Abstract
Pulmonary arterial hypertension (PAH) is characterized by a sustained and progressive elevation in pulmonary arterial pressure and pulmonary vascular remodelling leading to right heart failure and death. Prognosis is poor and novel therapeutic approaches are needed. The serotonin hypothesis of PAH originated in the 1960s after an outbreak of the disease was reported among patients taking the anorexigenic drugs aminorex and fenfluramine. These are indirect serotinergic agonists and serotonin transporter substrates. Since then many advances have been made in our understanding of the role of serotonin in the pathobiology of PAH. The rate-limiting enzyme in the synthesis of serotonin is tryprophan hydroxylase (Tph). Serotonin is synthesized, through Tph1, in the endothelial cells of the pulmonary artery and can then act on underlying pulmonary arterial smooth muscle cells and pulmonary arterial fibroblasts in a paracrine fashion causing constriction and remodelling. These effects of serotonin can be mediated through both the serotonin transporter and serotonin receptors. This review will discuss our current understanding of ‘the serotonin hypothesis' of PAH and highlight possible therapeutic targets within the serotonin system.
Keywords: pulmonary arterial hypertension, serotonin, tryptophan hydroxylase, serotonin transporter, serotonin receptors, dexfenfluramine
Introduction
Pulmonary arterial hypertension (PAH) is characterized by constriction and remodelling of the pulmonary arterial bed, leading to a progressive increase in pulmonary arterial pressure, right heart failure and death. Idiopathic PAH (iPAH) (formerly known as primary PAH) describes a form of the disease for which there is no known cause. PAH can also occur secondary to global hypoxia as seen in patients with chronic obstructive pulmonary disease or following chronic exposure to high altitude. Various drugs and toxins have also been associated with the development of PAH, as has HIV infection (Simonneau et al., 2004). Familial PAH (fPAH) transmits as an autosomal dominant trait that exhibits genetic anticipation but also markedly reduced penetrance (20%) (Loyd et al., 1995). The primary genetic defect of fPAH, (present in ∼70% of cases), is a mutation in the gene-encoding bone morphogenetic protein receptor type II (BMPR-II), a member of the transforming growth factor-β (TGF-β) superfamily (Lane et al., 2000; Machado et al., 2001). Genetic heterogeneity may occur in some cases of severe unexplained PAH. For example, mutations in the TGF-β receptor, activin receptor-like kinase-1, have been reported in families with hereditary haemorrhagic telangiectasia and severe pulmonary hypertension (Trembath et al., 2001). The true prevalence of BMPR-II mutations in iPAH is unknown, with reports ranging from 10 to 40% of patients (Thomson et al., 2000; Sankelo et al., 2005; Baloira et al., 2008; Fujiwara et al., 2008). The cause of the variable phenotypic expression of PAH among carriers of mutated BMPR-II genes and patients is unclear, and likely related to additional environmental and/or genetic modifiers. Increased activation of the serotonin system has been proposed as a ‘second hit' risk factor.
The ‘serotonin hypothesis of PAH' arose in the 1960s when patients taking the anorexigen aminorex fumarate were associated with an increased risk of developing PAH. Subsequently, in the early 1980s French investigators reported a cluster of PAH among patients using fenfluramine derivatives (Abenhaim et al., 1996; Kramer and Lane, 1998). Both aminorex and fenfluramine are serotonin transporter (SERT) substrates and increase extracellular concentrations of serotonin (Rothman et al., 1999). Serotonin is thought to mediate PAH by promoting both vasoconstriction and remodelling of the pulmonary vasculature (MacLean et al., 2000). Serotonin induces proliferation of both pulmonary arterial fibroblasts and pulmonary arterial smooth muscle cells (Lee et al., 1994; Welsh et al., 2004). This results in a thickening of the medial layer and a narrowing of the lumen of the pulmonary artery and contributes to the pulmonary vascular remodelling associated with PAH. Clinically, pulmonary arterial smooth muscle cells from PAH patients proliferate faster than those from controls when stimulated with serotonin, and elevated circulating peripheral serotonin has been associated with the development of PAH (Herve et al., 1995; Eddahibi et al., 2001). Experimentally, exogenous serotonin can potentiate the development of hypoxia-induced PAH in rats (Eddahibi et al., 1997), and inhibition of serotonin receptors or SERT can inhibit the development of PAH in animal models (Keegan et al., 2001; Hironaka et al., 2003; Marcos et al., 2003; Guignabert et al., 2005). Fawn hooded rats, which have an inherited platelet storage defect to serotonin and increased expression of SERT, have increased susceptibility to PAH (Morecroft et al., 2005).
Although many advances have been made in recent years, the pathophysiology of PAH is still not fully understood. Prognosis is poor and current therapies are not effective in the long-term. This review will examine our current understanding of the role of serotonin in the pathobiology of PAH, and discuss possible therapeutic targets.
Tryptophan hydroxylase 1
Tryptophan hydroxylase (Tph) is the rate-limiting enzyme in the synthesis of serotonin. Recently, it was demonstrated that there are two isoforms of Tph, now termed Tph1 and Tph2. Tph1 is present mainly in the gut and mediates generation of serotonin in the periphery, whereas Tph 2 is present exclusively in the central nervous system (Walther and Bader, 2003; Walther et al., 2003a). Expression of the Tph1 gene is increased in lungs and pulmonary arterial endothelial cells from patients with iPAH. Medium collected from human pulmonary arterial endothelial cell cultures induces marked proliferation of human pulmonary arterial smooth muscle cells, an effect reduced in the presence of the Tph inhibitor p-chlorophenylalanine. This effect is greater when using pulmonary artery endothelial cells and pulmonary arterial smooth muscle cells from iPAH patients than when using cells from control subjects (Eddahibi et al., 2006). Taken together these data suggest that the increased Tph1 expression/activity in pulmonary artery endothelial cells, and subsequent paracrine effects of serotonin on pulmonary arterial smooth muscle cells, may play a role in PAH (see Figure 1 for summary). Moreover, there is also evidence to suggest that Tph1 is involved in PAH secondary to hypoxia. Hypoxia-induced increases in both right ventricular pressure and pulmonary vascular remodelling are severely ablated in mice deficient in Tph1 indicating that de novo synthesis of serotonin is essential for the development of hypoxia-induced PAH (Izikki et al., 2007; Morecroft et al., 2007). Both hypoxia and mechanical stretch have been shown to increase Tph1 expression and serotonin release in rabbit lung (Pan et al., 2006). These observations suggest that chronic hypoxia itself may induce Tph1 synthesis in the pulmonary arterial endothelium, which can then act on underlying pulmonary arterial smooth muscle cells and pulmonary arterial fibroblasts in a paracrine fashion. Mice lacking Tph1 have a reduced risk of thrombosis and thromboembolism (Walther et al., 2003b) and so targeting Tph1 for the treatment of PAH is of interest.
