Abstract
Background and purpose:
Duchenne muscular dystrophy (DMD) is a severe muscle wasting disorder caused by the absence of the cytoskeletal protein dystrophin. This leads to muscle cell death accompanied by chronic inflammation. Cyclosporin A (CsA) is a powerful immunosuppressive drug, which has been proposed for DMD treatment. CsA also directly regulates the mitochondrial permeability transition pore (mPTP), which participates in cell death pathways through the inhibition of cyclophilin D. Here, we evaluated whether Debio 025, a cyclophilin inhibitor with no immunosuppressive activity, improves the dystrophic condition in a mouse model of DMD, through regulation of mPTP.
Experimental approach:
The potency of Debio 025 to protect mouse dystrophic cells against mitochondria-mediated death was assessed by caspase-3 activity and calcium retention capacity assays. Mdx5Cv mice (3-week-old) were treated daily by gavage for 2 weeks with Debio 025 (10, 30 or 100 mg kg−1), CsA (10 mg kg−1) or placebo. The effects on muscle necrosis and function were measured.
Key results:
In vitro investigations showed protective effect of low concentrations of Debio 025 against cell death. Histology demonstrated that Debio 025 partially protected the diaphragm and soleus muscles against necrosis (10 and 100 mg kg−1, respectively). Hindlimb muscles from mice receiving Debio 025 at 10 mg kg−1 relaxed faster, showed alteration in the stimulation frequency-dependent recruitment of muscle fibres and displayed a higher resistance to mechanical stress.
Conclusions and implications:
Debio 025 partially improved the structure and the function of the dystrophic mouse muscle, suggesting that therapies targeting the mPTP may be helpful to DMD patients.
Keywords: dystrophic mdx mouse, cyclophilin D, mitochondrial permeability transition pore, cyclosporin A, myotube cell death, Duchenne muscular dystrophy, pharmacotherapy
Introduction
Debio 025 (D-MeAla3EtVal4-cyclosporin) is a cyclophilin inhibitor with potent anti-human immunodeficiency and anti-hepatitis C virus activities (Noser et al., 2006; Flisiak et al., 2007). It has recently passed phase Ib and IIa clinical trials where it showed a strong anti-hepatitis C effect (Flisiak et al., 2007). It binds to cyclophilin A (CyPA) at a nanomolar concentration and inhibits its prolyl cis–trans isomerase activity. However, unlike cyclosporin A (CsA), Debio 025 does not interact with calcineurin (Hansson et al., 2004) and, therefore, is not immunosuppressive.
Duchenne muscular dystrophy (DMD) is a severe muscular dystrophy that affects approximately 1 in 3500 male births. The first symptoms appear in early childhood. The disease is characterized by progressive muscle wasting associated with respiratory complications and cardiac dysfunction leading to death by the age of 20–30 years. DMD is caused by the absence of the structural protein dystrophin that is encoded by the X chromosome (Xp21). Dystrophin is a large protein of 427 kDa that links the extracellular matrix through the transmembrane glycoprotein complex of dystroglycans to the intracellular F-actin network, thus providing mechanical stability to muscle cells during contraction (Petrof et al., 1993).
Although the gene encoding dystrophin was identified in 1987 (Hoffman et al., 1987), the mechanisms leading to disease manifestation still remain unclear. The dystrophic condition is associated with a wide variety of cellular dysfunctions, including membrane instability (mislocation or absence of many of the dystrophin-associated proteins), deregulation in Ca2+ homoeostasis, increased susceptibility to oxidative damage, enhanced proteolytic activity and apoptosis, and impaired energy metabolism (Blake et al., 2002). Altogether, these events lead to muscle fibre death followed by infiltration of activated lymphocytes and macrophages, progressive replacement of muscle tissue by fibrotic and adipose tissue, and culminate in the loss of functional muscle mass.
Despite considerable efforts to replace the damaged gene by stem cell transfer or gene therapy (Nowak and Davies, 2004; Chakkalakal et al., 2005), these approaches have not yet led to a cure mainly because of safety issues concerning vectors and limitations with targeting all the muscles of the body. To date, the main treatments that improve the life expectancy and the quality of life of DMD patients consist of surgery, kinesitherapy, ventilatory assistance and pharmacological interventions. Currently, the only drugs used in DMD patients are the glucocorticoids, prednisolone and deflazacort (Muntoni et al., 2002). Their action on inflammation, promotion of muscle-specific gene expression, correction of deregulated Ca2+ homoeostasis (Metzinger et al., 1995; Leijendekker et al., 1996) and activation of the calcineurin/nuclear factor of activated T cells (NF-AT) pathway (St-Pierre et al., 2004) might explain their therapeutic effects. However, in some cases, side effects inherent to this class of drugs were reported in treated DMD patients (Fisher et al., 2005). Recently, with increasing knowledge of the mechanisms underlying the disease process, new therapeutic approaches have been proposed as palliative treatments for DMD, essentially based on investigations using the dystrophic mdx (muscular dystrophy X-linked) mouse model (Khurana and Davies, 2003; Chakkalakal et al., 2005; Cossu and Sampaolesi, 2007). These include the treatment with creatine to increase muscle energetic balance and help with correcting Ca2+ deregulation (Pulido et al., 1998; Passaquin et al., 2002), specific calpain inhibitors to counteract the increased Ca2+-dependent proteolytic activity (Lescop et al., 2005), lipid peroxidation inhibitors to blunt nuclear factor-κB activation (Messina et al., 2006), antioxidants such as green tea extract to counteract oxidative stress (Buetler et al., 2002; Dorchies et al., 2006), deacetylase inhibitors to induce muscle growth (Minetti et al., 2006) or phosphodiesterase inhibitors to prevent ischaemia (Dorchies et al., 2006; Rolland et al., 2006; Asai, 2007). To block tissue infiltration by activated lymphocytes, anti-inflammatory and immunosuppressive drugs, such as glucocorticoids (Muntoni et al., 2002) and CsA (Sharma et al., 1993; De Luca et al., 2005) have been tested in dystrophic mice and in DMD patients. In our view, a general immunosuppression with CsA is not a suitable treatment for DMD as the patients would need to be protected from infections, and also because CsA has significant adverse effects (Mason, 1990).
Another approach is to target apoptosis that may be primary or secondary. Primary apoptosis will remove damaged cells before the cell is disrupted and induce an immune response. This is the classical apoptotic pathway that serves to protect the tissue as a whole. Secondary apoptosis generally occurs after primary tissue damage (necrosis) and is mostly caused by reactive oxygen species (Tidball and Wehling-Henricks, 2007). By comparing biopsies from healthy and DMD patients, the mitochondrial content was found to be similar, but more ultrastructural abnormalities such as aberrant morphology of the mitochondria were observed in DMD patients (Watkins and Cullen, 1987). Moreover, the Krebs cycle intermediate α-ketoglutarate is elevated only in the muscle of DMD patients, suggesting predominant oxidative metabolism (Sharma et al., 2003). A specific decrease in all mitochondrial inner membrane enzymes, most probably as a result of Ca2+ overload, has been postulated to be the cause for the bioenergetic deficits in dystrophin-deficient skeletal muscle (Kuznetsov et al., 1998). In addition to the established role of mitochondria in energy metabolism, regulation of cell death has emerged as a major function of these organelles (Bouchier-Hayes et al., 2005; Orrenius et al., 2007). Taking into account the key role played by mitochondrial Ca2+ handling in the control of cell death, these organelles are potential targets of impaired Ca2+ homoeostasis in muscular dystrophy (Robert et al., 2001). Calcium deregulation induces mitochondrial depolarization and apoptosis (Hajnoczky et al., 2003). Indeed, depolarization of the inner mitochondrial membrane results in the opening of mitochondrial permeability transition pore (mPTP) and Ca2+ efflux from mitochondrial matrix through both the mPTP and the Na+/Ca2+ exchanger (Bernardi, 1999).
