Abstract
Background and purpose:
Kinins are acknowledged as important regulators of intestinal function during inflammation; however, their effects on human intestinal ion transport have not been reported. Here, we used muscle-stripped human colonic tissue and cultured T84-cell monolayers to study bradykinin (BK) actions on human intestinal ion transport.
Experimental approach:
Ion transport was measured as changes in short-circuit current (Isc) across colonic epithelia mounted in Ussing chambers.
Key results:
In intact tissue, there was a distinct polarity to BK-elicited Isc responses. Whereas basolateral BK stimulated sustained responses (EC50=0.5±0.1 μM), those to apical BK were more rapid and transient (EC50=4.1±1.2 nM). In T84 cells, responses to both apical and basolateral BK were similar to those seen upon apical addition to intact tissues. Cross-desensitization between apical and basolateral domains was not observed. BK-induced responses were largely due to Cl− secretion as shown by their sensitivity to bumetanide and removal of Cl− from the bathing solution. Studies using selective agonists and antagonists indicate responses to BK are mediated by B2 receptors. Finally, responses to basolateral BK in intact tissues were inhibited by tetrodotoxin (1 μM), atropine (1 μM), capsaicin (100 μM) and piroxicam (10 μM). BK-stimulated prostaglandin (PG)E2 release from colonic tissue.
Conclusions:
BK stimulates human colonic Cl− secretion by activation of apical and basolateral B2 receptors. Responses to apical BK reflect a direct action on epithelial cells, whereas those to basolateral BK are amplified by stimulation of enteric nerves and PG synthesis.
Keywords: bradykinin, kinin receptors, epithelium, chloride secretion, colon.
Introduction
One of the primary functions of the intestinal epithelium is to transport fluid to and from the gut lumen. Normally, absorptive processes predominate, enabling conservation of the large volumes of water that enter the intestine each day. However, secretion is also an ongoing process that is required for appropriate hydration of the mucosal surface. Epithelial fluid transport is wholly dependent on the active transport of ions with absorption being driven by cations, anions and coupled nutrient transport, and secretion being driven by anions, predominantly Cl− (Barrett and Keely, 2006). Many pathological conditions, including intestinal inflammation, can disrupt the finely tuned balance between absorption and secretion so that secretion predominates, leading to the clinical manifestation of diarrhoea. Under such conditions, Cl− secretion is believed to be promoted by a wide array of hormonal, neuronal and immunological factors including histamine, 5-hydroxytryptamine, eicosanoids, vasoactive intestinal peptide and ACh that are released from activated immunocytes and nerves within the mucosa (Montrose et al., 2003).
Bradykinin (BK) is a non-apeptide member of the family of kinins and is generated from inactive precursors, kininogens, by the actions of the enzyme, kallikrein (Margolius, 1989; Joseph and Kaplan, 2005). Each component of this ‘kininogen–kallikrein–kinin axis' is present within the gut mucosa and kinins fulfil several of the criteria necessary to be considered as potential mediators of inflammation in the intestine. Indeed, alterations in the expression of kinins have been shown to occur in inflammatory bowel disease, suggesting they are likely to be important in disease progression (Stadnicki et al., 2003; Devani et al., 2005). Classically, kinins exert their actions through the activation of Gq-protein-coupled receptors of which two subtypes have been identified (Leeb-Lundberg et al., 2005). B2 receptors (receptor nomenclature follows Alexander et al., 2008) are constitutively expressed and mediate most of the biological effects of kinins throughout the body, whereas B1 receptors are typically induced under conditions of tissue trauma and inflammation. Activation of kinin receptors can exert a wide variety of biological effects including alterations in visceral smooth muscle contraction, vascular tone and endothelial permeability. Studies in animal models suggest that kinins may also promote intestinal Cl− secretion (Cuthbert, 2001). In many cases, the prosecretory effects of kinins appear to be largely indirect, involving stimulation of eicosanoid synthesis and activation of enteric neurons (Cuthbert et al., 1984; Diener et al., 1988; Phillips and Hoult, 1988; Green et al., 2003). However, there are likely to be some species differences in the nature of kinin-induced secretory responses, although some studies have demonstrated a lack of neuronal involvement (Manning et al., 1982). Furthermore, studies of kinin effects on cultured epithelial cells demonstrate that they can also act directly on intestinal epithelia to promote Cl− secretion (Cuthbert et al., 1985, 1987). Interestingly, these studies also indicate that kinins may have the ability to stimulate transport responses from either the apical or basolateral aspect of human colonic epithelia, suggesting a bilateral distribution of functional kinin receptors that is unique to humans. However, these observations had not been confirmed in native human colonic tissue.
These studies were carried out to characterise the actions of BK on ion transport across native human colonic tissue in vitro. In particular, we aimed to examine the ‘sidedness' of responses to kinins, the identity of the kinin receptors involved and whether kinins exert their effects by direct or indirect actions on the epithelium.
Methods
Tissue preparation and electrical measurements
Ethical approval for this study was obtained from the Ethics Committee, St Vincent's University Hospital. Segments of human colon were obtained during surgical resection for colonic carcinoma. Tissues from patients with inflammatory bowel disease or diverticular disease were excluded from these studies. All tissues were free of overt pathology and considered to be ‘normal' as ascertained by gross examination by a pathologist. Tissues were transferred in preoxygenated Krebs–Henseleit buffer (in mM: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2MgSO4, 1.2 KH2PO4, 25 NaHCO3 and 11.1 D-glucose) to the laboratory and were then stripped of their underlying smooth muscle layers by blunt dissection to yield mucosal preparations consisting of the epithelial cell layer and attendant lamina propria. Tissues were then mounted in Ussing chambers (window area=0.63 cm2) and were bathed in circulating, oxygenated (95% O2–5% CO2) Krebs–Henseleit solution at 37 °C. Tissues were voltage clamped to zero potential difference using DVC-1000 voltage clamps (World Precision Instruments, New Haven, CT, USA). Short circuit current (Isc) and potential difference were monitored using silver/silver chloride electrodes with agar cross-bridges. Adjacent pieces of tissues from single specimens of colon were used to permit a paired experimental design. All Isc responses were normalized to μA cm−2. In experiments where the effects of Cl− substitution in the bathing solution were examined, NaCl was replaced with equimolar Na+-gluconate and Ca2+ was increased from 2.5–4 mM in the bathing solution to compensate for Ca2+ chelation by gluconate.
