Abstract
Background and purpose:
Breast cancer, the most common cancer in women in most countries, is a highly stressful disease. Catecholamines released during stress bind to adrenoceptors and we have recently described α2-adrenoceptors in human breast cell lines, linked to enhanced cell proliferation. The purpose was to assess the in vivo effects of compounds acting on α2-adrenoceptors in a reliable model of breast cancer.
Experimental approach:
The expression of α2-adrenoceptors was confirmed by immunocytochemistry, immunofluorescence and reverse transcription-PCR in the mouse mammary tumour cell line MC4-L5. Proliferation was assessed by [3H]thymidine incorporation and tumours were measured daily. Apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated dUTP digoxigenin nick-end labelling.
Key results:
Incubation for 2 days with α2-adrenoceptor agonists (clonidine and dexmedetomidine) significantly enhanced proliferation of the mouse mammary tumour cell line MC4-L5. These agonists also significantly stimulated tumour growth of the progestin-dependent tumour C4-HD even in the presence of medroxyprogesterone acetate (MPA). In every tumour tested (C4-HD, CC4-2-HD and CC4-3-HI), regardless of MPA sensitivity, clonidine significantly enhanced tumour growth in the absence of MPA. The α2-adrenoceptor antagonists, yohimbine and rauwolscine, completely reversed the effects of clonidine. However, the group receiving yohimbine alone showed a nonsignificant but constant increase in tumour growth, whereas rauwolscine alone diminished tumour growth significantly, behaving as a reverse agonist. In CC4-3-HI tumours, rauwolscine treatment enhanced apoptosis and diminished the mitotic index, whereas clonidine had the inverse effect.
Conclusions and implications:
α2-Adrenoceptor agonists enhanced tumour growth and rauwolscine behaved in vivo as a reverse agonist, suggesting that it may be tested for adjuvant treatment.
Keywords: mammary tumour, clonidine, rauwolscine, yohimbine, mouse
Introduction
Breast cancer, the most common cancer among women in the majority of countries (Parkin, 2004), is among the most stressful of diseases, the threat being particularly serious for women with family histories of the disease (James et al., 2004). The principal effectors of the stress system include, among others, the catecholamines adrenaline and noradrenaline (Charmandari et al., 2005). The catecholamines bind to adrenoceptors, which may be divided into three types α1, α2 and β, and each of these types is composed of three receptor subtypes (Philipp and Hein, 2004; Knaus et al., 2007). Our group has recently described α2-adrenoceptors in different human tumour and non-tumour breast cell lines by reverse transcription (RT)-PCR, immunocytochemistry and binding assays. Moreover, the stimulation by α2-adrenoceptor agonists was associated with an increase in [3H]thymidine incorporation into these cells (Vazquez et al., 2006).
An experimental model in which ductal, progestin-dependent, metastatic mammary carcinomas were induced by the continuous administration of medroxyprogesterone acetate (MPA) has been developed in Balb/c mice (Lanari et al., 1986). These tumours express high levels of oestrogen and progestin receptors and are maintained through serial syngeneic passages in MPA-treated mice (Lanari et al., 1986, 1989). Several cell lines were obtained from these tumours (Lanari et al., 2001). One of these cell lines was chosen as an in vitro model for murine mammary tumours. MC4-L5 is an epithelial cell line which, although expressing oestrogen and progesterone receptors, does not respond in vitro to these hormones (Lanari et al., 2001). By transplantation to untreated mice, progestin-independent tumour lines that retain the expression of oestrogen and progestin receptors have been generated (Lanari and Molinolo, 2002).
The aim of the present study was to assess whether the mitogenic action observed in vitro for α2-adrenoceptor agonists in human breast cancer cell lines is reflected in tumour growth and whether any antagonist is able to inhibit tumour growth, which could allow the possibility of therapeutic intervention with this type of compound.
Materials and methods
Animals
Animal care and manipulation were in agreement with institutional guidelines and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences and National Research Council, 1996). Balb/c female virgin mice (2 month old) were used. The animals were fed ad libitum and kept in air-conditioned rooms at 20±2 °C with a 12 h light–dark period.