The mPTP, the structure of which is still not established (Forte and Bernardi, 2005), behaves as a voltage-dependent, high-conductance and nonspecific megachannel of the inner mitochondrial membrane. In the fully open state, its apparent diameter is about 3 nm and the open–closed transitions of the pore are highly regulated by multiple effectors. Indeed, pore opening can be promoted by a high concentration of matrix Ca2+ levels, oxidative stress, pyridine nucleotide oxidation, thiol oxidation, alkalinization or low transmembrane potential (Bernardi, 1999; Crompton, 1999). Persistent mPTP opening is followed by equilibration of ionic gradients and of species with molecular weight lower than 1.5 kDa, which may cause swelling, cristae unfolding and outer membrane rupture associated with the release of cytochrome c and pro-apoptotic factors (Petronilli et al., 2001). Opening of the mPTP is inhibited by CsA after its binding to cyclophilin D (CyPD), a member of the cyclophilin family of cis–trans isomerases (Halestrap and Davidson, 1990). CyPD is involved in modulation of the mPTP affinity for Ca2+, and conversely Ca2+ modulates the efficacy of mPTP inhibition by CsA (McGuinness et al., 1990; Bernardi, 1992). The mPTP is activated early in apoptosis and its inhibition has been shown to protect against apoptosis (Bernardi et al., 2001). Recently, it has been reported that mitochondria-mediated apoptosis/cell death can be reduced by CsA in liver diseases (Soriano et al., 2004), in a model of Ullrich congenital muscular dystrophy (Angelin et al., 2007), as well as in experimental models of ischaemia-reperfusion injury of the heart (Gateau-Roesch et al., 2006) and of neuronal injury (Forte et al., 2007).
Here, we investigated whether Debio 025 could prevent or reduce the massive necrotic episode, which affects skeletal muscle of 3-week-old dystrophic mice. Because Debio 025 is capable of selectively and strongly inhibiting CyPD (Gomez et al., 2007), we hypothesized that this compound would block the mPTP, prevent cell death and reduce muscle wasting. The advantage of Debio 025 over CsA lies in its increased affinity for cyclophilins and its very low calcineurin inhibitory activity (about 7000-fold less than CsA) (Hansson et al., 2004). Indeed, calcineurin activity is required for the initiation of skeletal muscle differentiation (Friday et al., 2000), and has been shown to be essential for successful dystrophic muscle regeneration (Chakkalakal et al., 2004; Stupka et al., 2004). In the current study, the use of CsA and Debio 025 allowed discrimination between effects mediated by the calcineurin/NF-AT pathway and direct CyPD effects that are likely to be linked to the inhibition of mitochondria-mediated cell death. Here, using in vitro and in vivo investigations, we have shown that the CyPD inhibitor Debio 025 has a therapeutic potential in the dystrophic mouse.
Methods
In vitro studies
Materials
Media and foetal calf serum were purchased from Invitrogen (Basel, Switzerland), except Ham's F10 nutrient mixture, and horse serum from Sigma (Buchs, Switzerland). Collagen type I and collagenase were from Sigma, dispase from Roche Molecular Diagnostics (Basel, Switzerland), and Matrigel from BD Biosciences. Other chemicals were from Sigma, unless stated otherwise.
Cell culture
Primary cultures of skeletal muscle cells were established from hindlimb muscles of 3- to 4-day-old newborn mice by collagenase–dispase digestion (100 and 2.4 U mL−1, respectively). The cells were re-suspended in Ham's F10 supplemented with 20% foetal calf serum and 5 ng mL−1 basic fibroblast growth factor (FGF2) (PeProTech, London, UK) and plated onto Petri dishes (Falcon; BD Biosciences, Basel, Switzerland) coated with collagen type I (3 μg cm−2) and Matrigel (1 μg cm−2). After 24 h, the medium was replaced by a 1:1 (vol:vol) mixture of Dulbecco's modified Eagle's medium (DMEM) and Ham's F10, supplemented with 10% foetal calf serum and 2.5 ng mL−1 FGF2 for another 24-h period. Then, primary cultures were used for the preparation of purified myoblasts. Myoblast separation was performed based on the expression of α7 integrin subunit on myoblast cell surface (Blanco-Bose et al., 2001). Primary muscle cells were re-suspended with trypsin, incubated with a rat monoclonal anti-α7 integrin (a gift from Dr Randall Kramer, UCSF, USA), and then with magnetic beads coated with sheep anti-rat Ig (DynaBeads M-450; Dynal Biotech, Compiègne, France). Tagged myoblasts were purified with a magnetic device, and re-suspended in a 2:1 mixture of Ham's F10/DMEM supplemented with 10% foetal calf serum and 5 ng mL−1 FGF2. The cells were plated (7000 cells cm−2) in both 35- or 60-mm diameter Petri dishes (Falcon; BD Biosciences) coated with a mixture of collagen type I and Matrigel as described above. After 2 days, differentiation was induced by switching to DMEM containing 2% horse serum. The cells were kept in a humidified incubator gassed with 95% air–5% CO2 at 37 °C.
Drug treatment
CsA, Debio 025 (Debiopharm, Lausanne, Switzerland), FK-506 and staurosporine (STS) (Alexis, Lausen, Switzerland) were prepared in dimethylsulphoxide (DMSO) as 10−2 or 10−3 M stock solutions and frozen at −20 °C. Cultures were treated in a range from 1 nM to 10 μM using freshly thawed stock aliquots. The final DMSO concentration was 0.1%. Cultures receiving 0.1% DMSO without any test substance served as a control.
For toxicity assays, CsA or Debio 025 was added on either primary cultures or pure myotube cultures on day 4 of differentiation, the medium was refreshed daily by 50% until day 6 of differentiation, and cells were analysed on day 7. STS was added on pure myotube cultures on day 4 of differentiation for 24 h.
For caspase-3 activity assays, pure myotube cultures were pretreated with the compound (CsA, Debio 025 or FK-506) for 1 h on day 4 before being challenged with STS at 100 nM for 15 h.
Survival assay
Cell survival was assessed by the measurement of acid phosphatase activity (Connolly et al., 1986). The cells were washed twice with phosphate-buffered saline, and lysed in 0.1 M citrate buffer pH 5.5 containing 0.1% Triton X-100 (Applichem, Axonlab, Le Mont-sur-Lausanne, Switzerland) and 1 mg mL−1 p-nitrophenylphosphate as a substrate for phosphatase. After incubation for 20–30 min at room temperature, the reaction was stopped by the addition of 0.1 volume of 1 N NaOH and absorbance was read at 405 nm (Labsystem Multiscan MS, Allschwil, Switzerland). The yellow colour that appeared is considered proportional to the amount of cells in the sample. Results were expressed as a percentage of the value of the control culture.
Measurement of caspase-3 activity
Caspase-3 activity was measured in cell homogenates. The cells were washed with phosphate-buffered saline and scraped into lysis buffer (100 mM HEPES pH 7.4, 10% sucrose, 20 mM 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulphonate (CHAPS), 1 mM EDTA (Fluka), 200 mM NaCl, 10 mM dithiothreitol and protease inhibitor mixture), and repeatedly passed through a 26-gauge needle. The lysates were centrifuged at 14 000 g for 10 min and the supernatants were collected. Protein content was determined using the Bradford method (Bio-Rad, Reinach, Switzerland). Reaction mixtures (100 μL final) were prepared containing 100 μg protein, 20 μM of the fluorigenic substrate N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-AMC) (Biomol, Anawa Trading, Zürich, Switzerland), 100 mM HEPES pH 7.4 and protease inhibitor mixture. After 10 min of stabilization, caspase-3 activity was measured by monitoring the proteolytic cleavage of DEVD-AMC for 5 min at 30 °C using a FLUOstar OPTIMA fluorimeter (BMG Labtech, Champigny sur Marne, France) (excitation 380 nm, emission 460 nm). Calibration curves were constructed using AMC (1 nM to 1 μM) instead of DEVD-AMC in the same reaction buffer. One unit of enzyme activity was defined as the amount of AMC released per minute per microgram of protein. The caspase-3 inhibitor, N-acetyl-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-CHO) (Biomol), was used at 10 μM as a control.