Cells
Briefly, T84 cells were grown in DME/F12 media (JRH, Lenexa, KS, USA) supplemented with 5% newborn calf serum. Cells were passaged by trypsinization. For Ussing chamber/voltage clamp experiments, 106 cells were seeded onto collagen-coated polycarbonate filters (Nucleopore, Pleasanton, CA, USA) glued onto Lexan rings. Cells were cultured on filters for 7–10 days before use. Monolayers of T84 cells were mounted in Ussing chambers (window area=2 cm2) and bathed in oxygenated (95% O2/5% CO2) Ringers' solution at 37 °C. The composition of the Ringers' solution was (in mM): 140 Na+, 5.2 K+, 1.2 Ca2+, 0.8 Mg2+, 120 Cl−, 25 HCO3−, 2.4 H2PO4, 0.4 HPO42− and 10 glucose. Changes in Isc were monitored as described above.
Prostaglandin (PG)E2 assays
Sections of human colon (approx. 3 mg protein) were prepared as described above. After a 20 min equilibration period in oxygenated Krebs–Henseleit solution at 37 °C, the sections of mucosal tissue were transferred to individual wells of a 24-well plate containing increasing concentrations of BK. Tissues were incubated for a period of 15 min after which they were removed and assayed for protein content. Incubation media were stored at −70 °C until assayed for PGE2 content by a commercially available radioimmunoassay kit (Amersham International, UK).
Data analysis
All results are expressed as mean±s.e.m. for a series of n independent experiments. Data from paired preparations were compared by Wilcoxon's signed-rank test. Two-way ANOVA was employed to compare control and test concentration response curves.
Materials
Bradykinin, lysyl-BK, des-Arg9-BK, des-Arg10-lysyl-BK, bumetanide, tetrodotoxin (TTX), piroxicam, carbachol, atropine, histamine and capsaicin were all obtained from Sigma Chemical Co., UK or USA. PGE2 radioimmunoassay kits were obtained from Amersham International, UK. HOE 140 was a gift from Hoechst-Roussell. All other chemicals were of analytical grade.
Results
BK stimulates ion transport in intact human colon mucosal strips
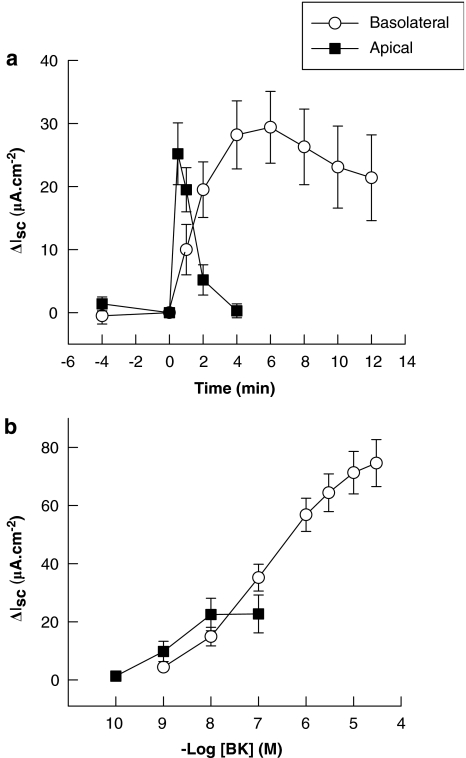
Basal electrical parameters of human colon were similar to thosedescribed previously (Keely et al., 1995). Addition of BK (1 μM) to the basolateral domain of human colon strips resulted in the stimulation of a sustained inward Isc response, which reached a maximum effect after approximately 5 min (Figure 1a). Apical addition of BK (10 nM) stimulated a more rapid in onset and transient Isc response. Responses to both apical and basolateral BK were concentration-dependent (Figure 1b) with an EC50 for basolateral addition of 0.5±0.1 μM (n=34) and for apical addition of 4.1±1.2 nM (n=12). Basolateral treatment with a maximally effective concentration of BK (30 μM) did not alter subsequent responses to apical addition of the peptide (10 nM), which were 14.8±3.7 μA cm−2 in control tissues compared to 25.2±9.5 μA cm−2 in tissues pretreated with basolateral BK (n=6). Likewise, apical addition of BK did not alter subsequent responses to basolateral addition (data not shown). Although the maximal Isc responses (left colon=78.2±9.5 μA cm−2; n=24; right colon=65.7±15.7 μA cm−2; n=10) and EC50 values (left colon=0.45±0.11 μM; right colon=0.59±0.28 μM) for basolateral BK were similar in tissues derived from left or right colon, data from both regions were pooled for the purposes of these studies.
Figure 1.
Bradykinin (BK) stimulates ion transport in intact human colon mucosal strips in vitro. (a) Time course of the effects of BK on Isc in human colon. BK was added at time zero to either the apical (10 nM; n=12) or basolateral (1 μM; n=10) side of voltage clamped human colon. (b) Concentration-dependence of the responses to basoalateral or apical BK. BK was added cumulatively to either the basolateral or apical side of intact human colon. Responses were allowed to reach their maximum (approximately 1 min for apical and 5 min for basolateral addition) before the subsequent additions of BK. Calculated EC50 s were 0.5±0.1 μM (basolateral; n=34) and 4.1±1.2 nM (apical; n=12). All values are expressed as ΔIsc induced by BK addition and are means±s.e.m. for the number of experiments noted.
