrageenan-induced hyperalgesia, oedema, plasma protein extravasation and capsaicin-induced neuropeptide release via peripheral CB1 receptor activation (Richardson et al., 1998b). More recently, CB2 receptor activation has been demonstrated to inhibit acute nociception, inflammatory hyperalgesia, and the allodynia and hyperalgesia produced in models of neuropathic pain (Hanus et al., 1999; Clayton et al., 2002; Malan et al., 2002; Elmes et al., 2005).
There is still very little information on CB2 receptors on peripheral sensory nerves in the airways. We have previously demonstrated, using a commercially available cannabinoid CB2 receptor agonist (JWH 133), that activation of the CB2 receptor subtype inhibits capsaicin-induced depolarization of the guinea-pig and human vagus (Patel et al., 2003). Moreover, we have also demonstrated inhibition of sensory nerve function in vivo with CB2 agonists in a conscious guinea-pig model of cough (Patel et al., 2003). However, confirmation of the role of the CB2 receptor as a target for antitussive therapies awaits the use of more selective CB2 agonists and experiments demonstrating the sensitivity of this inhibitory response to blockade by a selective CB2 receptor antagonist. Identification of a particular receptor as being responsible for a certain effect is achieved with greater certainty using selective antagonists rather than simply relying on the selectivity of agonists. This is particularly true when high concentrations of agonists have been used to elicit an effect, which may no longer be selective in vivo. This is clearly a key set of experiments to perform given that our original study (Patel et al., 2003) is in complete contrast to a recent paper in which they highlight an antitussive role for the CB1 receptor in a model of cough (Calignano et al., 2000).
In the experiments described, we have initially characterized the effect of the novel selective CB2 receptor agonist (GW 833972A) (see Figure 2 for structure) on sensory nerve activity in vitro by directly measuring nerve depolarization elicited by capsaicin before and after superperfusion with the compound. Furthermore, using this methodology, we have characterized the response of guinea-pig vagal sensory nerves to the sensory nerve stimulants, hypertonic saline (Lalloo et al., 1995; Fox et al., 1996, 1997), capsaicin (Lalloo et al., 1995; Fox et al., 1996) and prostaglandin E2 (PGE2) (Roberts et al., 1985; Smith et al., 1998) and compared this to responses in the human vagus and shown them to be similar. These experiments then describe the effect of GW 833972A on the activation of vagal sensory nerves by other tussive agents such as PGE2 and hypertonic saline. This is an important finding given that PGE2 is an endogenous mediator that is known to elicit cough in man. Human vagus nerve preparations were then used to confirm the activity of GW 833972A on capsaicin-induced depolarization to provide the appropriate validation of the target in man and to determine clinical relevance. We then used GW 833972A and receptor-selective receptor antagonists to assess whether the inhibitory effects of CB2 receptor activation were evident on in vivo functional responses elicited by airway sensory nerves in a model of cough and plasma protein leakage. These studies were performed in an attempt to end the controversy surrounding the role of the CB1 versus the CB2 receptor in the antitussive effects of cannabinoids highlighted by two recent papers (Calignano et al., 2000; Patel et al., 2003).
Methods
Animals
Male Dunkin–Hartley outbred guinea-pigs (300–500 g; David Hall, Staffordshire, UK) were housed in a temperature-controlled (21 °C) room with food and water freely available for at least 1 week before commencement of the experiments. The experiments were performed in accordance with the UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) act 1986.
Compound
GW 833972A was tested for activity at the CB1 and CB2 receptor using recombinant human and rat receptors expressed with human G proteins in a yeast cell line. Details of the assay and its readouts are described in Dowell and Brown (2002). The compound was found to be a full agonist. The pEC50 for GW 833972A at the human CB2 receptor was 7.3 (pEC50 at the rat CB2 receptor=7.5) and it was approximately 1000-fold selective for the CB2 receptor over the CB1 receptor (pEC50 for CB1 receptor=4.5) (GlaxoSmithKline, unpublished data).
HU210 is a highly potent cannabinoid receptor agonist (Ki values are 0.061 and 0.52 nM at cloned human CB1 and CB2 receptors, respectively). CP 55,940 is a cannabinoid agonist that displays high and roughly equal affinity for both central and peripheral cannabinoid receptors (Ki=3.7 and 2.6 nM at CB1 and CB2 receptors, respectively) (Felder et al., 1995). GW 833972A was found to be approximately 15-fold less potent than HU210 at the CB2 receptor when it was profiled in the same assay and so GW 833972A, although more selective for the CB2 receptor over the CB1 receptor, is less potent than the non-selective ligands CP55,940 and HU210. Unfortunately, comparable data were not generated against the guinea-pig CB1 and CB2 receptors but there is currently no reason to assume that these should be vastly different.