Isolation of mouse skeletal muscle mitochondria
Mouse skeletal muscle mitochondria were prepared as described previously (Madsen et al., 1996) with slight modifications. Mice were killed by cervical dislocation and skeletal muscles (diaphragm and limb muscles) were rapidly removed and transferred into ice-cold isolation medium (250 mM sucrose, 10 mM Tris-HCl, 0.1 mM EGTA (Fluka), pH 7.4). Muscles were cleaned of visible fat and connective tissue, and minced with scissors. Muscle pieces were transferred to 20 mL of isolation medium supplemented with 0.2% BSA and Nagarse (Fluka) (0.2 mg mL−1). After 2 min, muscles were homogenized using a motor-driven Teflon-glass potter, transferred to 80 mL of isolation medium supplemented with 0.2% BSA and centrifuged at 900 g for 6 min to pellet cellular debris. The supernatant was harvested and centrifuged at 10 000 g for 10 min. The resulting pellet was re-suspended in isolation medium and centrifuged at 7000 g for 6 min. The final mitochondrial pellet was re-suspended in 0.3 mL of the isolation medium at a final protein concentration of about 20 mg mL−1 before being used for calcium retention capacity (CRC) assay. All procedures were carried out at 0–4 °C.
CRC
The extra-mitochondrial Ca2+ concentration was measured at room temperature in a 2 mL cuvette placed in a thermostated Perkin-Elmer LS-5B spectrofluorimeter (excitation 505 nm, emission 535 nm) equipped with magnetic stirring, using the Ca2+ fluorescent indicator Calcium Green-5N (Invitrogen) (Ichas et al., 1997). For each measurement, a suspension of mitochondria corresponding to 0.5 mg per mL protein was prepared in a medium containing 250 mM sucrose, 10 mM 3-[N-morpholino] propanesulphonic acid, 10 μM EGTA, 1 mM ortho-phosphoric acid (Merck, VWR International, Dietikon, Switzerland), 5 mM glutamate, 2.5 mM malate, pH 7.4. Then, the test substance and subsequently the probe were added to the suspension at a final concentration of 1 μM each. After 1 min, Ca2+ concentration was raised stepwise by successive additions of 10 μL of 1 mM CaCl2, one addition every minute. At the end of each measurement, the maximum fluorescence was checked by the addition of carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) at a final concentration of 300 nM. Calibration of the signal was achieved by the addition of known amounts of Ca2+.
In vivo studies
Animal groups and drug treatment
All of the procedures involving animals were conducted in accordance with the Swiss Federal Veterinary Office's guidelines, based on the Swiss Federal Law on Animal Welfare, and were approved by the Cantonal Veterinary Office. The study was performed on dystrophic mdx5Cv mice (Im et al., 1996). Breeding pairs of dystrophic mdx5Cv animals were kindly provided by Dr Serge Braun (Transgene, Strasbourg, France) with the agreement of The Jackson Laboratory (Bar Harbor, ME, USA). The colonies were thereafter maintained in our animal facility. Animals were housed in plastic cages containing wood granule bedding, maintained with 10 h dark/14 h light cycles and unlimited access to food and water throughout the study. Roughly equal numbers of males and females were used in each group. Litters of 3-week-old animals were treated daily by gavage for 2 weeks and analysed at 5 weeks of age. Animals received CsA 10 mg kg−1, Debio 025 10, 30, or 100 mg kg−1. The substance suspensions were freshly prepared from a cremophor-based concentrate formulation provided by Debiopharm. The placebo group consisted of animals receiving the formulation alone. Substance and placebo solutions were prepared in a way to contain the same concentration of excipients (of which cremophor was at 4.1% final concentration), and were administered at 10 μL g−1 of body weight. Treatments were performed at 12±2 h. Data from 7 to 9 animals per group were analysed.
Monitoring of mouse body weight and food intake
Mice were weighed every day. Animals were kept with the dam until weaning (28 days of age) and were isolated in separate cages thereafter. Known amounts of food pellets were delivered on day 28, and the amount of remaining pellets was determined on day 35 (day of death). Food consumption over this 7-day period was expressed as the mass of pellets consumed in gram per gram of body weight (on day 35) per day.
Isometric force recordings
At the end of the treatment period, animals were anaesthetized by i.p. injection of a mixture of urethane (1.5 g kg−1) and diazepam (5 mg kg−1). The Achilles tendon of the right hindlimb was exposed and linked to a force transducer coupled to a LabView interface for trace acquisition and analysis. The knee joint was firmly immobilized. Two thin steel electrodes were inserted into the triceps surae muscle (comprising the fast-twitch glycolytic gastrocnemius and plantaris muscles, and the slow-twitch oxidative soleus muscle). Muscles were electrically stimulated with 0.5 ms pulses of controlled intensity and frequency. The stimulation-recording protocol was performed as follows. After manual settings of optimal muscle length and optimal current intensity, a phasic twitch was recorded to measure the absolute phasic twitch force (Pt), the time to peak (TTP) and the time for half relaxation from the peak (RT1/2). After a 3-min pause, muscles were subjected to a force–frequency test, where tetanic force was recorded using 200 ms bursts of increasing frequency (from 20 to 100 Hz by increments of 10 Hz) with one burst every 30 s. The strongest response was taken as the absolute optimal tetanic force (Po). Finally, after another 3-min pause, muscles were submitted to a fatigue test for 5 min: frequency was set at 60 Hz, and the decrease in tetanic muscle force was recorded while 60 stimulation sequences were delivered, each consisting of a 2-s tetanic burst and a 3-s rest. The responses were expressed as a percentage of the maximal tension. Absolute phasic and tetanic forces were converted into specific forces (millinewtons per square millimetre of muscle section) after normalization for the muscle cross-sectional area. The cross-sectional area (in square millimetre) was determined by dividing the triceps surae muscle mass (in milligram), by the product of optimal muscle length (in millimetre) and d, the density of mammalian skeletal muscle (d=1.06 mg mm−3).
Plasma creatine kinase level
After the measurement of the muscle mechanical contractile properties, the mice were thoracotomized, received an intracardiac injection of heparin (approximately 30 U heparin per mL of blood) and the aorta was cut 20–30 s later. Whole blood was collected from the chest cavity in heparinized centrifuge tubes in a time window of 24±4 h after the last administration of test substance. Fresh blood fractions were saved for creatine kinase (CK) determination. The blood was centrifuged at 3000 g for 10 min at 4 °C and plasma was stored at 2–6 °C. CK determination was performed in four animals per group by spectrophotometric analysis using Catachem diagnostic kit (Investcare Vet, Middlesex, UK) according to the manufacturer's instructions within 48 h of plasma preparation.
Tissue sampling
Immediately after blood collection, the extensor digitorum longus (EDL), soleus, gastrocnemius and plantaris muscles were dissected bilaterally and weighed for determination of muscle mass. Heart, liver, kidneys and spleen were also collected on some animals and weighed. EDL, soleus muscles from the left leg and the left hemi-diaphragm were embedded in Tissue Freezing Medium (Polysciences, Brunschwig, Basel, Switzerland), frozen in liquid nitrogen-cooled isopentane and stored at −80 °C for histological analysis.