Tumours
MPA-induced mammary ductal carcinomas maintained by in vivo syngeneic transplantation were used in all the experiments (Lanari et al., 1989). The tumours analysed were C4-HD, CC4-2-HD (progestin dependent) and CC4-3-HI (progestin sensitive but not dependent). Fragments of each tumour were transplanted subcutaneously. The C4-HD tumour and, when stated, also the CC4-2-HD tumour were transplanted simultaneously with MPA (20 mg depot s.c.) (Gador Laboratories, Buenos Aires, Argentina) in the contralateral flank. Tumour growth was monitored every day. The two major diameters were measured with a calliper and the volume was calculated with the formula 4 × 3−1 × π × minor radius2 × major radius (Wendel et al., 1996).
Treatments
At 1 day after transplantation of the tumour, animals received daily injections of the drugs: clonidine group (0.1 mg kg−1 day−1), dexmedetomidine group (0.05 mg kg−1 day−1) and yohimbine and rauwolscine groups (0.5 mg kg−1 day−1). The control group in all cases received physiological saline.
Cell culture
The MC4-L5 cell line was routinely cultured in phenol red-free (Berthois et al., 1986) Dulbecco's modified Eagle's medium (DMEM):Ham's F12 medium (F12) (1:1) supplemented with 10% foetal calf serum (FCS), 2 mM glutamine, 100 IU mL−1 penicillin, 100 μg mL−1 streptomycin and 15 mM HEPES. Cells were subcultured once weekly after trypsinization (0.25% trypsin–0.025% EDTA). The medium was changed twice weekly (Lanari et al., 2001).
Primary cultures
Tumours were aseptically removed, minced and washed with DMEM:F12 medium. The tissue was suspended in 5 mL of enzymic solution (2.5 mg mL−1 trypsin, 5 mg mL−1 albumin and 850 U mL−1 collagenase type II in phosphate-buffered saline (PBS)) and incubated at 37 °C for 40 min, under continuous stirring. Enzyme action was stopped by adding DMEM:F12 medium with 5% FCS. Epithelial and fibroblastic cells were separated as previously described (Lamb et al., 1999). Briefly, the cell suspension obtained was resuspended in 25 mL of medium with 10% FCS and allowed to sediment for 20 min. The sedimented cells correspond to the epithelial-enriched fraction, which was resuspended again in 25 mL of medium and allowed to sediment for another 20 min. The upper 15 mL was discarded and this procedure was repeated more or less 10 times until no fibroblasts were detected in the supernatant. The cells were plated in culture flasks with 10% FCS medium and allowed to attach for 24 h. The medium was then removed and replaced by fresh medium. This medium was changed every 2–3 days.
Proliferation assays
Cells were seeded at 7000 cells per well in 96-well plates and incubated in phenol red-free DMEM:F12 medium supplemented with 10% FCS, 2 mM glutamine, 100 IU mL−1 penicillin, 100 μg mL−1 streptomycin, 250 ng mL−1 amphotericin B and 15 mM HEPES. After 24 and 48 h, the medium was changed to a similar one but with FCS replaced by 1% charcoal-stripped FCS. α2-Adrenoceptor agonists/antagonists were added as well. All solutions of these compounds were prepared in 10 mM ascorbic acid, frozen and diluted immediately before use. [3H]Thymidine at 0.2 μCi per well was added with the latest change of medium. After 24 h, cells were harvested in a Nunc Cell Harvester 8, and filters were counted in a liquid scintillation counter. The cells were trypsinized prior to harvesting.
Immunocytochemical studies
The cells were cultured in Lab-Tek Chamber Slide System (Nunc) with 10% FCS in phenol red-free DMEM:F12 medium; 2 days before immunocytochemistry the media were changed to 1% charcoal-stripped FCS medium. The cells were fixed in 10% buffered formalin containing 0.2% Triton X-100 in PBS for 20 min at room temperature and washed three times with PBS for 5 min each. The chambers were incubated for 20 min at room temperature with 3% H2O2 in distilled water to quench endogenous peroxidase activity, washed extensively with PBS and incubated for 40 min in 2% albumin–PBS. Cells were incubated for 60 min at 37 °C with anti-human α2A (A-271) from Sigma-Aldrich (St Louis, MO, USA), α2B (H-96) rabbit polyclonal or α2C-RA (C-20) goat polyclonal antibodies (from Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:100, 1:50 and 1:60, respectively, in 1% albumin–PBS. These antibodies were raised against peptides corresponding to amino acids on the internal carboxy-terminal regions of α2-adrenoceptors. The chambers were washed with PBS and successively incubated for 40 min at room temperature with the corresponding secondary antibody–peroxidase conjugated (labeled streptavidin biotin (LSAB)+system). Primary antibodies were omitted in controls.