Measurement of sensory nerve depolarization of isolated vagus nerve preparations in vitro
Male Dunkin–Hartley guinea-pigs (250–300 g) were killed by cervical dislocation and the neck opened by midline incision to expose the trachea and thorax. Segments of vagus nerve, 20–30 mm long caudal to the nodose ganglion, were removed with fine forceps. Human trachea, with branches of the cervical vagus still attached, was obtained from donor tissue (one male, 45 years of age and one female, 37 years of age for human versus guinea-pig comparison studies, n=2; two males, 54 years of age and 45 years of age, n=2 for studies using GW 833972A) for heart/lung transplantation. Relevant approvals were obtained from the Royal Brompton and Harefield Trust Ethics Committee. Both human and guinea-pig vagus nerve trunk segments were placed in oxygenated Krebs solution (Krebs Henseliet) of the following composition (mM): NaCl 118, KCl 5.9, MgSO4 1.2, CaCl2 2.5, NaH2PO4 1.2, NaHCO3 25.5 and glucose 5.6 and bubbled with 95% O2/5% CO2. Immediately after dissection, the desheathed nerve trunk was mounted in a ‘grease-gap' recording chamber. The nerve was drawn longitudinally through a narrow channel (2 mm diameter, 10 mm in length) in a Perspex block. The centre of the channel was filled with petroleum jelly, injected through a sidearm when the nerve was in place, onto the middle of the vagus, creating an area of high resistance and electrically isolating the extracellular space between the two ends of the nerve. One end of the nerve emerged into a wider channel and was constantly superfused with Krebs solution with a flow rate of approximately 2 mL min−1. The other nerve ending remained throughout the study in a second, smaller chamber containing oxygenated Krebs. Ag/Ag Cl electrodes (Mere 2 Flexible reference electrodes; World Precision Instruments (WPI), Stevenage, Hertfordshire, UK) filled with Krebs solution made contact at either end of the nerve trunk and recorded dc potential via a DAM 50 differential amplifier (WPI); dc voltages were amplified × 10, filtered at 1000 Hz and sampled at 5 Hz. The temperature of the perfusate was maintained at 37 °C by a water bath (Patel et al., 2003).
Sensory nerve activity that is nerve depolarizations were induced by superfusion of the vagus nerve with hypertonic saline, capsaicin or PGE2. The stimulants were applied for a period of up to 2 min, after which the tissue was washed until the baseline response of the nerve was regained. Non-cumulative concentration–response curves were obtained to hypertonic saline (1, 2, 4 and 8% NaCl), capsaicin (0.1–100 μM) and PGE2 (concentrations varying from 0.3 to 30 μM). Drugs were applied at known concentrations into the perfusing solution of the first channel only and depolarizing responses recorded onto a chart recorder (Lectromed Multi-Trace 2; Welwyn Garden City, Hertfordshire, UK).
In compound studies, nerve depolarizations of the vagus nerve were induced by superfusion of capsaicin (1 μM), hypertonic saline (2% m/v) or PGE2 (10 μM) for 2 min. The nerves were then washed with Krebs until the responses had returned to baseline. Following two reproducible depolarization responses to capsaicin, hypertonic saline or PGE2, the nerves were superfused with GW 833972A (0.3–300 μM) or vehicle (0.1% dimethyl sulphoxide (DMSO) final concentration) for 10 min. The responses to capsaicin, hypertonic saline or PGE2 were then measured in the presence of GW 833972A/vehicle. After washing, recovery was observed by superfusing the nerves again with the depolarizing agents for 2 min.
For antagonist studies, the preparations were superfused for 10 min with the CB1 antagonist rimonabant (N-piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR 141716A), 0.01 μM), the CB2 antagonist N-[(1S)-endo-1,3,3,-trimethylbicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide (SR 144528) (0.01 μM) or vehicle (0.01% DMSO) before perfusion with GW 833972A (0.3–300 μM) or vehicle. Appropriate concentrations of these antagonists were determined previously (Patel et al., 2003). Superfusion with compounds/vehicle was maintained during the subsequent application of capsaicin, hypertonic saline or PGE2. Owing to the limited availability of human vagus nerve, key experiments were performed in this tissue to confirm the observations seen with guinea-pig vagus nerves and to provide the appropriate validation of the target in man and to determine clinical relevance.
Measurement of cough in conscious guinea-pigs
Each conscious unrestrained guinea-pig was individually placed in a plastic transparent whole-body plethysmograph chamber (Buxco, Wilmington, NC, USA). Cough was detected both by pressure change and by sound and recorded by the Buxco Cough Analyser. The chamber was fitted with a microphone, which was connected to an external speaker, allowing the cough sound to be magnified and the number of coughs to be confirmed by manual counting. A cough was identified by a characteristic high sound coupled with a quick, large abdominal movement of the guinea-pig. The cough produces a transient increase in airflow over and above the normal flow, which is detected by the Buxco software and appears on the screen. A bias flow generator supplied air to each chamber at a rate of 2 L min−1 and withdrew air at a rate of 2.5 L min−1. The Buxco Cough Analyser utilized a specific algorithm to count coughs in 10 min by recognition of a box flow waveform that crosses a positive threshold to a negative one within a given maximum time period. Using these criteria together, cough was easily distinguished from sneezes and augmented breaths. In previous studies, animals have been treated with terbutaline sulphate (0.05 mg kg−1, i.p.) 10 min before the cough challenge to minimize respiratory distress due to bronchoconstriction (Patel et al., 2003). However, we have found that terbutaline at the dose used (0.05 mg kg−1) produced an inhibitory effect on the cough reflex. Although the effect was not statistically significant, it amounted to about 38% inhibition and so animals were not pretreated in the present study.
The tussive agent (citric acid 0.3 M) was delivered by aerosol via a nebulizer (De Vilbiss, Somerset, PA, USA). GW 833972A (30 mg kg−1, i.p., 2 mL kg−1; n=20) or vehicle (0.5% methyl cellulose with 0.2% Tween 80 in saline, i.p., n=20) was administered 30 min before exposure to the tussive agent. Citric acid (0.3 M) was administered for 10 min, during which time the number of coughs were counted. In subsequent studies (not shown), a dose–response experiment was performed (at 3, 10 and 30 mg kg−1) to determine the lowest dose of GW 833972A that had a biologically relevant effect so that a dose could be determined for use in antagonist studies.