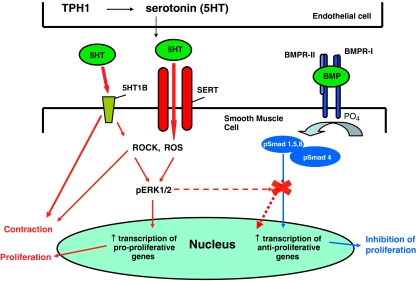
Figure 1.
Serotonin is synthesized in the pulmonary arterial endothelial cells by tryptophan hydroxylase1 (Tph1). Serotonin can then influence pulmonary vascular smooth muscle proliferation and/or contraction via activity at the serotonin transporter (SERT) and serotonin receptors (particularly the 5-HT1B receptor in humans). Intracellular accumulation of serotonin and activation of the 5-HT1B receptor may induce reactive oxygen species (ROS), activation of Rho-kinase (ROCK), leading to phosphorylation and nuclear translocation of extracellular-regulated kinase (ERK)1/2. Once inside the nucleus phosphorylated ERK1/2 can increase transcription of nuclear growth factors and mediate cellular proliferation. Activation of the bone morphogenetic receptor type II (BMPR-II) leads to signalling through the Smad 1/5/8 pathway. Smads1, 5 and 8 must dimerize with Smad 4 to enter the nucleus where they can activate anti-proliferative transcription factors. Serotonin may antagonize BMPR-II signalling as phosphorylated ERK1/2 can phosphorylate the linker region of Smad 1and inhibit nuclear translocation.
Serotonin transporter
The gene-encoding SERT is located on chromosome 17q11.2 and has a variant in the promoter region. This polymorphism affects SERT expression and function. The long (L) allele induces an increased rate of SERT transcription over the short (S) allele (Lesch et al., 1996). The LL genotype was found to be more prevalent in a small sample of primary PAH patients than in the control group (Eddahibi et al., 2001). However, other groups studying larger cohorts of PAH patients suggest that variation of the SERT gene alone is unlikely to mediate susceptibility to PAH, although PAH patients with the LL genotype may present earlier than those without (Machado et al., 2006; Willers et al., 2006). The LL genotype has also been associated with exaggerated PAH in patients with chronic obstructive lung disease (Eddahibi et al., 2003), and with an increased risk of developing PAH at high altitudes (Long et al., 2002). Further evidence for a role for SERT in PAH exists in animal models of the disease. Mice overexpressing SERT (SERT+ mice) develop increased pulmonary pressures and are more susceptible to hypoxia-induced PAH, whereas mice deficient for the SERT are less susceptible (Eddahibi et al., 2000; MacLean et al., 2004; Guignabert et al., 2006). Inhibition of SERT protects against PAH secondary to both hypoxia (Marcos et al., 2003) and monocrotaline injection (Guignabert et al., 2005). Moreover, drugs such as aminorex, dexfenfluramine and methamphetamine, all of which are SERT substrates and compete for SERT, mediating release of serotonin via SERT, have been associated with an increased risk of developing PAH (Rothman et al., 1999; Chin et al., 2006). Indeed, dexfenfluramine-induced pulmonary vascular remodelling is exaggerated in SERT+ mice compared with wild-type controls (Dempsie et al., 2008). There is evidence to suggest that SERT may mediate the proliferative effects of serotonin, as inhibition of SERT reduces proliferation of human and bovine pulmonary arterial smooth muscle cells (Lee et al., 1994; Marcos et al., 2004), and rat pulmonary arterial fibroblasts (Welsh et al., 2004). Moreover, both serum- and serotonin-induced proliferation is increased in pulmonary arterial smooth muscle cells from PAH patients compared with controls, and this is due to increased expression of SERT (Eddahibi et al., 2001). Although the precise mechanism by which SERT mediates proliferation of pulmonary arterial smooth muscle cells and pulmonary arterial fibroblasts still remains unclear, evidence suggests that serotonin transport into the cell via SERT results in the production of reactive oxygen species (ROS). ROS then mediates phosphorylation and /or nuclear translocation of extracellular signal-regulated kinase 1/2 (ERK1/2), which leads to activation of transcription factors, such as GATA 4, and cellular proliferation (Lee et al., 1999, 2001a; Suzuki et al., 2003; Liu et al., 2004; Lawrie et al., 2005).
Classical vasodilators cannot be used to treat PAH due to their systemic hypotensive effects. To avoid systemic hypotension, pharmacological targets for PAH need to be pulmonary-specific. Serotonin-induced proliferation of systemic SMCs is through the 5-HT2A receptor (Sharma et al., 1999). Hence, serotonin-induced proliferation mediated by SERT activity is a pulmonary-specific mechanism, making SERT an attractive target for PAH. However, we have observed increased serotonin-induced contraction in the presence of SERT inhibitors in isolated pulmonary arteries from animals showing increased SERT expression, that is, fawn hooded rats and SERT+ mice (Morecroft et al., 2005; Dempsie et al., 2008). Therefore, if SERT inhibitors are considered for the treatment of PAH, these may have to be administered in conjunction with serotonin receptor antagonists. This is discussed further below.