BK stimulates ion transport in T84 cells
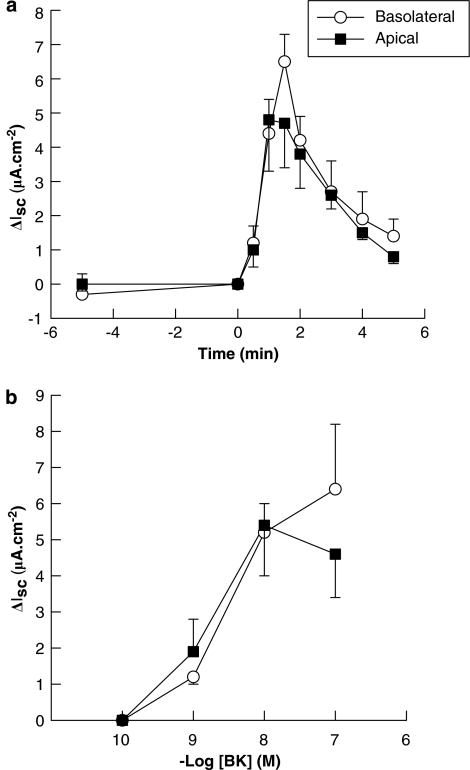
In contrast to its effects in intact colonic preparations, BK-stimulated identical Isc responses in T84-cell monolayers, on apical or basolateral addition (Figure 2a). Moreover, the time courses for both apical and basolateral responses in these cultured epithelial cells were comparable to those obtained upon apical addition to intact colon. Concentration response curves for apical and basolateral BK in T84 cells were also similar (Figure 2b) and comparable to those obtained upon apical addition to intact colon. As observed in intact tissue, basolateral BK (10 nM) did not alter subsequent responses to apical addition in T84 cells (control response=5.9±1.5 μA cm−2; test response=4.9±0.7 μA cm−2; n=6). Likewise, apical BK (10 nM) did not alter subsequent responses to basolateral addition (control response=5.6±0.8 μA cm−2; test response=6.8±2.0 μA cm−2; n=6).
Figure 2.
Bradykinin (BK) stimulates ion transport across T84 monolayers. (a) Time course of the effects of BK (10 nM) on Isc in T84 cells. BK was added at time zero to either the apical (n=8) or basolateral (n=6) side of T84 monolayers. (b) Concentration-dependence of responses to basolateral or apical BK in T84 cells. Each monolayer was challenged with a single concentration of BK on either the basolateral or apical side (n=3–8 monolayers for each concentration tested). Both effects had calculated EC50 s of approximately 5 nM. Data are expressed as mean±s.e.m. for n experiments.
BK mediates its effects on human colonic ion transport through the activation of B2 receptors
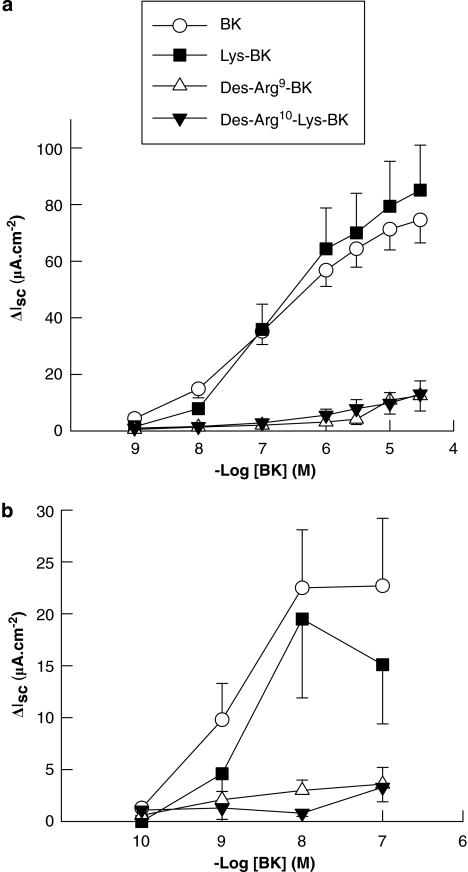
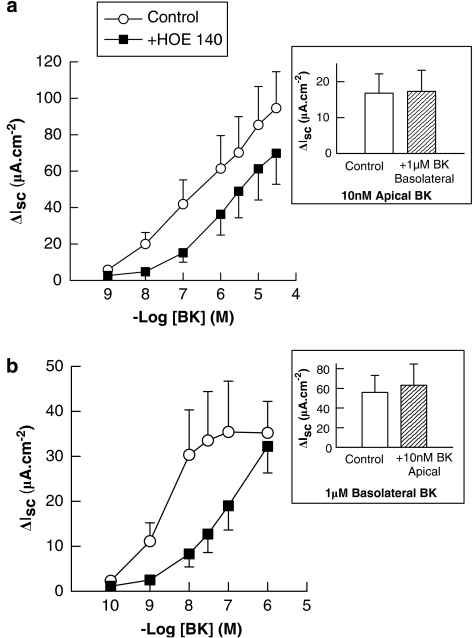
Responses of intact colonic mucosae to both apical and basolateral BKs were mimicked by lysyl-BK, but not by the B1-receptor agonists, des-Arg9-BK or des-Arg10-kallidin (Figure 3), indicating that the responses to BK are mediated predominantly by B2 receptors. This is supported by the finding that responses to both apical and basolateral BK were attenuated by the B2-receptor antagonist, HOE 140 (Figure 4). Responses to apical BK were unaffected by basolateral treatment with HOE 140. Likewise, responses to basolateral BK were unaffected by apical treatment with the antagonist. HOE 140 was also without effect on Isc responses to PGE2 (1 μM) or the cholinergic agonist, carbachol, (100 μM) demonstrating selectivity of the antagonist for BK receptors. Control responses to PGE2 and carbachol were 24.8±7.0 and 52.0±10.8 μA, respectively compared to 28.8±6.9 and 60.2±4.4 μA in HOE 140-pretreated tissues. HOE 140 was also without effect on basal Isc (data not shown).