In the next series of experiments, the effect of the CB1 (rimonabant, SR 141716A) or the CB2 (SR 144,528) receptor antagonist (administered 20 min before the agonist or vehicle; both at 10 mg kg−1 i.p., 1 mL kg−1) or vehicle (0.5% methyl cellulose with 0.2% Tween 80 in saline) was evaluated on the antitussive activity of GW 833972A (30 mg kg−1, i.p., administered 30 min before citric acid challenge) in the guinea-pig (n=10 in each group).
Measurement of plasma protein extravasation in vivo
Measurement of plasma leakage/extravasation in vivo in guinea-pigs was performed as previously described (Belvisi et al., 1989; Birrell et al., 2002). The study was conducted over 2 days in equal parts with n=3 animals from each group on each day. Male Dunkin–Hartley guinea-pigs weighing 300–400 g received GW 833972A (a total dose of 30 mg kg−1 i.p.—dosed 10 mL kg−1 at two sites, n=6) or vehicle (0.5% methylcellulose plus 0.2% Tween 80 in saline, i.p., n=6) 30 min before capsaicin administration. Animals were anaesthetized (urethane 2 g kg−1 i.p., 2.0 mL of a 25% solution given at two sites followed by a further 0.5–1 mL when supine) 20 min before capsaicin or vehicle and placed on a heated blanket to maintain body temperature. They received capsaicin (0.3 mg kg−1 i.v.) 1 min after Evans blue (20 mg kg−1 i.v.). The capsaicin dose (0.3 mg kg−1 i.v.) was established in a previous dose–response study. The first dose in the response curve to elicit a significant increase in plasma extravasation was selected.
The trachea was cannulated, using a short cannula, as near to the head as possible and the animals artificially respired at 70 strokes per min and at a volume of 2–4 mL depending on body weight. The jugular veins were exposed to allow i.v. substance administration by passing the injection needle through the pectoralis major to prevent bleeding on withdrawal. Five minutes after capsaicin administration, animals were killed and the tissue content of Evans blue dye assessed after the systemic circulation had been perfused with saline to remove intravascular dye.
The heart and the lungs were removed en bloc. The trachea, bronchi and lungs were dissected free and the parenchyma scraped from the intrapulmonary airways. The lower trachea, bronchi and intrapulmonary airways were separated and placed in 2 mL of formamide for 18 h at 37 °C to facilitate the extraction of Evans blue dye. Dye concentration in the extracts was determined at the absorbance maximum of 620 nm wavelength using a spectrophotometer (Philips Spectrophotometer, Cambridge, UK) and its tissue content (ng dye per mg wet weight tissue) calculated from a standard curve of Evans blue dye concentrations in the range of 0.3125–20 μg mL−1.
Materials
All Krebs salts were obtained from BDH (Dorset, UK) and Krebs Henseliet solution was made fresh on a daily basis. GW 833972A (for structure, see Figure 1), SR 141716A and SR 144528 were kind gifts from GlaxoSmithKline (Stevenage, UK). All other chemicals were obtained from Sigma Aldrich (St Louis, MO, USA). Stock concentration of PGE2 was diluted in 100% ethanol and stock concentrations of capsaicin, SR 141716A (10 mM) and SR 144528 (10 mM) were made in 100% DMSO.
Figure 1.
Characterization of the depolarization responses elicited by human vagus nerve preparations in response to tussive agents: comparison with responses obtained in guinea-pig tissue. Values are presented as mean±s.e.mean of the percentage change in depolarization responses before and after drug superfusion of n=2–4.
For the in vivo experiments, all drug solutions were freshly prepared on the day of each experiment. GW 833972A, SR 144528 and SR 141716 (rimonabant) were suspended in 0.5% methylcellulose with 0.2% Tween 80 in saline (vehicle) and diluted in the appropriate vehicle to give a dosing volume of 1 mL kg−1. The dose of the antagonists used was chosen based on activity in in vivo models of inflammation (Clayton et al., 2002; Kehl et al., 2003). Evans blue dye (20 mg mL−1 in saline) was filtered through a Minisart (Sartorius, Gottingen, Germany) membrane of 0.2 μm pore diameter.
Statistical analysis
For the in vitro studies, two vagal preparations were obtained from each animal. Only one concentration of one agonist and/or antagonist was tested per vagus nerve preparation and experiments were randomized such that different concentrations of different drugs were tested on vagi from the same animal on the same day. Nerve depolarization responses were measured from the time of stimulant addition to its peak at 2 min and then expressed as mV depolarization. Submaximal (approximate EC50) concentrations were determined for GW 833972A on depolarizations by the tussive agents. Approximate EC50 concentrations are defined as 50% of the maximal response obtained. In experiments in which tissues were treated, responses were expressed as mV before (control response) and after drug additions and then expressed as a percentage change from control. Submaximal (approximate EC50) concentrations were used in experiments and as the response to a stimulant was measured before and after drug intervention, within the same nerve, the data were analysed by a paired two-tailed t-test with significance P<0.05 denoted by *. For the in vivo experiments, the Mann–Whitney U-test was used when comparing drug-treated versus vehicle-treated animals in the cough and leak studies (P<0.05 denoted by *). All the values in the figures and text are expressed as mean±s.e.mean of n=4 unless otherwise stated.