The 5-HT1B receptor
The 5-HT1B receptor mediates serotonin-induced constriction in human small and large pulmonary arteries (MacIntyre et al., 1992; MacLean et al., 1996; Morecroft et al., 1999). Moreover, pulmonary arteries removed from PAH patients show increased expression of the 5-HT1B receptor (Launay et al., 2002). The 5-HT1B receptor is a Gi-coupled receptor and we have previously demonstrated that 5-HT1B receptor-mediated vasoconstriction can be increased through pharmacological synergy (MacLean, 1999). For example hypoxia, reduced cGMP levels and raised vascular tone all markedly increase 5-HT1B-mediated responses in pulmonary arteries. As all these stimuli exist in PAH, it is likely that there will be increased 5-HT1B-mediated responses in pulmonary arteries from PAH lungs. Experimentally, the 5-HT1B receptor has been shown to be involved in the development of PAH in rats and mice exposed to chronic hypoxia, as well as in the increased contractile response to serotonin observed in the pulmonary arteries from these animals (Keegan et al., 2001). 5-HT1B receptor expression is enhanced in pulmonary arteries from monocrotaline-treated rats (Wang et al., 2001), and in a pig model of pulmonary hypertension (Rondelet et al., 2003). Serotonin mediates vasoconstriction of most systemic resistance arteries via the 5-HT2A receptor (Hoyer et al., 1994). Therefore, the 5-HT1B receptor could be a pulmonary selective target for PAHs. The 5-HT1B receptor and SERT activity have been shown to co-operate in pulmonary vascular contraction of rat pulmonary arteries (Morecroft et al., 2005). In pulmonary resistance arteries isolated from fawn-hooded rats, the vascular response to serotonin can be enhanced by the SERT inhibitors fluoxetine and citalopram. However, in the presence of the combined 5-HT1B receptor/SERT antagonist LY393558 the contractile response to serotonin is reduced. In pulmonary resistance arteries from both normoxic and chronically hypoxic rats the 5-HT1B receptor antagonist SB224289 and SERT inhibitor fluoxetine both inhibited the serotonin-induced contractile response. However, LY393558 was the most potent inhibitor of serotonin-induced constriction and there was synergy between the effects of fluoxetine and SB224289 when given simultaneously. Synergy between the 5-HT1B receptor and SERT has also been described in proliferation of bovine and human pulmonary arterial smooth muscle cells (Liu et al., 2004; Lawrie et al., 2005). Therefore, dual blockade of the 5-HT1B receptor and SERT would likely be the optimal therapeutic approach. Indeed unpublished data from our laboratory suggests that LY393558 is more effective than citalopram at preventing PAH secondary to SERT overexpression and hypoxia.
The 5-HT2A receptor
The 5-HT2A receptor has also been implicated in PAH. Antagonism of the 5-HT2A receptor inhibits monocrotaline-induced PAH in mice (Hironaka et al., 2003), and also inhibits serotonin-induced pulmonary vasoconstriction in vessels from both normoxic and hypoxic rats (Morecroft et al., 2005; Cogolludo et al., 2006). Moreover, the 5-HT2A receptor mediates serotonin-induced proliferation of rat pulmonary arterial fibroblasts (Welsh et al., 2004). The 5-HT2A receptor is present in human pulmonary arteries; however, it only contributes to vasoconstriction when serotonin concentrations are much higher than the physiological range (Morecroft et al., 1999). Moreover, the 5-HT2A receptor also mediates vasoconstriction of the systemic circulation. The 5-HT2A receptor antagonist ketanserin has proved to be clinically effective in the treatment of systemic hypertension. Hence, there is no specificity for the pulmonary circulation and the systemic effects have limited its use in either primary or secondary pulmonary hypertension, where it fails to improve pulmonary haemodynamics significantly (Frishman et al., 1995).
The 5-HT2B receptor
The 5-HT2B receptor is upregulated in pulmonary arteries removed from pulmonary hypertensive patients, and the development of hypoxia-induced PAH is inhibited in mice deficient for the 5-HT2B receptor (Launay et al., 2002). This receptor may also control plasma serotonin levels in mice (Callebert et al., 2006). However, there is currently no evidence of 5-HT2B receptor-mediated constriction of human pulmonary arteries or 5-HT2B receptor-mediated proliferation of human pulmonary arterial smooth muscle cells or pulmonary arterial fibroblasts. In addition, loss of 5-HT2B receptor function may pre-dispose to fenfluramine-associated PAH in man (Blanpain et al., 2003).
Serotonin signalling in PAH
Much progress has been made in recent years in identifying mechanisms downstream of serotonin that are involved in the pathogenesis of PAH. Unravelling the serotonin-induced signalling pathways is important to identify further possible therapeutic targets. As discussed above, serotonin transport through SERT has been shown to be involved in pulmonary arterial smooth muscle cell and pulmonary arterial fibroblast proliferation (Lee et al., 1994; Marcos et al., 2004; Welsh et al., 2004), and this involves ROS and ERK1/2 activation (Figure 1). Studies on human pulmonary arterial smooth muscle cells provide evidence that it may be the breakdown of serotonin by monoamine oxidase which results in the generation of ROS (Lawrie et al., 2005), whereas studies on bovine pulmonary arterial smooth muscle cells suggest serotonin induces ROS through activation of NADPH oxidase (Lee et al., 1998; Liu and Folz, 2004). Serotonin-induced activation of NADPH oxidase has been postulated to occur via activation of small GTPase proteins such as Rac-1 (Lee et al., 1998). In platelets, internalized serotonin is transamidated to small GTPases by transglutaminases, rendering these GTPases constitutively active (Walther et al., 2003b). The mechanism by which ERK1/2 is phosphorylated and translocated to the nucleus is also controversial and may be species-specific. In bovine pulmonary arterial smooth muscle cells, ROS has been proposed to mediate phosphorylation of ERK1/2, whereas activation of Rho-kinase (ROCK) via the 5-HT1B receptor mediates nuclear translocation of phosphorylated ERK1/2 (Lee et al., 1999, 2001a; Liu et al., 2004). Studies in human pulmonary arterial smooth muscle cells show phosphorylation of ERK1/2 to occur via activation of the 5-HT1B receptor, with ROS responsible for nuclear translocation of phosphorylated ERK1/2 (Lawrie et al., 2005). After phosphorylated ERK1/2 has been translocated to the nucleus it can increase DNA binding of transcription factors such as GATA-4, Egr-1 and Elk-1, and thus increase expression of proteins, which are involved in cellular proliferation (Liu et al., 2004). One such protein is S100A4/Mts1, a calcium-binding protein that is involved in proliferation of human pulmonary arterial smooth muscle cells (Lawrie et al., 2005). S100A4/Mts1 is upregulated in the neointima in remodelled vessels from patients with PAH and a subset of mice overexpressing S100A4/Mts1 develop occlusive pulmonary arterial lesions similar to those seen in patients with PAH (Greenway et al., 2004).