For immunofluorescence, the cells were similarly cultured. They were treated with 10% FCS and permeated with 0.1% Triton X-100 in 4% FCS. After overnight incubation at 4 °C with the antibodies already described at a concentration of 1:50, and several PBS washes, the cells were incubated with secondary rabbit anti-goat IgG or donkey anti-rabbit IgG in 4% FCS, both conjugated to fluorescein isothiocyanate. Primary antibodies were omitted in the controls. Nuclei were stained with 0.1 μg mL−1 propidium iodide for 1 min, and the cells were mounted with Vectashield H-1000. The sections were analysed under a Nikon laser confocal microscope.
Apoptosis was assessed in CC4-3-HI tumours fixed in 4% buffered formaldehyde. For apoptosis quantification, tumour sections were processed for in situ immunohistochemical localization of nuclei exhibiting DNA fragmentation by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP digoxigenin nick-end labelling (TUNEL) technique with use of an apoptosis detection kit (ApopTag plus peroxidase in situ apoptosis detection kit). The sections were treated according to the manufacturer's instructions and described by Meresman et al. (2000) and Lanari's group (Vanzulli et al., 2002). Briefly, the sections were deparaffinized and rehydrated with xylene and ethanol, and the tissue was pretreated with proteinase K (20 μg mL−1). Endogenous peroxidase was quenched by coating the samples with 3% H2O2 in PBS. The sections were rinsed with PBS and then immersed for 60 min in working strength TdT enzyme at room temperature. This enzyme was diluted as described by the manufacturer (70% dilution buffer 30% TdT enzyme). Then the sections were incubated for 30 min at room temperature with the anti-digoxigenin peroxidase conjugate, followed by the peroxidase substrate (39-diaminobenzidine tetrahydrochloride). Finally, the sections were counterstained with haematoxylin for 10 s. As a negative control, a number of tumour samples were subjected to treatment without TdT. The numbers of apoptotic cells were determined by counting labelled cells in × 400 magnification, in randomly selected fields and expressed as a percentage of apoptotic cells/total cells counted. Only stained cells displaying morphological features of apoptosis were considered apoptotic.
The mitotic index was assessed in haematoxylin and eosin-stained tumour samples also as a percentage of mitotic cells/total cells counted. Cells displaying mitotic characteristics were considered mitotic.
RT-PCR
The RT-PCR was performed as described by Čikoš et al. (2007) with modifications. When the cells attained 80% confluence, they were incubated with a medium without serum for additional 48 h. They were washed with PBS and total RNA was extracted by incubation with Tri reagent for 5 min at 30° C, according to the manufacturer's instructions. Chloroform (0.2 mL) was added per millilitre of initial extract. These samples were incubated at 30 °C for 3 min and centrifuged again at 10 464 g for 15 min at 4 °C. The aqueous phase was then incubated with cold isopropanol for 10 min at 30 °C and centrifuged again at 10 464 g for 10 min. The RNA pellet was washed with 75% ethanol, centrifuged at 3360 g for 5 min, and resuspended in diethylpyrocarbonate-treated (DEPC) water and the concentration was measured with a spectrophotometer. The RNA was reverse transcribed at 42 °C for 15 min in 20 μL containing 200 U of Superscript II reverse transcriptase, 4 mM oligo-dT18, 50 mM Tris-HCl pH 8.3, 3 mM MgCl2, 75 mM KCl, 10 mM dithiothreitol, 500 mM dNTPs (dATP, dTTP, dCTP and dGTP), 40 U RNase OUT (recombinant RNase inhibitor). The reaction was terminated by heating at 99 °C for 5 min and resting for 5 °C for 5 min.