Statements
All drug/molecular target nomenclature conforms with British Journal of Pharmacology guide to Receptors and Channels (Alexander et al., 2008).
Results
The effect of various sensory nerve stimulants on guinea-pig and human vagus nerves
Hypertonic saline (1, 2, 4 and 8% NaCl) produced a concentration-dependent depolarization of guinea-pig (Figure 1a) and human vagus nerves (Figure 1b). Application of capsaicin (0.1–100 μM) also produced a concentration-dependent depolarization of both guinea-pig (Figure 1a) and human vagi (Figure 1b). In guinea-pig tissue, the peak response occurred with 10 μM capsaicin, whereas in the human vagus nerve, the response was not maximal at 100 μM. Similarly, PGE2 also produced concentration-dependent nerve depolarizations of the guinea-pig vagus nerve (Figure 1a). The depolarization induced by 3 or 30 μM PGE2 of the guinea-pig vagus was similar to that elicited by the same concentrations in human vagus (Figure 1b). The vehicle for capsaicin (0.1% DMSO) or PGE2 (0.1% ethanol) did not evoke any nerve depolarization. On the basis of these studies, we selected concentrations of each ligand to elicit submaximal nerve depolarizations for future experiments investigating the action of test compounds.
Effect of the CB2 agonist GW 833972A on guinea-pig vagal nerve depolarization induced by capsaicin, hypertonic saline or PGE2
The selective CB2 receptor agonist GW 833972A was used as a tool compound for these investigations (see Figure 2 for structure). GW 833972A exhibited >1000-fold selectivity for the CB2 over the CB1 receptor (GSK in house data).
Figure 2.
Chemical structure of GW 833972A.
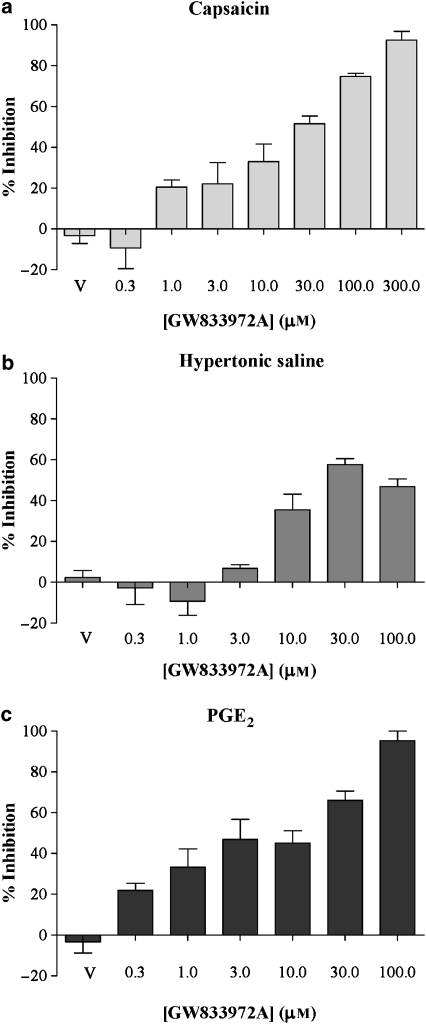
Capsaicin (1 μM), hypertonic saline (2%) or PGE2 (10 μM) induced reproducible depolarizations of the guinea-pig vagus nerve, with these concentrations of the depolarizing agents having produced submaximal responses (Figure 1). The CB2 agonist GW 833972A (0.3–300 μM) induced a concentration-dependent inhibition of the guinea-pig vagus nerve activity, stimulated by either capsaicin, hypertonic saline or PGE2 (Figure 3). Capsaicin-induced depolarizations were totally abolished by GW 833972A at 300 μM (Emax=92.6±7.5% inhibition, n=4), with an EC50 of 33.9±4.2 μM (Figure 3a). Hypertonic saline-induced depolarizations were also inhibited by GW 833972A, although the maximal inhibition reached only 57.7±5.1% at 30 μM (Emax, n=4) and the effect of GW 833972A became bell-shaped at higher concentrations (Figure 3b). Although 100% inhibition was not reached, we calculated an ‘apparent EC50' to determine the submaximal concentration to use in the antagonists experiments: 50% of the maximal possible inhibition was reached for GW 833972A at 6.5±1.0 μM. PGE2-induced depolarizations were totally abolished by GW 833972A at 100 μM (Emax=95.2±8.2% inhibition, n=4), with an EC50 of 15.9±2.5 μM (Figure 3c). In all the experiments, we found that the GW 833972A vehicle (0.1% DMSO) had no effect by itself.
Figure 3.
The effect of the selective CB2 receptor agonist (GW 833972A) on capsaicin-, hypertonic saline- and PGE2-evoked depolarization of the guinea-pig vagus nerve. GW 833972A (0.03–30 μM), inhibited (a) capsaicin (1 μM)-, (b) hypertonic saline (2%)- and (c) PGE2 (10 μM)-induced depolarization of guinea-pig vagus nerve in a concentration-dependent manner. Values are presented as mean±s.e.mean of the percentage change in depolarization responses before and after drug superfusion of n=4. V refers to the vehicle control group. PGE2, prostaglandin E2.