Under hypoxic conditions it would appear that serotonin-induced proliferation of pulmonary arterial fibroblasts is mediated via the p38 mitogen-activated protein (MAP) kinase pathway, rather than the ERK1/2 pathway (Welsh et al., 2004). The p38 MAP kinase pathway has frequently been associated with hypoxic-induced proliferation of pulmonary arterial fibroblasts and has recently been linked to activation of the transcription factor hypoxia-inducible factor-1α (Das et al., 2001; Welsh et al., 2001, 2006). It is interesting to note that hypoxia mediates upregulation of the α and γ isoforms of p38 MAP kinase and also of hypoxia-inducible factor-1α in the pulmonary but not the systemic circulation, thus providing possible future pulmonary selective targets for the treatment of PAH (Welsh et al., 2006; Mortimer et al., 2007).
ROCK has been implicated in both pulmonary vascular contraction and pulmonary vascular remodelling (Fukumoto et al., 2007). Inhibition of ROCK attenuates chronic hypoxic-induced PAH in mice, chronic hypoxic, monocrotaline and high flow-induced PAH in rats, decreases susceptibility to PAH in fawn-hooded rats, and is involved in the beneficial effect of sildenafil on PAH (Abe et al., 2004; Fagan et al., 2004; Guilluy et al., 2005; Hyvelin et al., 2005; Nagaoka et al., 2006; Li et al., 2007a). In two small groups of PAH patients, the ROCK inhibitor fasudil decreased pulmonary arterial pressure and pulmonary vascular resistance. Although decreased systemic vascular resistance was also reported in one of these studies, neither study reported systemic hypotension (Fukumoto et al., 2005; Ishikura et al., 2006). Interestingly, conventional pulmonary vasodilators such as prostacyclin do not mediate vasodilatation of the pulmonary vasculature through inhibition of ROCK (Abe et al., 2005), and the combination of ROCK inhibitor fasudil with beroprost, an oral prostacyclin analogue, was more effective than each drug alone in attenuating monocrotaline-induced PAH in rats (Tawara et al., 2007). The ROCK pathway can also be inhibited by members of the statin family of drugs, and various statins have beneficial effects in animal models of PAH. Atorvastatin, which can inhibit monocrotaline-induced PAH (Laudi et al., 2007), also inhibits serotonin mediated activation of the ROCK pathway and subsequent nuclear translocation of phosphorylated ERK1/2 in bovine PASMCs (Li et al., 2007b). Simvastatin can inhibit chronic hypoxic-induced PAH and also hypoxic-induced ROCK expression (Girgis et al., 2007). Statins are widely used as cholesterol-lowering agents, and the safety and tolerability of these drugs have been established making statin treatment of PAH an interesting possibility.
Dexfenfluramine and PAH
The original ‘serotonin hypothesis of PAH' was derived from the observation that obese patients using appetite suppressants such as aminorex and dexfenfluramine were at increased risk of developing PAH (Abenhaim et al., 1996; Kramer and Lane, 1998). The appetite-suppressant effect of fenfluramine derivatives is thought to be dependent upon inhibition of neuronal serotonin reuptake, increased serotonin release and subsequent serotonin receptor stimulation (Caccia et al., 1993; Rothman et al., 1999). As neurones, platelets, pulmonary endothelial and smooth muscle cells share the same SERT encoded by a single gene (Lesch et al., 1996), one school of thought is that it is this action of dexfenfluramine that promotes PAH (Celada et al., 1994). Indeed, increased serotonin plasma levels have been observed during treatment with fenfluramine derivatives (Celada et al., 1994). In addition, the metabolite, nor-dexfenfluramine is an agonist at 5-HT2A, 5-HT2B and 5-HT2C receptors (Rothman and Baumann, 2002). However, dexfenfluramine also has direct effects on pulmonary vessels including inhibition of potassium channels (Weir et al., 1996), increased intracellular calcium (Reeve et al., 1999), vasoconstriction (albeit with very low potency) (Higenbottam et al., 1999; Patnaude et al., 2000) and proliferation (Lee et al., 2001b). Intriguingly, attempts to elucidate the mechanism of dexfenfluramine-induced PAH in animal models had produced varied results, with dexfenfluramine being reported not only to exacerbate PAH (Launay et al., 2002) but also to protect against both hypoxia- and monocrotaline-induced PAH (Mitani et al., 2002; Rochefort et al., 2006) However, we have now shown that although dexfenfluramine induced PAH in wild-type mice, dexfenfluramine had no effects on the development of PAH in mice deficient in Tph1, the rate limiting enzyme in the synthesis of peripheral serotonin (Dempsie et al., 2008). Hence, we have definitively shown that dexfenfluramine mediates the development of PAH through a peripheral serotinergic mechanism rather than through non-serotinergic effects. This provides further evidence that peripheral serotonin plays a causative role in the development of PAH. Moreover, dexfenfluramine inhibited hypoxic-induced proliferation of pulmonary arterial fibroblasts derived from SERT+ mice, through inhibition of p38 MAP kinase, providing a possible mechanism for the protective effects of dexfenfluramine on hypoxic-induced PAH (Dempsie et al., 2008). As p38 MAP kinase has also been linked to vascular proliferation associated with monocrotaline-induced PAH (Lu et al., 2004), and SERT is overexpressed in pulmonary arteries from monocrotaline-treated rats (Laudi et al., 2007) our results also provide a potential mechanism for the protective effects of dexfenfluramine on PAH secondary to monocrotaline injection. Understanding the pharmacology of dexfenfluramine is essential to identify other drugs, which may also be risk factors for PAH. This is of current importance in light of recent reports on the increased abuse of amphetamine-like stimulants, such as methamphetamine, which have also been associated with the development of PAH (Chin et al., 2006).