Figure 3.
Other kinin agonists stimulate ion transport in human colon. BK analogues were added to either the basolateral (a) or apical (b) aspect of voltage clamped human colon and on Isc responses were measured. Lys-BK (kallidin) was approximately equipotent with BK in stimulating ion transport when added to either the basolateral (calculated EC50=0.4±0.1 μM; n=8) or to the apical domain (calculated EC50=5.1±1.3 nM; n=5). The B1 agonists, des-Arg9-BK and des-Arg10-Lys-BK, were practically without the effect when added either basolaterally or apically (n=5–6 throughout). Values are mean±s.e.m. for n experiments.
Figure 4.
HOE 140 inhibits Isc responses to bradykinin (BK) in human colon. (a) Basolateral addition of the BKB2 receptor antagonist, HOE 140 (100 nM) significantly attenuated responses to basolateral BK (n=8; P<0.05) but did not alter the responses to apical BK (10 nM) added at the end of each experiment (inset). (b) Apical addition of HOE 140 (100 nM) also attenuated the responses to apical addition of BK (n=6; P<0.05) but did not affect responses to basolateral BK (1 μM) added at the end of each experiment (inset). All values are mean±s.e.m. for n experiments.
Identification of the charge-carrying ion
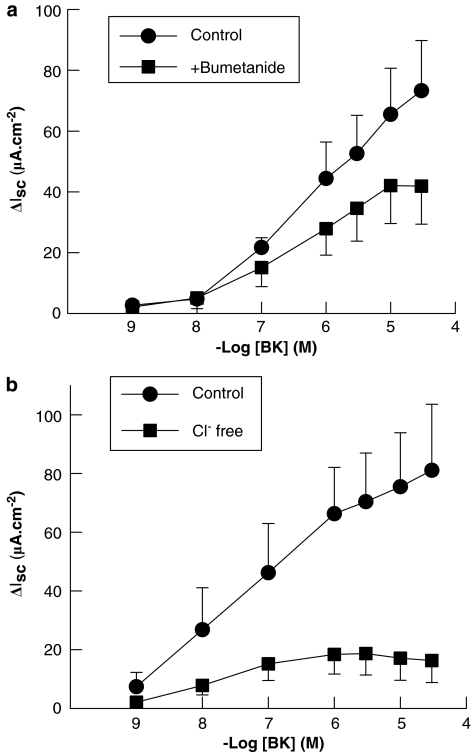
Responses to basolateral BK in intact human colon were significantly attenuated by the loop diuretic, bumetanide (100 μM), and were practically abolished when Cl− was replaced in the bathing solution with the impermeant anion, gluconate (Figure 5). Similarly, responses to apical BK (10 nM) in T84 cells were reduced by bumetanide. In control cells responses to apical BK were 11.0±3.5 μA cm−2 compared to 3.8±0.9 μA cm−2 in bumetanide-pretreated cells (n=5; P<0.05). These data indicate that Isc responses to apical and basolateral BK in human colon and T84 cells are both largely due to electrogenic Cl− secretion.
Figure 5.
Effects of bumetanide and chloride replacement on Isc responses to basolateral bradykinin (BK) in human colon. (a) The loop diuretic, bumetanide (100 μM), partially inhibited Isc responses to basolateral BK (n=5; P<0.05). (b) Responses to basolateral BK were also reduced by the removal of chloride ions from the bathing solution (n=6; P<0.05), indicating that these responses are due, at least in part, to the electrogenic chloride secretion. All values are mean±s.e.m. for n experiments.
The enteric nervous system and prostanoids participate in the prosecretory actions of basolateral BK in human colon
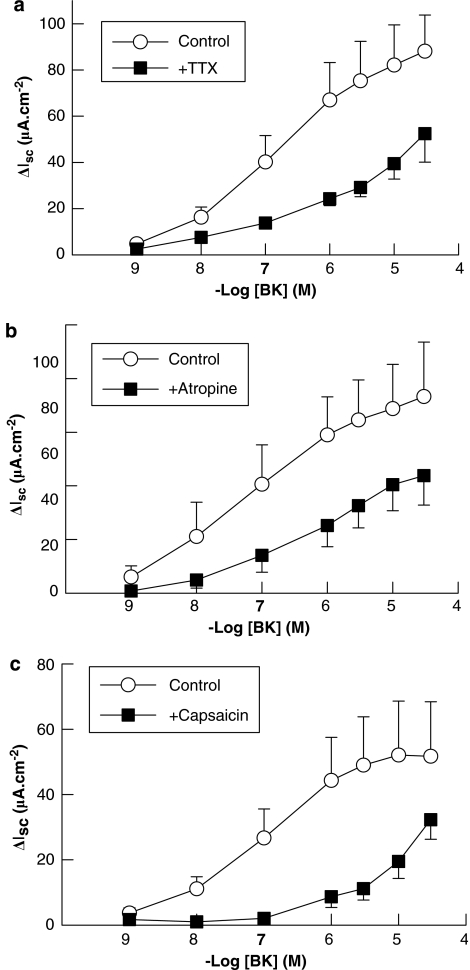
Finally, we investigated the potential role for indirect mechanisms in mediating prosecretory responses to BK in human colon, as has been reported in some animal models. The neurotoxin, TTX (1 μM) significantly reduced Isc responses to basolateral BK in intact human colonic preparations, indicating that these responses can be amplified by the activation of enteric nervous system (Figure 6a). The effects of TTX were not due to the loss of tissue viability, as it did not alter responses to carbachol (10 μM; n=5) added at the end of each experiment, which were 34.6±12.5 μA cm−2 in control tissues compared to 45.5±9.8 μA cm−2 in tissues pretreated with TTX. TTX significantly reduced basal Isc as reported previously (Keely et al., 1995). In further experiments, we investigated the potential involvement of cholinergic neurons in mediating responses to BK using the selective cholinergic receptor antagonist, atropine. Atropine (1 μM), which reduced responses to carbachol (100 μM) from 74.1±12.5–2.9±1.1 μA cm−2 (n=8; P<0.01), also significantly inhibited responses to BK (Figure 6b). The effects of atropine were not due to the loss of tissue viability as it did not alter responses to another GqPCR agonist, histamine (100 μM). Responses to histamine were 19.0±5.4 μA cm−2 in atropine-treated tissues compared to 18.1±5.6 μA cm−2 in controls (n=5).