Morphometrical analysis
Transverse sections (10 μm thick) were prepared with a HM 560M cryostat (Microm, Volketswil, Switzerland) and collected on SuperFrost Plus slides. Whole muscle cross sections taken near the mid-point of the muscle were stained with haematoxylin–eosin according to classical procedures. Pictures were taken with a Spot Insight QE digital camera (Visitron Systems, Puchheim, Germany) coupled to an inverted microscope (Axiovert 200M, Zeiss, Feldbach, Switzerland). One cross section was analysed per animal. The total cross-sectional area and the necrosis-regenerated surface (NRS) were delineated using the Adobe Photoshop software. Then, the surfaces were calculated using the MetaMorph software (Visitron Systems). Results were expressed as a percentage of the area occupied by necrotic and regenerated myofibres, and inflammatory cells to the total surface area. The samples were coded and analysed by an observer who was blinded to the study details.
Data analysis
The graphs were constructed and the data were analysed using the GraphPad Prism software (GraphPad, San Diego, CA, USA). Results were calculated as means±s.d. For clarity, data are shown as means±s.e.mean in figures. Statistical analysis was performed by using a two-tailed unpaired Student's t-test with the placebo mdx5Cv group used as a reference for comparison to any other group. Differences with P-values ⩽0.05 were considered significant.
Results
Effect of Debio 025 on mitochondria-mediated death
CRC
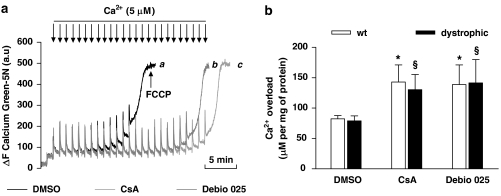
Mitochondria isolated from both normal and dystrophic skeletal muscle were investigated with respect to their ability to withstand incremental challenges of Ca2+ using a CRC assay (Figure 1). No significant difference in CRC was found between mitochondria isolated from wild-type (wt) or dystrophic muscle. The CRC was ∼70% higher when mitochondria were incubated with Debio 025 at 1 μM compared with vehicle (140 μM versus 82 μM, respectively). Addition of CsA at 1 μM, used as a positive control, increased CRC to the same extent as Debio 025.
Figure 1.
Effect of Debio 025 on the calcium retention capacity (CRC) of mitochondria isolated from mouse skeletal muscle. (a) CRC of isolated mitochondria from dystrophic mouse skeletal muscle. Mitochondria were repeatedly exposed to Ca2+ pulses. The ability of mitochondria to buffer Ca2+ was followed through the change in fluorescence of the Ca2+ indicator Calcium Green-5N. Muscle mitochondria were incubated with vehicle (trace a), 1 μM Debio 025 (trace b) or 1 μM cyclosporin A (CsA) (trace c). Pulses of 5 μM CaCl2 were added every minute (arrows). (b) Summary of four independent assays showing that the Ca2+ overload required to open the mitochondrial permeability transition pore (mPTP) was significantly higher in the presence of Debio 025 or CsA compared with control for both normal (wild type (wt)) and dystrophic muscle (n=4). *Significantly different from wt+vehicle, §significantly different from dystrophic+vehicle (P⩽0.05). Values represent means±s.e.mean.
Survival assay
Test substances were added for 2 days on either primary cultures or pure myotube cultures (four independent experiments for each phenotype). None of the test substances (1 nM to 10 μM) altered the viability of pure myotubes as assessed by acid phosphatase activity (not shown). On primary myotube cultures, 10 μM of either Debio 025 or CsA was slightly toxic (not shown). Of note, dystrophic myotubes presented a 4.3-fold higher sensitivity to STS compared with normal myotubes (IC50 ∼7 versus ∼30 nM, respectively) (not shown).
Caspase-3 activity
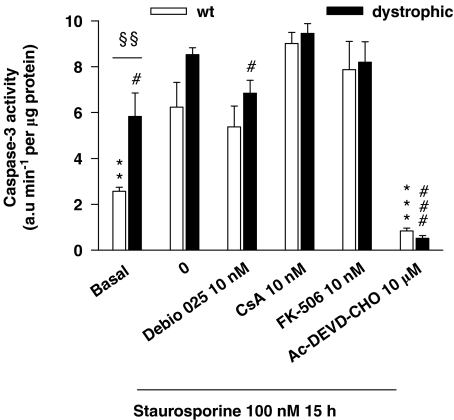
Caspase-3 activity was measured in cell homogenates using a fluorigenic substrate (Figure 2). Basal level of caspase-3 activity was 2.3-fold higher in dystrophic myotubes as compared with normal myotubes. STS increased caspase-3 activity in both normal and dystrophic myotubes. Debio 025 used at 10 nM partially decreased STS-induced caspase-3 activity in dystrophic myotubes, whereas CsA and FK-506 were ineffective at this concentration. The caspase-3 inhibitor Ac-DEVD-CHO, used as a control, fully prevented the STS-induced caspase-3 activity.
Figure 2.
Caspase-3 activity assay. Basal and staurosporine (STS)-induced caspase-3 activity was assessed in normal (wild type (wt)) and dystrophic pure myotubes. Cells were pretreated for 1 h with the substances and then exposed to 100 nM STS for 15 h in the presence of the test substance. Caspase-3 activity was measured in cell extracts by monitoring the proteolytic cleavage of the fluorigenic substrate N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-AMC) for 5 min at 30 °C. At the basal level, pure dystrophic myotubes presented a 2.3-fold higher caspase-3 activity compared with normal myotubes (§§P⩽0.01). STS increased caspase-3 activity in both normal (**P⩽0.01) and dystrophic (#P⩽0.05) myotubes. Debio 025 significantly decreased STS-induced activity (#P⩽0.05). The caspase-3 inhibitor N-acetyl-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-CHO), used as a control, abolished the STS-induced caspase-3 activity (***P⩽0.001, ###P⩽0.001). **,***Significantly different from wt+STS, #,###significantly different from dystrophic+STS (n=4). Values represent means±s.e.mean.
Effect of Debio 025 on food intake, body weight, muscle and organ weight
Food intake and mouse body weight
Animals (3-week-old) were treated daily by gavage for 2 weeks with CsA (10 mg kg−1), Debio 025 (10, 30, or 100 mg kg−1). Data from eight animals per group were analysed. Food consumption over the second week of the administration period was expressed as the mass of pellets consumed in gram per gram of body weight (on day 35) per day. Food intake was similar in all groups of mice (from 0.22±0.01 to 0.25±0.01 g food consumed per gram body weight per day). Animals in all groups had normal gait throughout the study, and the body weight increased similarly (from ∼8.8 g at day 28 to ∼16.5 g at day 35).
Corrected weight of muscles and organs
Muscle weight (eight animals per group) was expressed in milligram per gram body weight. No differences were observed in the weight of EDL (∼0.35 mg g−1), gastrocnemius (∼3.92 mg g−1), plantaris (∼0.50 mg g−1) and soleus (∼0.30 mg g−1) muscles between the various groups of treatment. Treatment for 2 weeks with CsA at 10 mg g−1 or Debio 025 (10, 30 and 100 mg g−1) did not alter the weight of heart, liver, kidney and spleen, collected from 5-week-old dystrophic mice.
Effect of Debio 025 on markers of muscle damage
Muscle histology
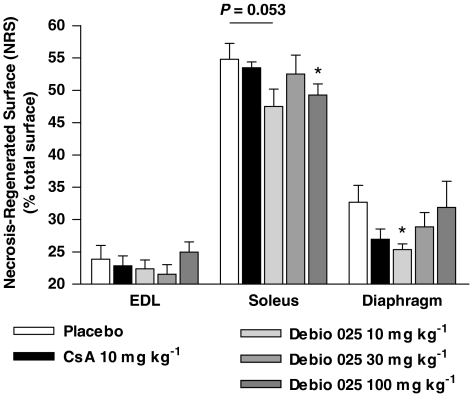
Data from 7 to 9 animals per group at 5 weeks of age were analysed. Normal and regenerated muscle fibres were readily identified by the presence of either peripheral or central nuclei after haematoxylin–eosin staining. Foci of necrotic fibres were characterized by the presence of infiltrated immune cells. The fractions of the muscle affected by necrosis, inflammation and regeneration are represented in Figure 3. Diaphragm muscles from placebo-treated mice exhibited 32.7±2.6% of NRS, an index of muscle damage. In contrast, in mice receiving Debio 025 at 10 mg kg−1, NRS dropped to 25.3±0.9%. This corresponds to 29% of protection compared with placebo-treated mice. However, this effect disappeared at higher doses of Debio 025 (30 and 100 mg kg−1). The slow oxidative soleus muscle was more affected with NRS values of 55.0±2.4%. Debio 025 at 100 mg kg−1 significantly diminished necrosis to 48.2±1.5%. NRS values reached 23.9±2.1% for the fast glycolytic EDL muscle of placebo-treated mice and were not changed by Debio 025 treatment.