PCR amplification was carried out in the presence of 30 μM of each oligonucleotide primer, 50 mM KCl, 10 mM Tris-HCl pH 8.3, 2 mM MgCl2, 0.2 mM dNTPs (dATP, dTTP, dCTP and dGTP) and 0.05 U mL−1 Taq DNA polymerase. cDNA (5 μL) was amplified in 20 μL PCR mix.
An initial denaturation step at 95 °C for 2 min was followed by 35 cycles at 95 °C for 35 s, 60 °C for 35 s and 72 °C for 1 min, followed by a unique incubation at 72 °C for 8 min. To check for the presence of cross-contamination, the reaction with water instead of cDNA was performed concurrently (control). Primers for the detection of mouse α2-adrenoceptors have been described and validated by Čikoš et al. (2007). α2A-Adrenoceptor: 5′-TTCTTTTTCACCTACACGCTCA-3′ and 5′-TGTAGATAACAGGGTTCAGCGA-3′, which amplifies a sequence of 112 bp; α2B-adrenoceptor: 5′-ACCTTCCCTTGCTGACTGTACT-3′ and 5′-TGGGAGGGAGGTATTCTAATCA-3′, also amplifying a sequence of 112 bp and α2C-adrenoceptor: 5′-GGCTGTGAACTTAGGGCTTTAG-3′ and 5′-ATAGGAAGTCAGCCCTTGCTC-3′, which amplifies a sequence of 105 bp. For β-actin transcript detection, β-actin primers (5′-GTGGGCCGCTCTAGGCACCAA-3′ and 5′-CTCTTTGATGTCACGCACGATTTC-3′), giving a 539-bp PCR product, served as a control for RNA integrity and the RT-PCR process. The PCR products were analysed using electrophoresis on a 9% polyacrylamide gel stained with silver nitrate. A 100-bp DNA ladder was used as a marker to determine the size of the PCR products.
Statistical analysis
Statistical analysis for the effect of agonists/antagonists and tumour growth was performed by ANOVA followed by Tukey–Kramer tests. For the apoptotic and mitotic indexes, values were transformed to logarithms and analysed by ANOVA followed by Dunnett's test against the control value (Dowdy and Wearden, 1983).
Drugs and chemicals
Foetal calf serum, culture media, antibiotics, collagenase, trypsin, Superscript II reverse transcriptase, RNase OUT (recombinant RNase inhibitor), Taq DNA polymerase and DNA ladder were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Glutamine, clonidine-HCl, yohimbine-HCl and rauwolscine-HCl were purchased from ICN Biomedicals Inc. (Costa Mesa, CA, USA). Methyl [3H]thymidine (NET 027E; specific activity: 20 Ci mmol−1) was from Dupont-New England Nuclear (Boston, MA, USA). Liquid scintillation cocktail was Optiphase ‘Hisafe' 3 (Wallac, Turku, Finland). All other reagents, including culture media were purchased from Sigma-Aldrich. Anti-human α2B (H-96) rabbit and α2C-RA (C-20) goat polyclonal antibodies were from Santa Cruz Biotechnology, whereas α2A (A-271) was from Sigma-Aldrich. Secondary antibodies, peroxidase conjugates and the catalysed signal amplification system (LSAB+system) were from Dako Cytomation (Carpinteria, CA, USA). Vectashield H-1000 was from Vector Laboratories (Burlingame, CA, USA). ApopTag plus peroxidase in situ apoptosis detection kit S7101 was from Chemicon International (Temecula, CA, USA); proteinase K from Fermentas Life Science (Burlington, ON, Canada); Tri Reagent from Molecular Research Center Inc. (Cincinnati, OH, USA).
Drug and receptor nomenclature
Drug and receptor nomenclature conforms to the Guide to Receptors and Channels (Alexander et al., 2008).