Serotonin and the BMPR-II receptor
Bone morphogenetic proteins are members of the TGF-β family of cytokines and act on a variety of cell types to regulate growth, differentiation and apoptosis. The BMPR-II receptor is a member of the TGF-β superfamily type II receptors, and forms a complex with an associated type I receptor on ligand stimulation (Miyazono et al., 2005; Morrell, 2006). Activated type I receptors can then phosphorylate and thus activate the Smad-signalling pathway. Bone morphogenetic proteins signal through Smads 1,5 and 8 which must dimerize with Smad 4 to enter the nucleus, and regulate transcription of target genes (Massague et al., 2005). Pulmonary arterial smooth muscle cells from patients with BMPR-II mutations have a reduced capacity to activate Smads 1 5 and 8 and BMP-4 has a reduced ability to suppress proliferation in these cells (Yang et al., 2005). Thus a decrease in signalling through the BMPR-II receptor may increase pulmonary arterial smooth muscle cell proliferation and mediate PAH. However, as mentioned previously, disease penetrance in carriers of a mutation in the BMPR-II gene is low. Therefore, it has been postulated that exposure to another risk factor is necessary for development of PAH (Newman et al., 2004). In line with this, male mice deficient in BMPR-II-signalling (BMPR-II+/− mice) do not develop spontaneous PAH, but rather need an added stimulus to uncover a PAH phenotype. Indeed, an infusion of serotonin has been shown to uncover a PAH phenotype in BMPR-II+/− mice and this was associated with a serotonin inhibition of phosphorylation of Smad 1/5/8. Expression of the inhibitor of DNA-binding 3 mRNA in response to BMP2 was lower in BMPR-II+/− mice than their wild-type counterparts and this was inhibited by serotonin in both wild-type and BMPR-II+/− mice (Long et al., 2006). These effects of serotonin might be expected to lead to a proliferative phenotype (decreased Smad 1/5/8 and decreased inhibitor of DNA binding 3). Interestingly, although receptor-mediated phosphorylation of the Smads at the carboxyl terminal enables association with Smad 4 and thus nuclear translocation, ERK1/2 can phosphorylate the linker region of Smad 1 thus inhibiting nuclear translocation (Morrell, 2006). As ERK1/2 functions downstream of serotonin, this may provide the mechanism by which serotonin can antagonize the BMP pathway (Figure 1). Indeed, pulmonary arterial smooth muscle cells from BMPR-II+/− mice show increased proliferation in response to serotonin, and this is associated with increased ERK1/2 signalling. Moreover, pulmonary resistance arteries from BMPR-II+/− mice show an increased contractile response to serotonin (Long et al., 2006). Interestingly, patients with the BMPR-II mutation who had developed PAH after exposure to fenfluramines, had a shorter duration of fenfluramine exposure before illness than patients without the mutation (Humbert et al., 2002). As fenfluramines mediate PAH through a serotinergic mechanism (Dempsie et al., 2008), this observation may provide further evidence for a link between serotonin and the BMPR-II pathways.
Conclusions
There is now a considerable and growing body of evidence to support the ‘serotonin hypothesis of PAH'. Antagonistic interactions between the serotonin and the BMP system have been reported, thus increased serotonin signalling may provide a ‘second hit' risk factor which can mediate PAH in patients with a mutation in the BMPR-II gene. The effects of serotonin on the pulmonary vasculature are mediated through both SERT and the serotonin receptors (particularly the 5-HT1B receptor). Indeed, SERT and the 5-HT1B receptor synergize in mediating constriction of the pulmonary artery and emerging evidence now suggests that SERT and the 5-HT1B receptor co-regulate components of the serotonin-signalling system. Therefore, targeting both of these may provide optimal therapy for PAH. Also emerging as a possible therapeutic target is the rate-limiting enzyme in the synthesis of serotonin, Tph1. Advances have been made in unravelling the serotonin-signalling system, uncovering further possible therapeutic targets. Much work remains to be carried out in this area and the challenge will be to specifically target signalling molecules within the pulmonary vasculature.
Acknowledgments
YD is funded by an Integrative Mammalian Biology Industrial Partnership Award (Biotechnology and Biological Sciences Research Council/Pfizer/British Pharmacological Society).
Abbreviations
- BMP
bone morphogenetic protein
- BMPR-II
bone morphogenetic protein receptor type II
- ERK
extracellular signal-regulated kinase
- fPAH
familial pulmonary arterial hypertension
- iPAH
idiopathic pulmonary arterial hypertension
- MAP kinase
mitogen-activated protein kinase
- PAH
pulmonary arterial hypertension
- ROCK
Rho-kinase
- ROS
reactive oxygen species
- SERT
serotonin transporter
- TGF-β
transforming growth factor beta
- Tph
tryptophan hydroxylase
Conflict of interest
The authors state no conflict of interest.
References
- Abe K, Morikawa K, Hizume T, Uwatoku T, Oi K, Seto M, et al. Prostacyclin does not inhibit rho-kinase: an implication for the treatment of pulmonary hypertension. J Cardiovasc Pharmacol. 2005;45:120–124. doi: 10.1097/01.fjc.0000151898.65260.6a. [DOI] [PubMed] [Google Scholar]
- Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- Baloira A, Vilarino C, Leiro V, Valverde D. Mutations in the gene encoding bone morphogenetic protein receptor 2 in patients with idiopathic pulmonary arterial hypertension. Arch Bronconeumol. 2008;44:29–34. doi: 10.1016/s1579-2129(08)60006-3. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Le Poul E, Parma J, Knoop C, Detheux M, Parmentier M, et al. Serotonin 5-HT2B receptor loss of function mutation in a patient with fenfluramine-associated primary pulmonary hypertension. Cardiovasc Res. 2003;60:518–528. doi: 10.1016/j.cardiores.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Caccia S, Anelli M, Ferrarese A, Fracasso C, Garattini S. The role of D-norfenfluramine in the indole-depleting effect of D-fenfluramine in the Rat. Eur J Pharmacol. 1993;233:71–77. doi: 10.1016/0014-2999(93)90350-q. [DOI] [PubMed] [Google Scholar]