Effect of CB1 or CB2 antagonist on the GW 833972A-induced inhibition of the guinea-pig vagus nerve activity
Submaximal (approximate EC50) concentrations of GW 833972A were chosen (30 μM for capsaicin, and 10 μM for 2% hypertonic saline and PGE2) to investigate the impact of the selective receptor antagonists. At 30 μM, GW 833972A induced a 55.7±4.1% of maximal inhibition of capsaicin-induced depolarizations (Figure 4a). At 10 μM, GW 833972A induced a 41.8±2.0 and 39.7±9.8% of maximal inhibition of hypertonic saline- and PGE2-induced depolarizations, respectively (Figures 4b and c). The inhibitory effects observed with GW 833972A at these submaximal concentrations were then investigated in the presence of the CB1 (rimonabant, 0.01 μM) and CB2 (SR 144528, 0.01 μM) antagonists. The CB2 antagonist SR 144528 totally blocked the effect of GW 833972A on depolarizations of the guinea-pig vagus nerve induced by capsaicin, hypertonic saline or PGE2, whereas the CB1 antagonist rimonabant had no effect (Figure 4). In all the experiments, the vehicle (0.1% DMSO for GW 833972A, 0.01% DMSO for rimonabant and SR 144528) had no effect by itself.
Figure 4.
The effect of CB1 and CB2 receptor-selective antagonists on the inhibition of capsaicin-, hypertonic saline- and PGE2-induced depolarization of the guinea-pig vagus nerve evoked by submaximal concentrations of the selective CB2 receptor agonist (GW 833972A). The inhibitory action of a submaximal concentration of (a) GW 833972A (30 μM) on capsaicin (1 μM)-induced depolarizations, (b) GW 833972A (10 μM) on hypertonic saline (2%)-induced depolarizations and (c) GW 833972A (10 μM) on PGE2 (10 μM)-induced depolarizations of the guinea-pig vagus was not affected by prior superfusion (10 min) with the CB1 receptor antagonist rimonabant, (SR 141716A, 0.01 μM), but was completely abolished in the presence of the CB2 receptor antagonist SR 144528 (0.01 μM). Values are presented as mean±s.e.mean of the percentage change in responses before and after drug superfusion of n=4. *Significant difference (P<0.05) compared with control responses to tussive stimuli before drug superfusion in the same preparation using a paired t-test. PGE2, prostaglandin E2.
The effect of GW 833972A on capsaicin-induced depolarization of human vagus nerve
GW 833972A (1 and 10 μM) produced a concentration-related inhibition of capsaicin (10 μM)-induced nerve depolarization of human vagus nerve (55% at 1 μM and 78% at 10 μM of GW 833972A, n=1/2) (Figure 5).
Figure 5.
Histogram and representative trace describing the effect of GW 833972A on capsaicin-evoked depolarization of human vagus nerve. GW 833972A (1 and 10 μM) inhibited capsaicin (10 μM)-evoked depolarization of the human vagus nerve: (a) representative trace from data obtained from a single human vagus nerve (male, 54 years old) and (b) a histogram illustrating the depolarization pre- and post-administration of the compound (prior superfusion for 10 min) and then following washout. Values are presented as mean of the depolarization responses before and after drug superfusion of n=1–2.
The effect of the CB2 agonist GW 833972A on citric acid-induced cough in the conscious guinea-pig
Justification for dose selection for GW 833972A was based on the results from a GSK in house pharmacokinetic study and the in vitro activity data produced on the guinea-pig vagal nerve preparation. In the pharmacokinetic study, following i.p. administration of GW 833972A 3, 10 and 30 mg kg−1 blood levels of this compound of 0.28±0.06, 1.28±0.97 and 4.65±2.1 μM, respectively (n=7), were achieved after 1 h. Significant inhibitory effects of GW 833972A on vagal nerve depolarization elicited by all three stimuli were observed from 3 μM. Therefore, to achieve this concentration in vivo, it was necessary to dose at 30 mg kg−1. The pharmacokinetic study was performed in rats and we assumed that similar compound exposure would be maintained in the guinea-pig.
In a dose–response study, GW 833972A 30 mg kg−1 was the minimum dose that coincided with an antitussive action as predicted by the pharmacokinetic profile and the in vitro potency of this compound.
GW 833972A (30 mg kg−1) inhibited the citric acid-induced tussive response (Figure 6). In this first study, there was some sedation (visually assessed as guinea-pigs appeared more drowsy and less active) at this dose in 3 out of 20 of the guinea-pigs. However, there was no correlation between the inhibitory response seen and the animals in which sedation was observed, and no sedation was seen in the following cough studies performed with the same compound. We are not sure why sedation was seen only in the first study but it underlies the need to perform comprehensive antagonist studies as this effect may be due to the compound starting to lose selectivity at this dose and act on CB1 receptors.
Figure 6.
The effect of GW 833972A on citric acid (0.3 M)-induced cough in conscious guinea-pigs. GW 833972A (30 mg kg−1, i.p., administered 30 min before citric acid challenge) inhibited the citric acid-induced cough in the guinea-pig. Values are presented as mean±s.e.mean of the number of coughs per min over the 10-min citric acid exposure period, n=20 in each group. *Significant difference (P<0.05) between vehicle-treated and compound-treated groups. The Mann–Whitney U-test was used when comparing vehicle- versus drug-treated animals in the cough studies.