Results
Immunocytochemistry, immunofluorescence and RT-PCR of α2-adrenoceptor
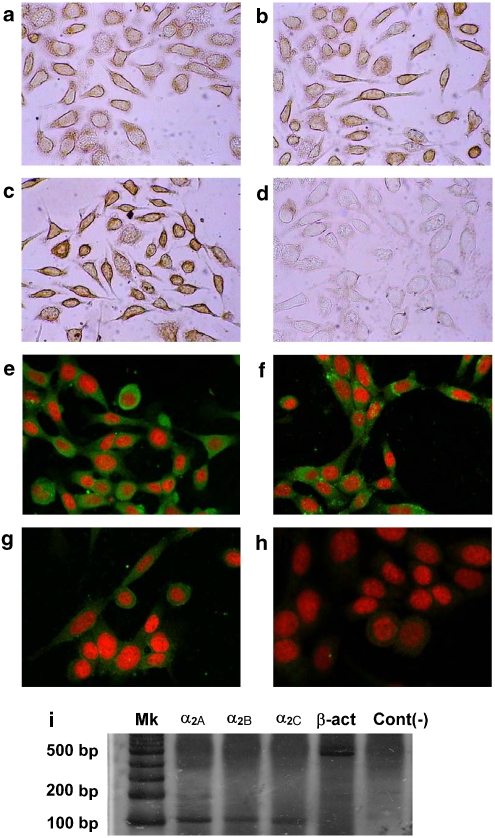
Previous study from our laboratory (Vazquez et al., 2006) showed that in cell lines from human breast cancer, α2B- and α2C-adrenoceptors were expressed in the majority of the cell lines analysed. In the present study, the expression of the different subtypes of α2-adrenoceptor was assessed by immunocytochemistry and confocal immunofluorescence microscopy in a mouse mammary tumour cell line. It may be concluded that α2-adrenoceptors are expressed by MC4-L5 cells. Although the immunocytochemistry showed that the α2A-adrenoceptors (Figure 1a) and α2B-adrenoceptors (Figure 1b) were localized at the cell membrane, even if the extensions of the cells are devoid of expression, the more precise confocal immunofluorescence microscopy (Figures 1e and f) showed that the distribution of these receptors was widely uniform in the cytoplasm and even in the nucleus. However, no colocalization of propidium iodide and α2-adrenoceptors was evident in any of the micrographs. As expected, the expression of the α2C-adrenoceptor was spread throughout the cell, both in the cytoplasm and the plasma membrane (Figures 1c and g). Figures 1d and h depict the negative controls. As the specificity of the commercially available polyclonal antibodies is not perfect to assess the different α2-adrenoceptor subtypes, their expression was assessed by semiquantitative RT-PCR. As can be seen in Figure 1i, all the subtypes were expressed in the cell line, confirming the results from immunocytochemistry and immunofluorescence.
Figure 1.
Immunocytochemistry (a–d), confocal immunofluorescence (e–h) and reverse transcription (RT)-PCR (i) of α2-adrenoceptors on MC4-L5 cells. Immunocytochemistry and confocal immunofluorescence were performed as stated in Materials and methods. Commercial specific polyclonal antibodies against α2A- (a, e), α2B- (b, f) or α2C-adrenoceptors (c, g) or controls (d, h) in the absence of primary antibody. Different controls were performed for the different secondary antibodies, but only one is shown for simplicity. For immunocytochemistry, the antibodies were used at a concentration of 1:100 for α2A, 1:50 for α2B and 1:30 for α2C-, whereas for immunofluorescence the concentration was 1:50. Original magnification was × 400. A representative result from three similar experiments. RT-PCR was performed as stated in Materials and methods. Silver-stained polyacrylamide electrophoresis of PCR products is shown in (i). A representative result from two similar experiments.
Tumour growth experiments
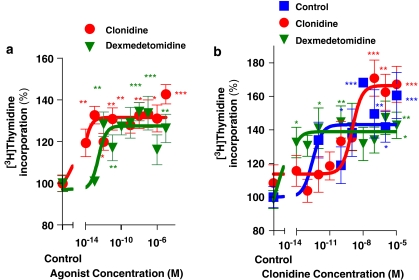
To assess whether the mitogenic effect of α2-adrenoceptor agonists previously found in human breast cancer cells was unique to that model, the mouse mammary tumour cell line MC4-L5 was incubated for 2 days in the presence of these agonists. As can be seen in Figure 2a, both clonidine and dexmedetomidine significantly enhanced [3H]thymidine incorporation into these epithelial cells. The EC50 values of 4.4 fM for clonidine and 0.263 pM for dexmedetomidine showed the cells to be highly sensitive to these compounds.