- Callebert J, Esteve JM, Herve P, Peoc'h K, Tournois C, Drouet L, et al. Evidence for a control of plasma serotonin levels by 5-hydroxytryptamine(2B) receptors in mice. J Pharmacol Exp Ther. 2006;317:724–731. doi: 10.1124/jpet.105.098269. [DOI] [PubMed] [Google Scholar]
- Celada P, Martin F, Artigas F. Effects of chronic treatment with dexfenfluramine on serotonin in rat-blood, brain and lung-tissue. Life Sciences. 1994;55:1237–1243. doi: 10.1016/0024-3205(94)00663-6. [DOI] [PubMed] [Google Scholar]
- Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension. Chest. 2006;130:1657–1663. doi: 10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeno L, Tamargo J, et al. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells—Role of 5-HT2A receptors, caveolin-1, and K(V)1.5 channel internalization. Circ Res. 2006;98:931–938. doi: 10.1161/01.RES.0000216858.04599.e1. [DOI] [PubMed] [Google Scholar]
- Das M, Bouchey DM, Moore MJ, Hopkins DC, Nemenoff RA, Stenmark KR. Hypoxia-induced proliferative response of vascular adventitial fibroblasts is dependent on G protein-mediated activation of mitogen-activated protein kinases. J Biol Chem. 2001;276:15631–15640. doi: 10.1074/jbc.M010690200. [DOI] [PubMed] [Google Scholar]
- Dempsie Y, Morecroft I, Welsh DJ, MacRitchie NA, Loughlin L, Nilsen M, et al. Converging evidence in support of the serotonin hypothesis of dzexfenfluramine-induced pulmonary hypertension using novel transgenic mice. Circulation. 2008;117:2928–2937. doi: 10.1161/CIRCULATIONAHA.108.767558. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Chaouat A, Morrell N, Fadel E, Fuhrman C, Bugnet AS, et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation. 2003;108:1839–1844. doi: 10.1161/01.CIR.0000091409.53101.E8. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, et al. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension—Critical role for serotonin-induced smooth muscle hyperplasia. Circulation. 2006;113:1857–1864. doi: 10.1161/CIRCULATIONAHA.105.591321. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, et al. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest. 2000;105:1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S, Raffestin B, Pham I, Launay JM, Aegerter P, Sitbon M, et al. Treatment with 5-HT potentiates development of pulmonary hypertension in chronically hypoxic rats. Am J Physiol-Heart Circ Physiol. 1997;41:H1173–H1181. doi: 10.1152/ajpheart.1997.272.3.H1173. [DOI] [PubMed] [Google Scholar]
- Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol-Lung Cell Mol Physiol. 2004;287:L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- Frishman WH, Huberfeld S, Okin S, Wang YH, Kumar A, Shareef B. Serotonin and serotonin antagonism in cardiovascular and non-cardiovascular disease. J Clin Pharmacol. 1995;35:541–572. doi: 10.1002/j.1552-4604.1995.tb05013.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Yagi H, Matsuoka R, Akimoto K, Furutani M, Imamura S, et al. Implications of mutations of activin receptor-like kinase 1 gene (ALK1) in addition to bone morphogenetic protein receptor II gene (BMPR2) in children with pulmonary arterial hypertension. Circ J. 2008;72:127–133. doi: 10.1253/circj.72.127. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91:391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto Y, Tawara S, Shimokawa H. Recent progress in the treatment of pulmonary arterial hypertension: expectation for Rho-kinase inhibitors. Tohoku J Exp Med. 2007;211:309–320. doi: 10.1620/tjem.211.309. [DOI] [PubMed] [Google Scholar]
- Girgis RE, Mozammel S, Champion HC, Li DC, Peng XQ, Shimoda L, et al. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol-Lung Cell Mol Physiol. 2007;292:L1105–L1110. doi: 10.1152/ajplung.00411.2006. [DOI] [PubMed] [Google Scholar]
- Greenway S, van Suylen RJ, Sarvaas GD, Kwan E, Ambartsumian N, Lukanidin E, et al. S100A4/Mts1 produces murine pulmonary artery changes resembling plexogenic arteriopathy and is increased in human plexogenic arteriopathy. Am J Pathol. 2004;164:253–262. doi: 10.1016/S0002-9440(10)63115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignabert C, Izikki M, Tu LI, Li ZL, Zadigue P, Barlier-Mur AM, et al. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res. 2006;98:1323–1330. doi: 10.1161/01.RES.0000222546.45372.a0. [DOI] [PubMed] [Google Scholar]
- Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, et al. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation. 2005;111:2812–2819. doi: 10.1161/CIRCULATIONAHA.104.524926. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guerin P, Sagan C, Pacaud P, et al. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol. 2005;146:1010–1018. doi: 10.1038/sj.bjp.0706408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- Higenbottam T, Marriott H, Cremona G, Laude E, Bee D. The acute effects of dexfenfluramine on human and porcine pulmonary vascular tone and resistance. Chest. 1999;116:921–930. doi: 10.1378/chest.116.4.921. [DOI] [PubMed] [Google Scholar]
- Hironaka E, Hongo M, Sakai A, Mawatari E, Terasawa F, Okumura N, et al. Serotonin receptor antagonist inhibits monocrotaline-induced pulmonary hypertension and prolongs survival in rats. Cardiovasc Res. 2003;60:692–699. doi: 10.1016/j.cardiores.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Humbert M, Deng Z, Simonneau G, Barst RJ, Sitbon O, Wolf M, et al. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J. 2002;20:518–523. doi: 10.1183/09031936.02.01762002. [DOI] [PubMed] [Google Scholar]
- Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circulation Res. 2005;97:185–191. doi: 10.1161/01.RES.0000174287.17953.83. [DOI] [PubMed] [Google Scholar]
- Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, et al. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- Izikki M, Hanoun N, Marcos E, Savale L, Barlier-Mur AM, Saurini F, et al. Tryptophan hydroxylase 1 (Tph1) knock-out and Tph2 polymorphism: effects on hypoxic pulmonary hypertension in mice. Am J Physiol - Lung Cell Mol Physiol. 2007;293:L1045–L1052. doi: 10.1152/ajplung.00082.2007. [DOI] [PubMed] [Google Scholar]
- Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT1B receptor to hypoxia-induced pulmonary hypertension—Converging evidence using 5-HT1B-receptor knockout mice and the 5-HT1B/1D-receptor antagonist GR127935. Circ Res. 2001;89:1231–1239. doi: 10.1161/hh2401.100426. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lane DA. Aminorex, dexfenfluramine, and primary pulmonary hypertension. J Clin Epidemiol. 1998;51:361–364. doi: 10.1016/s0895-4356(97)00289-8. [DOI] [PubMed] [Google Scholar]
- Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, Loyd JE, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- Laudi S, Trump S, Schmitz V, West J, McMurtry IF, Mutlak H, et al. Serotonin transporter protein in pulmonary hypertensive rats treated with atorvastatin. Am J Physiol - Lung Cell Mol Physiol. 2007;293:L630–L638. doi: 10.1152/ajplung.00110.2006. [DOI] [PubMed] [Google Scholar]
- Launay JM, Herve P, Peoc'h K, Tournois C, Callebert J, Nebigil CG, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8:1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- Lawrie A, Spiekerkoetter E, Martinez EC, Ambartsumian N, Sheward WJ, MacLean MR, et al. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circ Res. 2005;97:227–235. doi: 10.1161/01.RES.0000176025.57706.1e. [DOI] [PubMed] [Google Scholar]
- Lee SL, Simon AR, Wang WW, Fanburg BL. H(2)O(2) signals 5-HT-induced ERK MAP kinase activation and mitogenesis of smooth muscle cells. Am J Physiol - Lung Cell Mol Physiol. 2001a;281:L646–L652. doi: 10.1152/ajplung.2001.281.3.L646. [DOI] [PubMed] [Google Scholar]
- Lee SL, Wang WW, Fanburg BL. Superoxide as an intermediate signal for serotonin-induced mitogenesis. Free Radic Biol Med. 1998;24:855–858. doi: 10.1016/s0891-5849(97)00359-6. [DOI] [PubMed] [Google Scholar]
- Lee SL, Wang WW, Fanburg BL. Dexfenfluramine as a mitogen signal via the formation of superoxide anion. FASEB J. 2001b;15:1324–1325. doi: 10.1096/fj.00-0431fje. [DOI] [PubMed] [Google Scholar]
- Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol-Lung Cell Mol Physiol. 1999;277:L282–L291. doi: 10.1152/ajplung.1999.277.2.L282. [DOI] [PubMed] [Google Scholar]
- Lee SL, Wang WW, Lanzillo JJ, Fanburg BL. Regulation of serotonin-induced Dna-synthesis of bovine pulmonary-artery smooth-muscle cells. Am J Physiol - Lung Cell Mol Physiol. 1994;266:L53–L60. doi: 10.1152/ajplung.1994.266.1.L53. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li FH, Xia W, Li AW, Zhao CF, Sun RP. Long-term inhibition of Rho kinase with fasudil attenuates high flow induced pulmonary artery remodeling in rats. Pharmacol Res. 2007a;55:64–71. doi: 10.1016/j.phrs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Li M, Liu Y, Dutt P, Fanburg BL, Toksoz D. Inhibition of serotonin-induced mitogenesis, migration, and ERK MAPK nuclear translocation in vascular smooth muscle cells by atorvastatin. Am J Physiol-Lung Cell Mol Physiol. 2007b;293:L463–L471. doi: 10.1152/ajplung.00133.2007. [DOI] [PubMed] [Google Scholar]
- Liu JQ, Folz RJ. Extracellular superoxide enhances 5-HT-induced murine pulmonary artery vasoconstriction. Am J Physiol - Lung Cell Mol Physiol. 2004;287:L111–L118. doi: 10.1152/ajplung.00006.2004. [DOI] [PubMed] [Google Scholar]
- Liu YL, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res. 2004;95:579–586. doi: 10.1161/01.RES.0000141428.53262.a4. [DOI] [PubMed] [Google Scholar]
- Long L, Aldashev AA, Hensiek A, Eddahibi S, Adnot S, Trembath RC, et al. Preliminary identification of genetic loci associated with high altitude pulmonary hypertension by association mapping Thorax 200257S110(Suppl 3) [Google Scholar]
- Long L, MacLean MR, Jeffery TK, Morecroft I, Yang XD, Rudarakanchana N, et al. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res. 2006;98:818–827. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- Loyd JE, Butler MG, Foroud TM, Conneally PM, Phillips JA, Newman JH. Genetic anticipation and abnormal gender ratio at birth in familial primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;152:93–97. doi: 10.1164/ajrccm.152.1.7599869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Shimpo H, Shimamoto A, Chong AJ, Hampton CR, Spring DJ, et al. Specific inhibition of p38 mitogen-activated protein kinase with FR167653 attenuates vascular proliferation in monocrotaline-induced pulmonary hypertension in rats. J Thorac Cardiovasc Surg. 2004;128:850–859. doi: 10.1016/j.jtcvs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Machado RD, Koehler R, Glissmeyer E, Veal C, Suntharalingam J, Kim M, et al. Genetic association of the serotonin transporter in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;173:793–797. doi: 10.1164/rccm.200509-1365OC. [DOI] [PubMed] [Google Scholar]
- Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68:92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre PD, Bhargava B, Hogg KJ, Gemmill JD, Hillis WS. The effect of Iv Sumatriptan, a selective 5-Ht1-receptor agonist on central hemodynamics and the coronary circulation. Br J Clin Pharmacol. 1992;34:541–546. [PMC free article] [PubMed] [Google Scholar]
- MacLean MR. Pulmonary hypertension, anorexigens and 5-HT: pharmacological synergism in action. Trends Pharmacol Sci. 1999;20:490–495. doi: 10.1016/s0165-6147(99)01389-9. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Clayton RA, Templeton AGB, Morecroft I. Evidence for 5-HT1-like receptor-mediated vasoconstriction in human pulmonary artery. Br J Pharmacol. 1996;119:277–282. doi: 10.1111/j.1476-5381.1996.tb15982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean MR, Deuchar GA, Hicks MN, Morecroft I, Shen SB, Sheward J, et al. Overexpression of the 5-hydroxytryptamine transporter gene—effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation. 2004;109:2150–2155. doi: 10.1161/01.CIR.0000127375.56172.92. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Herve P, Eddahibi S, Adnot S. 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000;131:161–168. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos E, Adnot S, Pham MH, Nosjean A, Raffestin B, Hamon M, et al. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med. 2003;168:487–493. doi: 10.1164/rccm.200210-1212OC. [DOI] [PubMed] [Google Scholar]
- Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, et al. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res. 2004;94:1263–1270. doi: 10.1161/01.RES.0000126847.27660.69. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Mitani Y, Mutlu A, Russell JC, Brindley DN, DeAlmeida J, Rabinovitch M. Dexfenfluramine protects against pulmonary hypertension in rats. J Appl Physiol. 2002;93:1770–1778. doi: 10.1152/japplphysiol.00500.2002. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Morecroft I, Dempsie Y, Bader M, Walther DJ, Kotnik K, Loughlin L, et al. Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia-induced pulmonary hypertension. Hypertension. 2007;49:232–236. doi: 10.1161/01.HYP.0000252210.58849.78. [DOI] [PubMed] [Google Scholar]