Figure 6.
Neuronal involvement in Isc responses to basolateral bradykinin (BK) in human colon. (a) The neurotoxin, tetrodotoxin (TTX; 1 μM), inhibited the responses to basolateral BK (n=5; P<0.05) indicating that the neuronal involvement in Isc responses to BK. Responses to basolateral BK were also inhibited by (b) the cholinergic antagonist, atropine (1 μM; n=7; P<0.05) and (c) capsaicin (100 μM; n=7; P<0.05) indicating that both cholinergic and sensory peptidergic neurons are involved in amplifying secretory reponses to basolateral BK in human colon. All values are mean±s.e.m. for n experiments.
A possible role for sensory peptidergic neurons in mediating secretory responses to BK was investigated using capsaicin. Capsaicin (100 μM) alone, which activates sensory nerves and depletes them of their transmitters, stimulated a transient, inward Isc response of 21.4±7.8 μA cm−2 (n=5) which returned to values below baseline within 30 min. Capsaicin also attenuated subsequent responses to BK (Figure 6c) but did not alter responses to 100 μM carbachol (control response=55.4±6.3 μA cm−2; test response=60.9±14.4 μA cm−2; n=8).
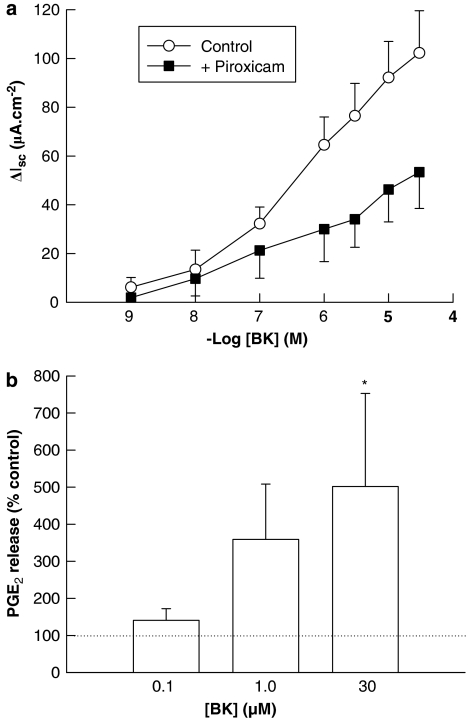
A potential role for PGs in mediating secretory responses to basolateral BK colon was investigated using piroxicam, a cyclooxygenase inhibitor. Pretreatment of tissues with piroxicam (10 μM) significantly attenuated subsequent responses to BK, indicating that the full expression of these responses involves PG synthesis (Figure 7a). This is supported by the finding that BK (100 nM–300 μM) stimulated the synthesis and release of PGE2 from the sections of human colon when compared to paired, unstimulated controls (Figure 7b).
Figure 7.
Involvement of prostaglandins in Isc responses to basolateral bradykinin (BK) in human colon. (a) The cyclooxygenase inhibitor, piroxicam (10 μM), significantly attenuated the responses to basolateral BK (n=5; P<0.05) indicating that the prostanoids are involved in the full expression of the responses to BK. (b) BK (100 nM–30 μM)-stimulated release of prostaglandin E2 (PGE2) from the segments of human colonic mucosa (n=5; P<0.05 by one-way ANOVA). Results are expressed as a percent of the release from paired control specimens not exposed to BK, as represented by the dashed line (100%; PGE2 release from the control tissues was 97±38 pg mg−1 tissue). All values are mean±s.e.m. for n experiments. *P<0.05 by the Student Newman–Keul post hoc test.
Discussion
Kinins are well established as mediators of intestinal inflammation but information regarding their role in regulating colonic ion transport have, to date, been limited to the studies in animal models and cultured epithelial cell lines. Here, we report for the first time the effects of kinins on ion transport across intact human colon in vitro. Although our data confirm that kinins are potent promoters of colonic epithelial transport, they also demonstrate a distinct polarity to BK-elicited transport responses in human colon that has not previously been observed in animal or cell culture models. Furthermore, our data suggest that although they stimulate ion transport from both the basolateral and apical aspects of human colon, it is the recruitment of additional extracellular factors that determine the full extent and duration of kinin-evoked responses.
Our studies suggest that the responses to apical and basolateral BK in human colon are likely to be due to the activation of separate populations of kinin receptors in each domain as (i) apical and basolateral BK evoke responses with distinctive time courses, implying differing mechanism(s) of action; (ii) responses to apical BK were not reduced by basolateral BK (and vice versa) and (iii) blockade of basolateral BK receptors specifically antagonized responses to basolateral, but not apical, BK (and vice versa). However, as responses to both apical and basolateral BK were mimicked by lysyl-BK but not the B1 agonists, des-Arg9-BK and des-Arg10-Lys-BK, it appears that the responses to BK on both sides of human colon are mediated by B2 receptors. This is further supported by the observations that the selective B2 receptor antagonist, HOE 140, antagonized responses to both apical and basolateral BK. This ability of kinins to stimulate ion transport from either side of colonic epithelia appears to be a feature unique to humans, as it has not been reported in other mammalian species (Cuthbert and Margolius, 1982; Manning et al., 1982; Rangachari et al., 1993; Cuthbert et al., 1994) and has only previously been shown in to occur in cultured epithelia derived from human colon (Cuthbert et al., 1985, 1987). This study did not determine why apical kinins are more potent in stimulating ion transport across intact tissues than basolateral addition; however, multiple factors are likely to be involved. Firstly, the connective tissue of the submucosa represents a diffusion barrier, which is likely to limit the access of kinins to their receptors within the tissue (Takeuchi et al., 2007), and secondly, kinin metabolizing enzymes are found within the lamina propria, which would serve to degrade exogenously added kinins thereby reducing their active concentrations (Duggan et al., 1989).