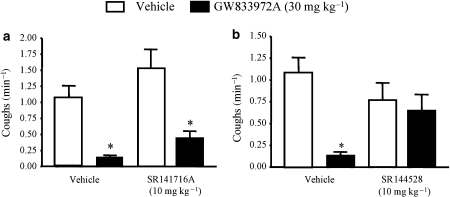
The effect of a CB1 (rimonabant, SR 141716A) and a CB2 (SR 144,528) receptor antagonist on the inhibitory effect of the CB2 agonist GW 833972A on citric acid-induced cough in the conscious guinea-pig
In this study, GW 833972A inhibited the citric acid-induced cough by 88.1% (Figure 7). In this study, no sedation was observed. It appears that the CB1 and the CB2 receptor antagonists had no significant effect alone on the cough reflex. However, the inhibitory effect evoked by the CB2 agonist (GW 833972A) was reduced by the CB2 antagonist (Figures 7a and b).
Figure 7.
The effect of CB1 and CB2 receptor antagonists on the inhibitory effect of GW 833972A on citric acid (0.3 M)-induced cough in conscious guinea-pigs. The CB2 (SR 144528) but not the CB1 (rimonabant, SR 141716A) receptor antagonist blocked the antitussive activity of GW 833972A (30 mg kg−1, i.p., administered 30 min before citric acid challenge) in the guinea-pig. Values are presented as mean±s.e.mean of the number of coughs per min over the 10-min citric acid exposure period, n=10 in each group. *Significant difference (P<0.05) between vehicle-treated and compound-treated groups. The Mann–Whitney U-test was used when comparing vehicle- versus drug-treated animals in the cough studies.
The effect of GW 833972A on capsaicin-induced plasma protein exudation in guinea-pig airways
Capsaicin evoked a significant increase in plasma protein exudation in the main bronchi compared with vehicle stimulation (20.2±1.5 increased to 90.6±10.4 ng per mg of tissue of Evans blue dye). Pretreatment with GW 833972A (30 mg kg−1 i.p.—dosed 10 mL kg−1 at two sites, n=6) or vehicle (0.5% methylcellulose plus 0.2% Tween 80 in saline, i.p., n=6) dosed 30 min before capsaicin had no significant effect on the leakage evoked by capsaicin (in the presence of GW 833972A, 72.6±7.9 ng per mg of tissue of Evans blue dye). GW 833972A (30 mg kg−1 i.p.—dosed 10 mL kg−1 at two sites, n=6) had no effect alone.
Discussion
Sensory nerve activity may be enhanced during inflammation so that protective central and local reflexes become exacerbated and deleterious and may contribute to the pathophysiology and symptoms of airway inflammatory diseases such as asthma and chronic obstructive pulmonary disease (Barnes, 2001). Stimulation of sensory nerves can evoke bronchoconstriction and cough via activation of an afferent central reflex pathway. Furthermore, activation of a particular subset of sensory fibres, the C-fibres, is known to evoke neurogenic inflammation in guinea-pigs and rodents but as yet it is still questionable whether this phenomenon occurs in man. This response is characterized by events such as microvascular leakage of plasma proteins and cellular infiltrate into the airways (Widdicombe, 1954; Karlsson, 1993; Belvisi, 2003).
We have previously demonstrated that the CB2 receptor agonist JWH 133 inhibited both capsaicin-induced depolarization of the vagus nerve and citric acid-induced cough in conscious guinea-pigs (Patel et al., 2003). Although JWH 133 is reported to possess 200-fold selectivity for the CB2 over the CB1 receptor, it is not clear how selective this agonist is in in vivo situations. This is an important consideration given that the reported antitussive activity of CB1 receptor agonists is probably due to their sedative activity (Calignano et al., 2000). Therefore, studies were performed utilizing a novel CB2 receptor agonist with a superior selectivity profile to JWH 133 to confirm the CB2-dependent nature of the response.
An isolated vagal preparation was used to facilitate pharmacological profiling of the novel CB2 agonist (GW 833972A). This sort of characterization is often simpler to perform initially in an isolated tissue preparation in which drug action is not complicated by pharmacokinetic issues. However, although the isolated vagus preparation presents us with the ideal opportunity to conduct a comprehensive pharmacological assessment, data using this preparation should be interpreted with some caution as the pharmacological agents are applied to the axon of the isolated vagus nerve in vitro and not to the peripheral nerve ending (see Patel et al., 2003 for more detailed discussion). However, excepting these caveats, we were able to demonstrate an inhibitory activity of GW 833972A on capsaicin-induced guinea-pig and human vagal sensory nerve activation in vitro, thus confirming previous data from our laboratory utilizing the non-selective CB agonist, CP 55,940, and the CB2-selective agonist, JWH 133 (Patel et al., 2003). However, when the EC50 concentrations for inhibition of capsaicin-induced depolarization of the guinea-pig vagus were compared, it appeared that although GW 833972A had improved selectivity it had reduced potency compared with the ligands we have used previously. The order of potency was as follows CP 55,940>JWH 133>GW 833972A (at approximately 1, 3 and 33 μM, respectively). These experiments also describe the effect of GW 833972A on the activation of vagal sensory nerves by other tussive agents such as PGE2 and hypertonic saline. This is an important facet of this paper given that PGE2 is an endogenous mediator that is known to elicit cough in man. Although the whole vagus preparation does not allow us to discriminate the actions of ligands on specific fibre types, the inhibitory activity of GW 833972A on PGE2- and hypertonic saline-induced depolarization suggests activity on C- and Aδ-fibres, respectively. Evidence to support this suggestion comes from electrophysiological recording studies of pulmonary C-fibre afferent activity, which has demonstrated that the sensitivity of these sensory endings to capsaicin challenge is potentiated by PGE2 (Lee and Morton, 1995) and that hypertonic saline excites fibres conducting in the Aδ-range (Fox et al., 1995).