Figure 3.
Effect of Debio 025 on the extent of necrosis and regeneration in dystrophic skeletal muscle. After a 2-week treatment with Debio 025, cyclosporin A (CsA) or placebo, whole transverse cross sections were prepared from extensor digitorum longus (EDL), soleus and diaphragm muscles, and stained with haematoxylin–eosin. The surface of the sections affected by necrosis and regeneration was measured and expressed as a percentage of total muscle section area (% necrosis-regenerated surface (NRS)). Diaphragm muscles from mice treated with 10 mg kg−1 of Debio 025 exhibited 29% of protection compared with placebo-treated mice. Debio 025 at 100 mg kg−1 significantly diminished necrosis in the soleus muscle compared with placebo (*P⩽0.05) (n=7–9). Values represent means±s.e.mean.
Plasma CK level
Creatine kinase level was determined in four animals per group at 5 weeks of age, as CK activity is a well-established index of muscle damage. As expected, placebo-treated dystrophic mice presented high levels of CK (10 670±3226 U L−1). Although Debio 025 treatment at 10 mg kg−1 tended to diminish CK levels (8790±1153 U L−1), statistical significance between groups was not reached (data not shown).
Effect of Debio 025 on isometric force parameters
Isometric force was recorded from 8 to 9 animals per group at 5 weeks of age.
Phasic twitch force and optimal tetanic force
The specific tension developed by triceps surae muscle is a parameter considered to be physiologically more relevant than absolute tension, which is influenced by changes in muscle weight and length. Pt (from 5.5±0.6 to 6.9±0.5 mN mm−2) and Po (from 26.1±1.6 to 32.6±2.1 mN mm−2) were not significantly altered by any of the treatments (data not shown). As a result, the phasic-to-tetanic ratios remained unchanged (from 0.20±0.01 to 0.24±0.01).
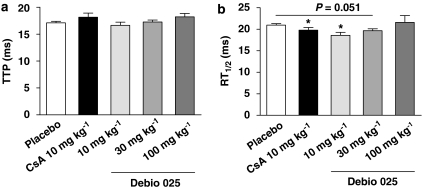
Kinetics of contraction and relaxation
Time to peak and half relaxation time (RT1/2) were determined from phasic twitch traces. TTP was ∼17.5 ms in all groups and no significant alterations were seen in the TTP upon treatments (Figure 4a). The RT1/2 of mice treated with CsA (19.8±0.6 ms) or Debio 025 at 10 mg kg−1 (18.6±0.7 ms) was significantly diminished compared with the placebo group (21.0±0.3 ms), indicating that triceps surae muscles from these treated mice relaxed faster (Figure 4b).
Figure 4.
Kinetics of contraction and relaxation. Electrically evoked isometric contractions of the triceps surae were recorded. (a) Time to peak (TTP) and (b) time for half-relaxation from the peak (RT1/2) were determined. TTP was similar in all groups. The triceps surae from mice treated with Debio 025 at 10 mg kg−1 relaxed significantly faster compared with the placebo-treated mice (*P⩽0.05) (n=8–9). Values represent means±s.e.mean.
Force–frequency relationship
Curves connecting tetanic tension output to stimulation frequency were established (Figure 5a). The fraction of maximal force developed by mice treated with 10 mg kg−1 of Debio 025 at low stimulation frequencies (30–50 Hz corresponding to the preferential recruitment of slow-twitch fibres) was less (from 36.4.±1.8 to 64.3±2.7%) than that of their placebo-treated counterparts (from 45.2±2.5% at 30 Hz to 74.2±3.8% at 50 Hz). This indicates a shift to the right of the force–tension relationship.
Figure 5.
Force–frequency relationship and resistance to mechanical stress. Tension output was expressed as a percentage of maximal response. (a) Tetanic tensions elicited by stimulation of increasing frequency were recorded at 30-s intervals on mice. The fraction of maximal force developed by mice treated with Debio 025 10 mg kg−1 at low stimulation frequency (30–50 Hz) that preferentially recruits slow-twitch fibres was less than in the placebo-treated counterparts. (b) Resistance to repetitive tetanizations was assessed. After a 2-week treatment with 10 mg kg−1 of Debio 025, the triceps surae was significantly more resistant than in the placebo-treated counterparts (*P⩽0.05). For both tests, the statistical significance of the test condition was calculated versus the placebo-treated mice (n=8–9). Values represent means±s.e.mean.
Resistance to mechanical stress
The resistance of triceps surae muscle to repetitive tetanization was assessed by a fatigue assay. As shown in Figure 5b, the tension abruptly decreased upon the first 15 tetanizations to reach a plateau over 150 s. The values measured at the end of the assay corresponded to the residual force after tetanus. At this stage, the contraction-induced damages within muscle were irreversible. The various groups presented similar profiles except for mice treated with Debio 025 at 10 mg kg−1. These animals were significantly less fatigable than their placebo counterparts. Residual force at the end of the fatigue protocol was 25.4±1.7% of maximal tension in the group with Debio 025 treated at 10 mg kg−1 compared with 18.8±2.4% in the placebo group. Thus, 10 mg kg−1 Debio 025 made the dystrophic muscles ∼37% more resistant to mechanical stress compared with untreated muscles.
Effect of agent used for the formulation
In the current in vivo study, cremophor was used to solubilize Debio 025 and CsA, which has been reported to have detrimental effects on mitochondria from muscle (Sanchez et al., 2001). Therefore, we performed a study using six mice per group with a non-cremophor-based formulation to investigate this possibility. Both formulations gave similar results (not shown), which may be due to the fact that the cremophor concentration used was still below the toxic level.
Discussion
The basal level of activated caspase-3, an end point in the caspase activation cascade leading to cell death, was found to be more than twofold higher in dystrophic myotubes than in normal myotubes. This is consistent with data obtained with muscle biopsies from DMD patients (Sandri et al., 2001), suggesting that caspase-3 might be an appropriate target for the pharmacological treatment of DMD. STS is a potent protein kinase inhibitor with a broad spectrum of activities (Ruegg and Burgess, 1989), known to induce apoptosis through a mitochondrial pathway (Yang et al., 1997) and to cause oxidative stress through generation of reactive oxygen species (Cai and Jones, 1998; Kruman et al., 1998). Pure dystrophic myotubes were almost fivefold more sensitive to STS than normal myotubes, which contrasts with findings by Rando et al. (1998). Interestingly, Debio 025 decreased STS-induced caspase-3 activity in cultured muscle cells, whereas CsA and FK-506 were ineffective at the concentration examined.