The magnitude and duration of the responses to BK in human colon differ depending on the side of the tissue to which it is exposed, implying that these responses are mediated by different intracellular signalling pathways (Barrett and Keely, 2000). The rapid onset and transient responses observed to apical BK are typical of calcium-mediated agonists and are consistent with BK having a direct action at epithelial B2 receptors, which are known to be coupled to elevations in intracellular calcium (Leeb-Lundberg et al., 2005). In contrast, although also mediated by B2 receptors, the more slow onset and sustained responses to basolateral addition of the peptide are typical of those mediated by cyclic nucleotides and imply that, in intact tissue, basolateral BK acts indirectly by promoting the release of cAMP-dependent secretagogues from cells within the lamina propria. These conclusions are supported by our findings with cultured colonic epithelial cells where, in the absence of accessory lamina propria cells, basolateral BK stimulates transient Isc responses that are similar to those seen upon apical addition. Studies from animal models and cultured epithelia support our conclusions in that in cultured epithelial cells, kinins stimulate Ca2+ mobilization but not the accumulation of cAMP, whereas in intact rat colonic tissue, kinins increase the intracellular cAMP (Cuthbert et al., 1984, 1987).
Thus, Although our data leave little doubt that kinins can act directly upon epithelial cells to promote ion transport, they also indicate that, as in animal models (Cuthbert et al., 1984; Warhurst et al., 1987; Diener et al., 1988; Phillips and Hoult, 1988; Wardle et al., 1993; Green et al., 2003), components of the attendant lamina propria are important in regulating kinin-induced responses in human colon. We found that the neurotoxin-, TTX-inhibited responses to basolateral BK, implying a neuronal involvement in these responses. In addition, the cholinergic antagonist, atropine and the peptidergic neurotoxin, capsaicin, also attenuated the responses to BK, indicating that these responses are mediated by both cholinergic and sensory peptidergic nerves. Furthermore, as responses to the cholinomimetic, carbachol, were unaltered by either TTX or capsaicin, it seems likely that ACh released from secretomotor neurons acts directly on epithelial cells and is the final step in the neuronal pathway activated by BK. Although it is tempting to speculate that cholinergic secretomotor neurons are indirectly activated by BK through capsaicin-sensitive, peptidergic neurons (Cooke, 1994), the identification of intermediate transmitter(s) released from such neurons was beyond the scope of this study. A comprehensive examination of the involvement of all possible neurotransmitters in the basolateral effect of BK would represent a considerable experimental undertaking and would thus more properly form the focus of a separate study. However, substance P is a strong candidate as capsaicin-sensitive sensory neurons contain this peptide as their primary transmitter and substance P stimulates TTX-sensitive transport responses in colonic tissues derived from a number of species, including humans (Yarrow et al., 1991; Cox et al., 1993; Riegler et al., 1999).
In addition to the activation of enteric nervous system, the sensitivity of BK-stimulated responses to the cyclooxygenase inhibitor, piroxicam, implies that PGs are also required for the full expression of responses to BK. This is further supported by the observation that BK rapidly stimulated release of PGE2 from sections of human colonic tissue in vitro. These results are in agreement with previous studies in mammalian intestine where products of both the cyclooxygenase and lipoxygenase pathways were implicated in BK-stimulated transport (Diener et al., 1988; Rangachari et al., 1993; Wardle et al., 1993; Riegler et al., 1999). Several cellular elements of the colonic mucosa have the capability to produce prostanoids upon activation by inflammatory mediators; however, studies from Bershneider and Powell (1992) suggest that the cells of the fibroblastic sheath, which lies in close proximity to the epithelium, are one likely source.
Previous studies in animal models (Perkins et al., 1988; Rangachari et al., 1993; Cuthbert and Huxley, 1998; Riegler et al., 1999) and colonic epithelial cell lines (Henderson and Cuthbert, 1993; MacVinish et al., 1993), suggest that ion transport responses evoked by basolateral BK are largely due to the electrogenic chloride secretion. Our studies in human colon confirm these findings as the responses to BK were sensitive to both the loop diuretic, bumetanide and to the removal of Cl− ions from the bathing solution. Furthermore, responses to apical BK in T84 cells were also found to be due to Cl− secretion. These data are important in the clinical setting as, in vivo, Cl− secretion is the primary driving force for fluid secretion into the gut lumen. Although intestinal inflammatory responses are known to be associated with increased kallikrein, kinin and kinin receptor expressions (Stadnicki et al., 2003, 2005; Devani et al., 2005; Hara et al., 2007) and that kinin receptor antagonists are known to alleviate symptoms of inflammatory bowel disease (Arai et al., 1999; Kamat et al., 2002), it is likely that dysregulated transport associated with such conditions are likely to be due, at least in part, to kinins. Kinins generated in inflammatory conditions are likely to originate from the epithelial cells themselves (Baird et al., 1991) and also from lamina propria mast cells and phagocytes, both of which are known to have kinin-generating capabilities (Figueroa et al., 1989; Shirasky et al., 1992). Furthermore, our observations that functional kinin receptors exist on the apical and basolateral side of human colonic epithelia are also likely to be important in the setting of intestinal inflammation, given that phagocytes are known to migrate through the colonic epithelium to form crypt abscesses during conditions of intestinal inflammation (Nash et al., 1991; Reaves et al., 2001).