- Morecroft I, Heeley RP, Prentice HM, Kirk A, MacLean MR. 5-hydroxytryptamine receptors mediating contraction in human small muscular pulmonary arteries: importance of the 5-HT1B receptor. Br J Pharmacol. 1999;128:730–734. doi: 10.1038/sj.bjp.0702841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecroft I, Loughlin L, Nilsen M, Colston J, Dempsie Y, Sheward J, et al. Functional interactions between 5-hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther. 2005;313:539–548. doi: 10.1124/jpet.104.081182. [DOI] [PubMed] [Google Scholar]
- Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling. Proc Am Thorac Soc. 2006;3:680–686. doi: 10.1513/pats.200605-118SF. [DOI] [PubMed] [Google Scholar]
- Mortimer HJ, Peacock AJ, Kirk A, Welsh DJ. P38 MAP kinase: essential role in hypoxia-mediated human pulmonary artery fibroblast proliferation. Pulm Pharmacol Ther. 2007;20:718–725. doi: 10.1016/j.pupt.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Gebb SA, Karoor V, Homma N, Morris KG, McMurtry IF, et al. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol. 2006;100:996–1002. doi: 10.1152/japplphysiol.01028.2005. [DOI] [PubMed] [Google Scholar]
- Newman JH, Trembath RC, Morse JA, Grunig E, Loyd JE, Adnot S, et al. Genetic basis of pulmonary arterial hypertension: current understanding and future directions J Am Coll Cardiol 20044333S–39.S [DOI] [PubMed] [Google Scholar]
- Pan J, Copland I, Post M, Yeger H, Cutz E. Mechanical stretch-induced serotonin release from pulmonary neuroendocrine cells: implications for lung development. Am J Physiol - Lung Cell Mol Physiol. 2006;290:L185–L193. doi: 10.1152/ajplung.00167.2005. [DOI] [PubMed] [Google Scholar]
- Patnaude LA, Undem BJ, O'Rourke ST. Dexfenfluramine-induced contraction of human and rat isolated pulmonary arteries. Eur J Pharmacol. 2000;401:229–234. doi: 10.1016/s0014-2999(00)00458-1. [DOI] [PubMed] [Google Scholar]
- Reeve HL, Archer SL, Soper M, Weir EK. Dexfenfluramine increases pulmonary artery smooth muscle intracellular Ca2+, independent of membrane potential. Am J Physiol. 1999;277:L662–L666. doi: 10.1152/ajplung.1999.277.3.L662. [DOI] [PubMed] [Google Scholar]
- Rochefort GY, Lemaire MC, Eder V, Hanton G, Hyvelin JM, Bonnet P, et al. Dexfenfluramine does not worsen but moderates progression of chronic hypoxia-induced pulmonary hypertension. Eur J Pharmacol. 2006;550:149–154. doi: 10.1016/j.ejphar.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Rondelet B, Van Beneden R, Kerbaul F, Motte S, Fesler P, McEntee K, et al. Expression of the serotonin 1b receptor in experimental pulmonary hypertension. Eur Respir J. 2003;22:408–412. doi: 10.1183/09031936.03.00036203. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates—Implications for primary pulmonary hypertension. Circulation. 1999;100:869–875. doi: 10.1161/01.cir.100.8.869. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther. 2002;95:73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Sankelo M, Flanagan JA, Machado R, Harrison R, Rudarakanchana N, Morrell N, et al. BMPR2 mutations have short lifetime expectancy in primary pulmonary hypertension. Hum Mutat. 2005;26:119–124. doi: 10.1002/humu.20200. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Zahradka P, Chapman D, Kumamoto H, Takeda N, Dhalla NS. Inhibition of serotonin-induced vascular smooth muscle cell proliferation by sarpogrelate. J Pharmacol Exp Ther. 1999;290:1475–1481. [PubMed] [Google Scholar]
- Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Day RM, Tan CC, Sandven TH, Liang QR, Molkentin JD, et al. Activation of GATA-4 by serotonin in pulmonary artery smooth muscle cells. J Biol Chem. 2003;278:17525–17531. doi: 10.1074/jbc.M210465200. [DOI] [PubMed] [Google Scholar]
- Tawara S, Fukumoto Y, Shimokawa H. Effects of combined therapy with a Rho-kinase inhibitor and prostacyclin on monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;50:195–200. doi: 10.1097/FJC.0b013e31806befe6. [DOI] [PubMed] [Google Scholar]
- Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. New Engl J Med. 2001;345:325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003a;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003b;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- Wang HL, Dong X, Zhang XH, Xing J. 5-HT1B receptor augmented 5-HT vasoconstrictor response of pulmonary artery in monocrotaline-induced pulmonary hypertensive rats. Acta Pharmacol Sin. 2001;22:269–273. [PubMed] [Google Scholar]
- Weir EK, Reeve HL, Huang JM, Michelakis E, Nelson DP, Hampl V, et al. Anorexic agents aminorex, fenfluramine, and dexfenfluramine inhibit potassium current in rat pulmonary vascular smooth muscle and cause pulmonary vasoconstriction. Circulation. 1996;94:2216–2220. doi: 10.1161/01.cir.94.9.2216. [DOI] [PubMed] [Google Scholar]
- Welsh DJ, Harnett M, MacLean M, Peacock AJ. Proliferation and signaling in fibroblasts—role of 5-hydroxytryptamine(2A) receptor and transporter. Am J Respir Crit Care Med. 2004;170:252–259. doi: 10.1164/rccm.200302-264OC. [DOI] [PubMed] [Google Scholar]
- Welsh DJ, Peacock AJ, MacLean M, Harnett M. Chronic hypoxia induces constitutive p38 mitogen-activated protein kinase activity that correlates with enhanced cellular proliferation in fibroblasts from rat pulmonary but not systemic arteries. Am J Respir Crit Care Med. 2001;164:282–289. doi: 10.1164/ajrccm.164.2.2008054. [DOI] [PubMed] [Google Scholar]
- Welsh DJ, Scott PH, Peacock AJ. p38 MAP kinase isoform activity and cell cycle regulators in the proliferative response of pulmonary and systemic artery fibroblasts to acute hypoxia. Pulm Pharmacol Ther. 2006;19:128–138. doi: 10.1016/j.pupt.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Willers ED, Newman JH, Loyd JE, Robbins IM, Wheeler LA, Prince MA, et al. Serotonin transporter polymorphisms in familial and idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;173:798–802. doi: 10.1164/rccm.200509-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, et al. Dysfunctional smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–1063. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]