It is generally accepted that degeneration of dystrophic skeletal muscle is associated with necrosis. However, several studies suggest that during the phase of acute muscle degeneration of mdx muscle, apoptosis precedes necrotic cell death (Tidball et al., 1995). The occurrence of apoptosis was estimated to be around 0.5–2% of total nuclei in mdx muscle (Matsuda et al., 1995; Sandri et al., 1995; Tidball et al., 1995). Moreover, dystrophin-deficient myotubes undergo apoptosis in mouse primary muscle cell culture after DNA damage (Sandri et al., 1998). L-Carnitine, which plays a role in the transport of fatty acids into mitochondria, was described as a potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure (Vescovo et al., 2002). In addition, the anti-apoptotic Bcl-2 protein may regulate the mPTP, thus preventing the release of pro-apoptotic factors from mitochondria (Mignotte and Vayssiere, 1998), and overexpression of Bcl-2 has been reported to suppress Ca2+ activation of the mPTP (Murphy et al., 2001). However, muscle-specific Bcl-2 overexpression did not ameliorate the dystrophic condition of mdx mice (Dominov et al., 2005), suggesting that therapies designed to treat DMD by inhibition of apoptosis are not appropriate. Thus, whereas CsA upregulates the Bcl-2 protein in a calcineurin-dependent manner (Genestier et al., 1995), it is likely not to be sufficient to completely prevent dystrophic cell death. In addition, investigations of CyPD-deficient mice indicated that CyPD is a regulator but not an intrinsic component of the mPTP, which can form and open in the absence of CyPD (Baines et al., 2005; Basso et al., 2005; Nakagawa et al., 2005). Therefore, in contrast to the conclusions by others (Halestrap, 2005), Bernardi et al. (2006) stressed that the in vivo studies with CyPD knockout mice can only be interpreted in terms of the role of CyPD, not of the mPTP, in cell death.
Our results show that CsA (10 mg kg−1) and Debio 025 (10, 30 and 100 mg kg−1) treatments of 3-week-old dystrophic mice for 2 weeks did not alter the weight of heart, liver, kidney and spleen, known to be targets of CsA as reported by others (Stupka et al., 2004). In this latter study, daily doses of 30 mg kg−1 CsA were given i.p. to 2-week-old mice for 2 weeks. As suggested by De Luca et al. (2005), this dose is expected to totally inhibit calcineurin activity in skeletal muscle. Calcineurin is a Ca2+/calmodulin-activated serine/threonine protein phosphatase involved in a number of processes (Crabtree, 2001). Both pro- and anti-apoptotic roles have been reported for its activation (Baines and Molkentin, 2005), likely to reflect the highly specialized role that this protein plays in different tissues. In the heart, calcineurin directly regulates myocyte growth, yet its overexpression in skeletal muscle had no effect on growth or fibre size (Molkentin, 2004). In contrast, calcineurin functions as a critical regulator of fibre-type switch in skeletal muscle, enhancing the formation of slow-type fibres (Naya et al., 2000). Calcineurin has also been implicated in regulating differentiation, developmental maturation and regeneration of skeletal muscle (Friday et al., 2000; Hong et al., 2002; Stupka et al., 2004). Moreover, calcineurin activity has been shown to attenuate the dystrophic progression in mdx muscle (Chakkalakal et al., 2004; Stupka et al., 2006). Therefore, it appears that 30 mg kg−1 of CsA is toxic to skeletal muscle, in particular to regenerating dystrophic skeletal muscle. The same dose of Debio 025 is not detrimental to this tissue. In addition, our results based on primary myotube cultures show that Debio 025 is not toxic at concentrations up to 1 μM (that is, about 1000 times more than the IC50 value).
Debio 025 at the low dose of 10 mg kg−1 protected the dystrophic mouse diaphragm. This result is of clinical relevance as the diaphragm is the most severely affected muscle in mdx mice and is considered the most pertinent muscle with respect to the human disease (Petrof et al., 1993). Besides, the mild protection of the soleus muscle observed with Debio 025 treatment is probably due to the oxidative metabolism inherent to this type of muscle. Moreover, it is well established that the density of mitochondria in this slow-twitch oxidative muscle is higher compared with fast-twitch glycolytic muscle. Of note, other substances such as creatine, green tea extract or pentoxifylline were found to be ineffective to protect the soleus muscle of dystrophic mice (Buetler et al., 2002; Passaquin et al., 2002; Dorchies et al., 2006).
However, CK activity, a well-known diagnostic marker for muscle injury, did not reach statistical significance between placebo and treated groups. This might be due to the surgical preparation prior to death, which through muscle damage may have attenuated a potential difference between placebo and treated mice.
The shortening of the time for relaxation by Debio 025 at 10 mg kg−1 appears most interesting as dystrophic muscle, similarly to fatigued muscle (Allen et al., 2008), is known to display marked slowing of relaxation as compared with normal muscle (Nicolas-Metral et al., 2001). Substances beneficial to mdx mice normalize the relaxation rate to the one of control mice (Dorchies et al., 2006). Both the faster relaxation and the alteration of the force–frequency relationship reported here suggest a change either in cytosolic Ca2+ handling or in the distribution of fibre types towards a faster phenotype.
The resistance to mechanical stress was improved with Debio 025 at 10 mg kg−1, whereas CsA had no effect. These findings are in contrast with results by others showing a functional improvement in the mdx mouse using the same dose of CsA (De Luca et al., 2005). This might be due to the use of a treadmill to accelerate muscle degeneration or to different ages and periods of treatment of the animals among studies.
In conclusion, our in vitro investigations showed a potent and efficacious protective effect of Debio 025 against mitochondria-mediated cell death. Daily oral treatment of 3-week-old dystrophic mice for 2 weeks with 10 mg kg−1 of Debio 025 resulted in partial improvement of the structure and the function of the dystrophic mouse muscle, and higher doses did not yield stronger effects, indicating high potency but limited efficacy in this model and under our conditions. Considering the high affinity of Debio 025 for CyPD, lower doses of Debio 025, for instance 1–3 mg kg−1, deserve to be investigated. While this paper was in the review process, a report was published showing similar results of Debio 025 injected s.c. at 50 mg kg−1 day−1 into mdx mice from 4 to 10 weeks of age (Millay et al., 2008). Of note, mitochondria prepared from Debio 025-treated muscle were still protected in in vitro tests, presumably several hours after in vivo exposure to this compound, highlighting the strong affinity of Debio 025 for the mPTP target. This in vivo treatment also reduced fibrosis and, to a minor extent (5–10%), central nucleation of mdx muscle fibres. As outlined above, the dystrophic pathology results from alterations in multiple molecular pathways, which together contribute to muscle fibre death. Therefore, our findings suggest that selective CyPD inhibitors are potential candidates for the symptomatic treatment of DMD and combinations with treatments targeting mechanical, immunological and signalling pathways should result in improved efficacy.
Acknowledgments
We thank Dr Randall Kramer, UCSF, USA, for the gift of α7 integrin antibody, Christian Haeberli for expert technical support and for maintenance of the isometric force set-up, Nicolas Demierre for developing the force recordings software and Dr Serge Braun for providing breeding pairs of dystrophic mice. This study was also supported by grants from the Swiss National Science Foundation (Grant 3100A0–109981), the ‘Association Française contre les Myopathies', the Duchenne Parent Project and the Swiss Foundation for Research on Muscle Diseases.
Abbreviations
- CK
creatine kinase
- CRC
calcium retention capacity
- CsA
cyclosporin A
- CyPD
cyclophilin D
- DMD
Duchenne muscular dystrophy
- EDL
extensor digitorum longus
- mdx
muscular dystrophy X-linked
- mPTP
mitochondrial permeability transition pore
- NRS
necrosis-regenerated surface
- RT1/2
time for half relaxation from the peak
- STS
staurosporine
- TTP
time to peak
- wt
wild type
Conflict of interest
The authors state no conflict of interest.