In conclusion, our studies demonstrate that kinins stimulate secretory responses across human colonic epithelia in vitro. Kinins have the capability to act from either the apical or basolateral domain through the activation of distinct populations of B2 receptors that do not cross-desensitize. Whereas the responses to apical BK are most likely to reflect a direct action on the epithelial cells themselves, the prosecretory effects of basolateral kinins appear to be largely indirect and involve the activation of enteric nervous system and the stimulation of eicosanoid synthesis. Our data support an important role for kinins in regulating human colonic epithelial transport and suggest that they are likely to contribute to transport disturbances associated with intestinal disease.
Acknowledgments
This work was supported by an award from the Health Research Board of Ireland to AWB, by NIH Grant DK 47756 to KEB and by a CCFA Senior Investigator Award to SJK.
Abbreviations
- BK
bradykinin
- CCh
carbachol
- Isc
short circuit current
- PGE2
prostaglandin E2
- TTX
tetrodotoxin
- ΔIsc
change in short circuit current
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 Suppl. 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y, Takanashi H, Kitagawa H, Wirth KJ, Okayasu I. Effect of icatibant, a bradykinin B2 receptor antagonist, on the development of experimental ulcerative colitis in mice. Dig Dis Sci. 1999;44:845–851. doi: 10.1023/a:1026694732602. [DOI] [PubMed] [Google Scholar]
- Baird AW, Miller DH, Schwartz DA, Margolius HS. Enhancement of kallikrein production and kinin sensitivity in T84 cells by growth in the nude mouse. Am J Physiol. 1991;261:C822–C827. doi: 10.1152/ajpcell.1991.261.5.C822. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Keely SJ. Chloride Secretion by the Intestinal Epithelium: Molecular Basis and Regulatory Aspects. Ann Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Keely SJ.Integrative physiology and electrophysiology of intestinal electrolyte transport Physiology of the Gastrointestinal Tract 2006Academic Press: San Diego, CA, USA; 1931–1951.In: Johnson L (ed) [Google Scholar]
- Bershneider H, Powell D. Fibroblasts modulate intestinal secretory responses to inflammatory mediators. J Clin Invest. 1992;89:484–489. doi: 10.1172/JCI115610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke HJ. Neuroimmune signalling in regulation of intestinal ion transport. Am J Physiol. 1994;266:G167–G178. doi: 10.1152/ajpgi.1994.266.2.G167. [DOI] [PubMed] [Google Scholar]
- Cox H, Tough I, Grayson K, Yarrow S. Pharmacological characterisation of neurokinin receptors mediating anion secretion in rat descending colon mucosa. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:142–147. doi: 10.1007/BF00164795. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. Kinins and epithelial ion transport in the alimentary tract. Biol Chem. 2001;382:57–60. doi: 10.1515/BC.2001.009. [DOI] [PubMed] [Google Scholar]
- Cuthbert A, Egleme C, Greenwood H, Hickman M, Kirkland S, MacVinish L. Calcium and cyclic-AMP-dependent chloride secretion in human colonic epithelia. Br J Pharmacol. 1987;91:503–515. doi: 10.1111/j.1476-5381.1987.tb11243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A, Evans M, Colledge W, MacVinish L, Ratcliff R. Kinin stimulated chloride secretion in mouse colon requires the participation of CFTR chloride channels. Brazilian J Med Biol Res. 1994;27:1905–1910. [PubMed] [Google Scholar]
- Cuthbert A, Halushka P, Margolius H, Spayne J. Mediators of the secretory response to kinins. Br J Pharmacol. 1984;82:597–607. doi: 10.1111/j.1476-5381.1984.tb10798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A, Kirkland S, MacVinish L. Kinin effects on ion transport in monolayers of HCA-7 cells, a line from human colonic adenocarcinoma. Br J Pharmacol. 1985;86:3–5. doi: 10.1111/j.1476-5381.1985.tb09428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A, Margolius H. Kinins stimulate net chloride secretion by the rat colon. Br J Pharmacol. 1982;75:587–598. doi: 10.1111/j.1476-5381.1982.tb09178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert AW, Huxley C. The primary and final effector mechanisms required for kinin-induced epithelial chloride secretion. Am J Physiol. 1998;274:G578–G583. doi: 10.1152/ajpgi.1998.274.3.G578. [DOI] [PubMed] [Google Scholar]
- Devani M, Vecchi M, Ferrero S, Avesani EC, Arizzi C, Chao L, et al. Kallikrein-kinin system in inflammatory bowel diseases: Intestinal involvement and correlation with the degree of tissue inflammation. Dig Liver Dis. 2005;37:665–673. doi: 10.1016/j.dld.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Diener M, Bridges R, Knobloch S, Rummel W. Indirect effects of bradykinin on ion transport in rat colon descendens: mediated by prostaglandins and enteric neurons. Naunyn-Schmiedeberg's Arch Pharmacol. 1988;337:69–73. doi: 10.1007/BF00169479. [DOI] [PubMed] [Google Scholar]
- Duggan KA, Mendelsohn FA, Levens NR. Angiotensin receptors and angiotensin I-converting enxyme in rat intestine. Am J Physiol. 1989;257:G504–G510. doi: 10.1152/ajpgi.1989.257.4.G504. [DOI] [PubMed] [Google Scholar]
- Figueroa C, MacIver A, MacKenzie J, Bhoola K. Identification of a tissue kallikrien in human polymorphonuclear leucocytes. Br J Haematol. 1989;72:321–328. doi: 10.1111/j.1365-2141.1989.tb07711.x. [DOI] [PubMed] [Google Scholar]
- Green B, Calvin A, O'Grady S, Brown D. Kinin-induced anion-dependent secretion in porcine ileum: characterization and involvement of opioid- and cannabinoid-sensitive enteric neural circuits. J Pharm Exp Ther. 2003;305:733–739. doi: 10.1124/jpet.102.047829. [DOI] [PubMed] [Google Scholar]