To conclusively determine which CB receptor subtype is involved in cannabinoid-mediated inhibition of stimuli-evoked sensory nerve depolarizations, the CB1 receptor-selective antagonist SR 141716A (rimonabant, Rinaldi-Carmona et al., 1994) and the CB2 receptor-selective antagonist SR 144528 (Rinaldi-Carmona et al., 1998) were used in conjunction with the cannabinoid agonist, GW 833972A. Antagonist affinity is the key factor when assessing receptor selectivity. The concentration of the antagonists used (0.01 μM) is similar to what has been used previously and close to the pA2 value for SR 141716A, rimonabant at the CB1 receptor (pA2=7.9; Rinaldi-Carmona et al., 1994). The effect of GW 833972A was blocked by the CB2-selective antagonist but not by the CB1-selective receptor antagonist confirming the selectivity of the tool compound.
The data obtained above would appear to confirm, at least in vitro, a role for the CB2 receptor as an interesting target for pathologies that may involve increased sensory nerve function (for example, cough, asthma, inflammatory pain). However, to confirm its potential as a target for human disease, it was necessary to perform target-validation experiments in the relevant human tissue. Encouragingly, the depolarization responses of guinea-pig and human isolated vagus nerves to the sensory nerve stimulants were all extremely similar between the two species. Hence, it is assumed that the guinea-pig is an appropriate surrogate model for the measurement of sensory nerve activity both in vitro and in vivo. The data shown here demonstrated the inhibitory activity of GW 833972A on capsaicin-induced depolarization of the human vagus at a similar concentration as had been shown effective in guinea-pig tissue. This study is unique given the opportunity we had to validate the target in the relevant human tissue that would be involved in evoking sensory nerve-mediated responses such as cough and plasma extravasation.
GW 833972A also demonstrated inhibitory activity on the citric acid-induced cough in the conscious guinea-pig model at a dose predicted by the in vitro data and pharmacokinetic studies. However, although the inhibitory activity in this model is encouraging with regard to CB2 receptors being a promising therapeutic target for chronic cough, there may still be concerns regarding the in vivo selectivity of this compound. Therefore, we felt it prudent to rule out an effect of the compound on the CB1 receptor (as any CNS sedative effects, like those seen in the first study described in this paper, may result in inhibition of reflex events such as cough) and to confirm a role for the CB2 receptor, we performed experiments with GW 833972A in the presence of selective CB1 and CB2 receptor antagonists. The antitussive activity of GW 833972A was blocked in the presence of the CB2, but not the CB1, receptor antagonist, confirming the CB2 receptor as a possible target for antitussive therapies.
We were interested to determine whether cannabinoids also impacted on other sensory nerve-mediated events. Plasma protein extravasation into the airways of experimental animals is one of the physiological responses to stimulation of the vagus nerve. This leakage is thought to be due to neuropeptides (in particular substance P) released from sensory nerve endings acting on neurokinin 1 receptors (Lei et al., 1992). In this study, we have shown that GW 833972A did not inhibit capsaicin-induced plasma protein exudation into guinea-pig main bronchi. At first glance, these data would appear to be at odds with the cough data. However, it could be that the sensory nerves that are involved in the activation of the cough reflex are a different population of fibres, with a differential cannabinoid expression profile than those that innervate post-capillary venules, and are involved in the capsaicin-evoked microvascular leakage response.
In conclusion, the lack of effect of GW 833972A on capsaicin-induced microvascular leakage suggests that not all airway sensory nerves can be modulated in a similar manner. In contrast to the microvascular leak data, we have shown conclusively that activation of the CB2 receptor subtype on peripheral airway sensory nerves inhibits the cough reflex evoked by citric acid in a conscious guinea-pig model. Moreover, the inhibitory action on capsaicin-induced depolarization of the human vagus nerve by the selective CB2 agonist GW 833972A provides proof of concept for the mechanism in man. These findings have important implications for the therapeutic potential of cannabinoids and confirm and extend our previous study describing the inhibitory potential of a CB2 receptor agonist on airway sensory nerves and its antitussive activity in a guinea-pig model (Patel et al., 2003). There is limited CB2 receptor expression in the CNS and hence the development of CB2 receptor-selective agonists (with improved potency over GW 833972A) will provide a new therapeutic strategy for the treatment of cough that should be devoid of the CNS-mediated side effects, which are normally associated with non-selective cannabinoid agonists.
Acknowledgments
Some of this work was funded by a research grant from GlaxoSmithKline, which partly paid salaries for HJP, DJH and MAB. The human tissue work was funded by an Experimental Medicine grant (G0502019) from the Medical Research Council, UK. The CB agonist and antagonists were a kind gift from GlaxoSmithKline. MGB is paid by a consultancy from GlaxoSmithKline.