References
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Angelin A, Tiepolo T, Sabatelli P, Grumati P, Bergamin N, Golfieri C, et al. Mitochondrial dysfunction in the pathogenesis of Ullrich congenital muscular dystrophy and prospective therapy with cyclosporins. Proc Natl Acad Sci USA. 2007;104:991–996. doi: 10.1073/pnas.0610270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai A. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS ONE. 2007;2:e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992;267:8834–8839. [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blalchy-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Petronilli V, Di Lisa F, Forte M. A mitochondrial perspective on cell death. Trends Biochem Sci. 2001;26:112–117. doi: 10.1016/s0968-0004(00)01745-x. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- Blanco-Bose WE, Yao CC, Kramer RH, Blau HM. Purification of mouse primary myoblasts based on alpha 7 integrin expression. Exp Cell Res. 2001;265:212–220. doi: 10.1006/excr.2001.5191. [DOI] [PubMed] [Google Scholar]
- Bouchier-Hayes L, Lartigue L, Newmeyer DD. Mitochondria: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2640–2647. doi: 10.1172/JCI26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetler TM, Renard M, Offord EA, Schneider H, Ruegg UT. Green tea extract decreases muscle necrosis in mdx mice and protects against reactive oxygen species. Am J Clin Nutr. 2002;75:749–753. doi: 10.1093/ajcn/75.4.749. [DOI] [PubMed] [Google Scholar]
- Cai J, Jones D. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Harrison M, Carbonetto S, Chin ER, Michel RN, Jasmin BJ. Stimulation of calcineurin signaling attenuates the dystrophic pathology in mdx mice. Hum Mol Genet. 2004;13:379–388. doi: 10.1093/hmg/ddh037. [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Thompson J, Parks RJ, Jasmin BJ. Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies. FASEB J. 2005;19:880–891. doi: 10.1096/fj.04-1956rev. [DOI] [PubMed] [Google Scholar]
- Connolly DT, Knight MB, Harakas NK, Wittwer AJ, Feder J. Determination of the number of endothelial cells in culture using an acid phosphatase assay. Anal Biochem. 1986;152:136–140. doi: 10.1016/0003-2697(86)90131-4. [DOI] [PubMed] [Google Scholar]
- Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 2007;13:520–526. doi: 10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Crabtree GR. Calcium, calcineurin, and the control of transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Nico B, Liantonio A, Didonna MP, Fraysse B, Pierno S, et al. A multidisciplinary evaluation of the effectiveness of cyclosporine A in dystrophic mdx mice. Am J Pathol. 2005;166:477–489. doi: 10.1016/S0002-9440(10)62270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominov JA, Kravetz AJ, Ardelt M, Kostek CA, Beermann ML, Miller JB. Muscle-specific BCL2 expression ameliorates muscle disease in laminin {alpha}2-deficient, but not in dystrophin-deficient, mice. Hum Mol Genet. 2005;14:1029–1040. doi: 10.1093/hmg/ddi095. [DOI] [PubMed] [Google Scholar]
- Dorchies OM, Wagner S, Vuadens O, Waldhauser K, Buetler TM, Kucera P, et al. Green tea extract and its major polyphenol (−)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol. 2006;290:C616–C625. doi: 10.1152/ajpcell.00425.2005. [DOI] [PubMed] [Google Scholar]
- Fisher I, Abraham D, Bouri K, Hoffman EP, Muntoni F, Morgan JE. Prednisolone-induced changes in dystrophic skeletal muscle. FASEB J. 2005;19:834–836. doi: 10.1096/fj.04-2511fje. [DOI] [PubMed] [Google Scholar]
- Flisiak R, Dumont J, Crabbe R. Cyclophilin inhibitors in hepatitis C viral infection. Expert Opin Investig Drugs. 2007;16:1345–1354. doi: 10.1517/13543784.16.9.1345. [DOI] [PubMed] [Google Scholar]
- Forte M, Bernardi P. Genetic dissection of the permeability transition pore. J Bioenerg Biomembr. 2005;37:121–128. doi: 10.1007/s10863-005-6565-9. [DOI] [PubMed] [Google Scholar]
- Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci USA. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol. 2000;149:657–665. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateau-Roesch O, Argaud L, Ovize M. Mitochondrial permeability transition pore and postconditioning. Cardiovasc Res. 2006;70:264–273. doi: 10.1016/j.cardiores.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Genestier L, Bonnefoy-Berard N, Rouault JP, Flacher M, Revillard JP. Tumor necrosis factor-alpha up-regulates Bcl-2 expression and decreases calcium-dependent apoptosis in human B cell lines. Int Immunol. 1995;7:533–540. doi: 10.1093/intimm/7.4.533. [DOI] [PubMed] [Google Scholar]
- Gomez L, Thibault H, Gharib A, Dumont J, Vuagniaux G, Scalfaro P, et al. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H1654–H1661. doi: 10.1152/ajpheart.01378.2006. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun. 2003;304:445–454. doi: 10.1016/s0006-291x(03)00616-8. [DOI] [PubMed] [Google Scholar]
- Halestrap A. Biochemistry: a pore way to die. Nature. 2005;434:578–579. doi: 10.1038/434578a. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Davidson AM. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson MJ, Mattiasson G, Mansson R, Karlsson JO, Keep MF, Ruegg UT, et al. The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J Bioenerg Biomembr. 2004;36:407–413. doi: 10.1023/B:JOBB.0000041776.31885.45. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Monaco AP, Feener CC, Kunkel LM. Conservation of the Duchenne muscular dystrophy gene in mice and humans. Science. 1987;238:347–350. doi: 10.1126/science.3659917. [DOI] [PubMed] [Google Scholar]
- Hong F, Lee J, Song J, Lee SJ, Ahn H, Cho JJ, et al. Cyclosporine blocks muscle differentiation by inducing oxidative stress and by inhibiting the peptidylprolyl-cis–trans-isomerase activity of cyclophilin A: cyclophilin A protects myoblasts from cyclosporine-induced cytotoxicity. FASEB J. 2002;16:1633–1635. doi: 10.1096/fj.02-0060fje. [DOI] [PubMed] [Google Scholar]
- Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Im WB, Phelps SF, Copen EH, Adams EG, Slightom JL, Chamberlain JS. Differential expression of dystrophin isoforms in strains of mdx mice with different mutations. Hum Mol Genet. 1996;5:1149–1153. doi: 10.1093/hmg/5.8.1149. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Davies KE. Pharmacological strategies for muscular dystrophy. Nat Rev Drug Discov. 2003;2:379–390. doi: 10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- Kruman I, Guo Q, Mattson M. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Winkler K, Wiedemann FR, Vonbossanyi P, Dietzmann K, Kunz WS. Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol Cel Biochem. 1998;183:87–96. doi: 10.1023/a:1006868130002. [DOI] [PubMed] [Google Scholar]
- Leijendekker WJ, Passaquin AC, Metzinger L, Ruegg UT. Regulation of cytosolic calcium in skeletal muscle cells of the mdx mouse under conditions of stress. Br J Pharmacol. 1996;118:611–616. doi: 10.1111/j.1476-5381.1996.tb15445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescop C, Herzner H, Siendt H, Bolliger R, Hennebohle M, Weyermann P, et al. Novel cell-penetrating [alpha]-keto-amide calpain inhibitors as potential treatment for muscular dystrophy. Bioorg Med Chem Lett. 2005;15:5176–5181. doi: 10.1016/j.bmcl.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Madsen K, Ertbjerg P, Pedersen PK. Calcium content and respiratory control index of isolated skeletal muscle mitochondria: effects of different isolation media. Anal Biochem. 1996;237:37–41. doi: 10.1006/abio.1996.0197. [DOI] [PubMed] [Google Scholar]
- Mason J. Pharmacology of cyclosporine (sandimmune). VII. Pathophysiology and toxicology of cyclosporine in humans and animals. Pharmacol Rev. 1990;41:423–434. [PubMed] [Google Scholar]