- Hara DB, Fernandes ES, Campos MM, Calixto JB. Pharmacological and biochemical characterization of bradykinin B2 receptors in the mouse colon: Influence of the TNBS-induced colitis. Reg Pept. 2007;141:25–34. doi: 10.1016/j.regpep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Henderson R, Cuthbert A. Activation of ion channels by lysylbradykinin in the HCA-7 colony 29 human adenocarcinoma cell line. Br J Pharmacol. 1993;108:479–483. doi: 10.1111/j.1476-5381.1993.tb12828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph K, Kaplan AP. Formation of bradykinin: a major contributor to the innate inflammatory response. Adv Immunol. 2005;86:159–208. doi: 10.1016/S0065-2776(04)86005-X. [DOI] [PubMed] [Google Scholar]
- Kamat K, Hayashi I, Mizuguchi Y, Arai K, Saeki T, Ohno T, et al. Suppression of dextran sulfate sodium-induced colitis in kininogen-deficient rats and non-peptide B2 receptor antagonist-treated rats. Jpn J Pharmacol. 2002;90:59–66. doi: 10.1254/jjp.90.59. [DOI] [PubMed] [Google Scholar]
- Keely SJ, Stack WA, O'Donoghue DP, Baird AW. Regulation of ion transport by histamine in human colon. Eur J Pharmacol. 1995;279:203–209. doi: 10.1016/0014-2999(95)00156-f. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg LMF, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International Union of Pharmacology. XLV. Classification of the Kinin Receptor Family: from Molecular Mechanisms to Pathophysiological Consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- MacVinish L, Reancharoen T, Cuthbert A. Kinin-induced chloride permeability changes in colony 29 epithelia estimated from 125I-efflux and MEQ fluorescence. Br J Pharmacol. 1993;108:469–483. doi: 10.1111/j.1476-5381.1993.tb12827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning D, Snyder S, Kachur J, Miller R, Field M. Bradykinin receptor-mediated chloride secretion in intestinal function. Nature. 1982;299:256–259. doi: 10.1038/299256a0. [DOI] [PubMed] [Google Scholar]
- Margolius H. Tissue kallikriens ands kinins: Regulation and roles in hypertensive and diabetic diseases. Ann Rev Pharmacol Toxicol. 1989;29:343–364. doi: 10.1146/annurev.pa.29.040189.002015. [DOI] [PubMed] [Google Scholar]
- Montrose MM, Keely SJ, Barrett KE.Electrolyte secretion and absoprtion: small intestine and colon Textbook of Gastroenterology 2003Lippencott, Raven: Philadelphia; 308–339.In: Yamada T, Alpers D, Kaplowitz N, Laine L, Owyang C, Powell D (eds) [Google Scholar]
- Nash S, Parkos C, Nusrat A, Delp C, Madara J. In vitro model of intestinal crypt abcess: a novel neutrophil-derived secretagogue activity. J Clin Invest. 1991;87:1474–1477. doi: 10.1172/JCI115156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M, Forster P, Dray A. The involvement of afferent nerve terminals in the stimulation of ion transport by bradykinin in rat isolated colon. Br J Pharmacol. 1988;94:47–54. doi: 10.1111/j.1476-5381.1988.tb11498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J, Hoult J. Secretory effects of kinins on colonic epithelium in relation to prostaglandins released from cells of the lamina propria. Br J Pharmacol. 1988;95:701–712. doi: 10.1111/j.1476-5381.1988.tb11696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari P, Berezin M, Prior T. Effects of bradykinin on the canine proximal colon. Regul Pept. 1993;46:511–522. doi: 10.1016/0167-0115(93)90252-4. [DOI] [PubMed] [Google Scholar]
- Reaves TA, Colgan SP, Selvaraj P, Pochet MM, Walsh S, Nusrat A, et al. Neutrophil transepithelial migration: regulation at the apical epithelial surface by Fc-mediated events. Am J Physiol. 2001;280:G746–G754. doi: 10.1152/ajpgi.2001.280.4.G746. [DOI] [PubMed] [Google Scholar]
- Riegler M, Castagliuolo I, So PTC, Lotz M, Wang C, Wlk M, et al. Effects of substance P on human colonic mucosa in vitro. Am J Physiol. 1999;276:G1473–G1483. doi: 10.1152/ajpgi.1999.276.6.G1473. [DOI] [PubMed] [Google Scholar]
- Shirasky H, Asakura K, Kojima T, Sohoma S, Katura A. The roles of histamine, leukotriene C4 and bradykinin on nasal vascular permeability in experimental nasal allergy of guinea pigs. Rhinology. 1992;30:41–48. [PubMed] [Google Scholar]
- Stadnicki A, Mazurek U, Plewka D, Wilczok T. Intestinal tissue kallikrein-kallistatin profile in inflammatory bowel disease. Int Immunopharmacol. 2003;3:939–944. doi: 10.1016/S1567-5769(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Stadnicki A, Pastucha E, Nowaczyk G, Mazurek U, Plewka D, Machnik G, et al. Immunolocalization and expression of kinin B1R and B2R receptors in human inflammatory bowel disease. Am J Physiol. 2005;289:G361–G366. doi: 10.1152/ajpgi.00369.2004. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Ham M, Mizumori M, Guth PH, Engel E, Kaunitz JD, et al. Solute diffusion through stripped mouse duodenum. J Physiol Pharmacol. 2007;58:767–791. [PubMed] [Google Scholar]
- Wardle T, Hall L, Turnberg L. Inter-relationships between inflammatory mediators released from colonic mucosa in ulcerative colitis and their effects on colonic secretion. Gut. 1993;34:503–508. doi: 10.1136/gut.34.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst G, Lees M, Higgs N, Turnberg L. Site and mechanisms of action of kinins in rat ileal mucosa. Am J Physiol. 1987;252:G293–G300. doi: 10.1152/ajpgi.1987.252.3.G293. [DOI] [PubMed] [Google Scholar]
- Yarrow S, Ferrar J, Cox H. The effects of capsaicin upon electrogenic ion transport in rat descending colon. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:557–563. doi: 10.1007/BF00170652. [DOI] [PubMed] [Google Scholar]