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol. 2001;125:145–154. doi: 10.1016/s0034-5687(00)00210-3. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Baraniuk JN, Belvisi MG. Neuropeptides in the respiratory tract. Am Rev Respir Dis. 1991;144:1187–1198. doi: 10.1164/ajrccm/144.5.1187. [DOI] [PubMed] [Google Scholar]
- Belvisi MG. Overview of the innervation of the lung. Curr Opin Pharmacol. 2002;2:211–215. doi: 10.1016/s1471-4892(02)00145-5. [DOI] [PubMed] [Google Scholar]
- Belvisi MG. Sensory nerves and airway inflammation: role of Aδ and C-fibres. Pulm Pharmacol Ther. 2003;16:1–7. doi: 10.1016/S1094-5539(02)00180-3. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Rogers DF, Barnes PJ. Neurogenic plasma protein extravasation: inhibition by morphine in guinea-pig airways in vivo. J Appl Physiol. 1989;66:268–272. doi: 10.1152/jappl.1989.66.1.268. [DOI] [PubMed] [Google Scholar]
- Birrell MA, Crispino N, Hele DJ, Patel HJ, Yacoub MH, Barnes PJ, et al. Effect of dopamine receptor agonists on sensory nerve activity: possible therapeutic targets for the treatment of asthma and COPD. Br J Pharmacol. 2002;136:620–628. doi: 10.1038/sj.bjp.0704758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder C, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Calignano A, Katona I, Desarnaud F, Giuffrida A, La Rana G, Mackie K, et al. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408:96–101. doi: 10.1038/35040576. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N, Marshall FH, Bountra C, O'Shaughnessy CT. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253–260. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. Pulmonary reflexes: neural mechanisms of pulmonary defence. Annu Rev Physiol. 1994;5:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- Coleridge JCG, Coleridge HM. Afferent Vagal C-Fibre innervation of the lungs and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Dowell SJ, Brown AJ. Yeast assays for G-protein-coupled receptors. Recept Channels. 2002;8:343–352. [PubMed] [Google Scholar]
- Elmes SJ, Winyard LA, Medhurst SJ, Clayton NM, Wilson AW, Kendall DA, et al. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118:327–335. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Fox AJ, Barnes PJ, Venkatesan P, Belvisi MG. Activation of large conductance potassium channels inhibits the afferent and efferent function of airway sensory nerves in the guinea-pig. J Clin Invest. 1997;99:513–519. doi: 10.1172/JCI119187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin-evoked sensitisation of airway sensory nerves: A mechanism for ACE-inhibitor cough. Nat Med. 1996;2:814–817. doi: 10.1038/nm0796-814. [DOI] [PubMed] [Google Scholar]
- Fox AJ, Urban L, Barnes PJ, Dray A. Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience. 1995;67:741–752. doi: 10.1016/0306-4522(95)00115-y. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchland J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Griffin G, Fernando SR, Ross RA, McKay NG, Ashford MLJ, Shire D, et al. Evidence for the presence of CB2 like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci USA. 1999;96:14228–14333. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Drew LJ, Chapman V. Spinal anandamide inhibits nociceptive transmission via cannabinoid receptor activation in vivo. NeuroReport. 2000;11:2817–2819. doi: 10.1097/00001756-200008210-00041. [DOI] [PubMed] [Google Scholar]
- Karlsson J-A. A role for capsaicin-sensitive, tachykinin containing nerves in chronic coughing and sneezing, but not in asthma: a hypothesis. Thorax. 1993;48:396–400. doi: 10.1136/thx.48.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J-A, Sant'Ambrogio G, Widdicome J. Afferent neural pathways in cough and reflex bronchoconstriction. J Appl Physiol. 1988;65:1007–1023. doi: 10.1152/jappl.1988.65.3.1007. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, et al. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–186. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea-pigs. J Appl Physiol. 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- Lee LY, Morton RF. Pulmonary chemoreflex sensitivity is enhanced by prostaglandin E2 in anesthetized rats. J Appl Physiol. 1995;79:1679–1686. doi: 10.1152/jappl.1995.79.5.1679. [DOI] [PubMed] [Google Scholar]
- Lei YH, Barnes PJ, Rodgers DF. Inhibition of neurogenic plasma protein exudation in guinea-pig airways by CP-96,345, a new non-peptide NK1 receptor antagonist. Br J Pharmacol. 1992;105:261–262. doi: 10.1111/j.1476-5381.1992.tb14243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Vanderah TW, Makriyannis A, Porreca F. Inhibition of pain responses by activation of CB2 cannabinoid receptors. Chem Phys Lipids. 2002;121:191–200. doi: 10.1016/s0009-3084(02)00155-x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterisation of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Patel HJ, Birrell MA, Crispino N, Hele DJ, Venkatesan P, Barnes PJ, et al. Inhibition of Guinea-pig and human sensory nerve activity and the cough reflex in Guinea-pigs by Cannabinoid (CB2) receptor activation. Br J Pharmacol. 2003;140:261–268. doi: 10.1038/sj.bjp.0705435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Aanonsen L, Hargreaves KM. Antihyperalgesic effects of spinal cannabinoids. Eur J Pharmacol. 1998a;345:145–153. doi: 10.1016/s0014-2999(97)01621-x. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998b;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Roberts AM, Schultz HD, Green JF, Armstrong DJ, Kaufman MP, Coleridge HM, et al. Reflex tracheal contraction evoked in dogs by bronchodilator prostaglandins E2 and I2. J Appl Physiol. 1985;58:1823–1831. doi: 10.1152/jappl.1985.58.6.1823. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Remmers JE, De Groot WJ, Callas G, Mortola JP. Localisation of rapidly adapting receptors in the trachea and main stem bronchus of the dog. Respir Physiol. 1978;33:359–365. doi: 10.1016/0034-5687(78)90062-2. [DOI] [PubMed] [Google Scholar]
- Smith JA, Amagasu SM, Eglen RM, Hunter JC, Bley KR. Characterisation of prostanoid receptor-evoked responses in rat sensory neurones. Br J Pharmacol. 1998;124:513–523. doi: 10.1038/sj.bjp.0701853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Receptors in the trachea and bronchi of the cat. J Physiol. 1954;123:71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]