- Matsuda R, Nishikawa A, Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem. 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- McGuinness O, Yafei N, Costi A, Crompton M. The presence of two classes of high-affinity cyclosporin A binding sites in mitochondria. Evidence that the minor component is involved in the opening of an inner-membrane Ca2+-dependent pore. Eur J Biochem. 1990;194:671–679. doi: 10.1111/j.1432-1033.1990.tb15667.x. [DOI] [PubMed] [Google Scholar]
- Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici MC, et al. Lipid peroxidation inhibition blunts nuclear factor-{kappa}B activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol. 2006;168:918–926. doi: 10.2353/ajpath.2006.050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzinger L, Passaquin AC, Leijendekker WJ, Poindron P, Ruegg UT. Modulation by prednisolone of calcium handling in skeletal muscle cells. Br J Pharmacol. 1995;116:2811–2816. doi: 10.1111/j.1476-5381.1995.tb15930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte B, Vayssiere J. Mitochondria and apoptosis. Eur J Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V, et al. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 2006;12:1147–1150. doi: 10.1038/nm1479. [DOI] [PubMed] [Google Scholar]
- Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Muntoni F, Fisher I, Morgan JE, Abraham D. Steroids in Duchenne muscular dystrophy: from clinical trials to genomic research. Neuromuscul Disord. 2002;12 Suppl 1:S162–S165. doi: 10.1016/s0960-8966(02)00101-3. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Schneider E, Kinnally KW. Overexpression of Bcl-2 suppresses the calcium activation of a mitochondrial megachannel. FEBS Lett. 2001;497:73–76. doi: 10.1016/s0014-5793(01)02440-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- Nicolas-Metral V, Raddatz E, Kucera P, Ruegg UT. mdx myotubes have normal excitability but show reduced contraction–relaxation dynamics. J Muscle Res Cell Motil. 2001;22:69–75. doi: 10.1023/a:1010384625954. [DOI] [PubMed] [Google Scholar]
- Noser JA, Towers GJ, Sakuma R, Dumont J, Collins MKL, Ikeda Y. Cyclosporine increases human immunodeficiency virus type 1 vector transduction of primary mouse cells. J Virol. 2006;80:7769–7774. doi: 10.1128/JVI.02427-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KJ, Davies KE. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment. EMBO Rep. 2004;5:872–876. doi: 10.1038/sj.embor.7400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Passaquin A, Renard M, Kay L, Challet C, Mokhtarian A, Wallimann T, et al. Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromuscul Disord. 2002;12:174–182. doi: 10.1016/s0960-8966(01)00273-5. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- Pulido SM, Passaquin AC, Leijendekker WJ, Challet C, Wallimann T, Ruegg UT. Creatine supplementation improves intracellular Ca2+ handling and survival in mdx skeletal muscle cells. FEBS Lett. 1998;439:357–362. doi: 10.1016/s0014-5793(98)01399-4. [DOI] [PubMed] [Google Scholar]
- Rando TA, Disatnik M-H, Yu Y, Franco A. Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromusc Disord. 1998;8:14–21. doi: 10.1016/s0960-8966(97)00124-7. [DOI] [PubMed] [Google Scholar]
- Robert V, Massimino ML, Tosello V, Marsault R, Cantini M, Sorrentino V, et al. Alteration in calcium handling at the subcellular level in mdx myotubes. J Biol Chem. 2001;276:4647–4651. doi: 10.1074/jbc.M006337200. [DOI] [PubMed] [Google Scholar]
- Rolland J-F, De Luca A, Burdi R, Andreetta F, Confalonieri P, Conte Camerino D. Overactivity of exercise-sensitive cation channels and their impaired modulation by IGF-1 in mdx native muscle fibers: beneficial effect of pentoxifylline. Neurobiol Dis. 2006;24:466–474. doi: 10.1016/j.nbd.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Ruegg UT, Burgess GM. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989;10:218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- Sanchez H, Zoll J, Bigard X, Veksler V, Mettauer B, Lampert E, et al. Effect of cyclosporin A and its vehicle on cardiac and skeletal muscle mitochondria: relationship to efficacy of the respiratory chain. Br J Pharmacol. 2001;133:781–788. doi: 10.1038/sj.bjp.0704129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Carraro U, Podhorska-Okolov M, Rizzi C, Arslan P, Monti D, et al. Apoptosis, DNA damage and ubiquitin expression in normal and mdx muscle fibers after exercise. FEBS Lett. 1995;373:291–295. doi: 10.1016/0014-5793(95)00908-r. [DOI] [PubMed] [Google Scholar]
- Sandri M, El Meslemani AH, Sandri C, Schjerling P, Vissing K, Andersen JL, et al. Caspase 3 expression correlates with skeletal muscle apoptosis in Duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment. J Neuropathol Exp Neurol. 2001;60:302–312. doi: 10.1093/jnen/60.3.302. [DOI] [PubMed] [Google Scholar]
- Sandri M, Lina MM, Marcello C, Emanuele G, Claudia S, Arslanb P, et al. Dystrophin deficient myotubes undergo apoptosis in mouse primary muscle cell culture after DNA damage. Neurosci Lett. 1998;252:123–126. doi: 10.1016/s0304-3940(98)00563-1. [DOI] [PubMed] [Google Scholar]
- Sharma KR, Mynhier MA, Miller RG. Cyclosporine increases muscular force generation in Duchenne muscular dystrophy X muscular dystrophy. Neurology. 1993;43:527–532. doi: 10.1212/wnl.43.3_part_1.527. [DOI] [PubMed] [Google Scholar]
- Sharma U, Atri S, Sharma MC, Sarkar C, Jagannathan NR. Skeletal muscle metabolism in Duchenne muscular dystrophy (DMD): an in-vitro proton NMR spectroscopy study. Magn Reson Imaging. 2003;21:145–153. doi: 10.1016/s0730-725x(02)00646-x. [DOI] [PubMed] [Google Scholar]
- Soriano ME, Nicolosi L, Bernardi P. Desensitization of the permeability transition pore by cyclosporin a prevents activation of the mitochondrial apoptotic pathway and liver damage by tumor necrosis factor-{alpha} J Biol Chem. 2004;279:36803–36808. doi: 10.1074/jbc.M405297200. [DOI] [PubMed] [Google Scholar]
- St-Pierre SJG, Chakkalakal JV, Kolodziejczyk SM, Knudson JC, Jasmin BJ, Megeney LA. Glucocorticoid treatment alleviates dystrophic myofiber pathology by activation of the calcineurin/NF-AT pathway. FASEB J. 2004;18:1937–1939. doi: 10.1096/fj.04-1859fje. [DOI] [PubMed] [Google Scholar]
- Stupka N, Gregorevic P, Plant DR, Lynch GS. The calcineurin signal transduction pathway is essential for successful muscle regeneration in mdx dystrophic mice. Acta Neuropathol (Berl) 2004;107:299–310. doi: 10.1007/s00401-003-0807-x. [DOI] [PubMed] [Google Scholar]
- Stupka N, Plant DR, Schertzer JD, Emerson TM, Bassel-Duby R, Olson EN, et al. Activated calcineurin ameliorates contraction-induced injury to skeletal muscles of mdx dystrophic mice. J Physiol. 2006;575:645–656. doi: 10.1113/jphysiol.2006.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball J, Albrecht D, Lokensgard B, Spencer M. Apoptosis precedes necrosis of dystrophin-deficient muscle. J Cell Sci. 1995;108:2197–2204. doi: 10.1242/jcs.108.6.2197. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J Appl Physiol. 2007;102:1677–1686. doi: 10.1152/japplphysiol.01145.2006. [DOI] [PubMed] [Google Scholar]
- Vescovo G, Ravara B, Gobbo V, Sandri M, Angelini A, Della Barbera M, et al. -Carnitine: a potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am J Physiol Cell Physiol. 2002;283:C802–C810. doi: 10.1152/ajpcell.00046.2002. [DOI] [PubMed] [Google Scholar]
- Watkins SC, Cullen MJ. A qualitative and quantitative study of the ultrastructure of regenerating muscle fibres in Duchenne muscular dystrophy and polymyositis. J Neurol Sci. 1987;82:181–192. doi: 10.1016/0022-510x(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]