Figure 2.
(a) Effect of the α2-adrenoceptor agonists clonidine and dexmedetomidine on [3H]thymidine incorporation into the mouse mammary tumour cells MC4-L5. Proliferation analysis was performed as described in Materials and methods. The cell line MC4-L5 was incubated with increasing concentrations of clonidine or dexmedetomidine. The value obtained in the absence of agonist was considered 100% for each cell line, expressing the values relative to this. Values are the mean±s.e.mean of 8 wells for treated and 16 for untreated cultures. *P<0.05, **P<0.01 and ***P<0.001 represent significant differences between groups, as analysed by ANOVA followed by Tukey–Kramer multiple test; n=2. (b) Sensitivity of primary cultures from mouse mammary tumours treated in vivo with clonidine, dexmedetomidine or saline solution (shown in Figure 3) to the agonist clonidine. Tumours from control-, clonidine- or dexmedetomidine-treated animals in primary culture were incubated with increasing concentrations of clonidine, as detailed in Materials and methods. The value obtained in the absence of agonist was considered 100% for each cell line, expressing the values relative to this. Values are the mean±s.e.mean of 8 wells for treated and 16 for untreated cultures. *P<0.05, **P<0.01 and ***P<0.001 represent significant differences between groups, as analysed by ANOVA followed by Tukey–Kramer multiple test; n=2.
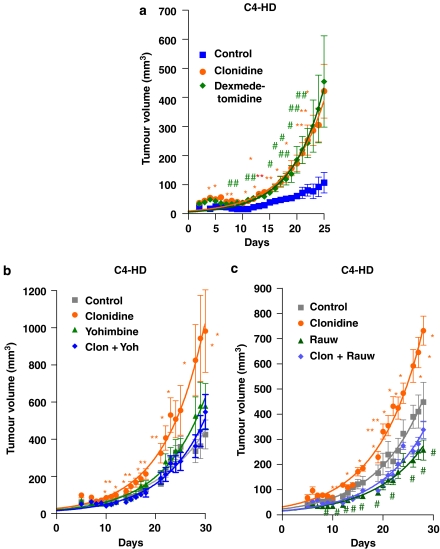
The action of these drugs in vivo was then investigated. For the first experiment, the progestin-dependent C4-HD tumour was used. As this tumour regresses in the absence of progestins, a depot preparation of MPA was injected into the animals. Figure 3a shows a significant stimulation of tumour growth even in the presence of MPA for both α2-adrenoceptor agonists. As the tumours could have lost sensitivity to the agonist during treatment, in vitro sensitivity to the α2-adrenoceptor agonist clonidine of the different primary cultures treated ex vivo with the agonists was studied (Figure 2b). As can be seen in this figure, the cells obtained from culturing the tumours of animals with saline treatment showed an EC50 value of 0.287 pM, the culture from dexmedetomidine-treated tumours showed an EC50 value of 1.08 fM, and the EC50 value of tumours from animals treated with clonidine was 1.02 nM. These results suggest that although there is an ex vivo regulation of receptor concentration, the tumours after 25 days of treatment continue to respond to the agonists.
Figure 3.
Effect of different treatments on mouse mammary tumour C4-HD volume. (a) Effect of the α2-adrenoceptor agonists clonidine and dexmedetomidine. (b) Effect of the agonist clonidine and the antagonist yohimbine and the combination of both. (c) Effect of the agonist clonidine and the antagonist rauwolscine and the combination of both. The tumours were inoculated and measured as detailed in Materials and methods. (a) The animals received medroxyprogesterone acetate (MPA) depot and a daily injection of clonidine (0.1 mg kg−1), dexmedetomidine (0.05 mg kg−1) or vehicle (saline solution). The animals in (b) were inoculated daily with clonidine (0.1 mg kg−1), yohimbine (0.5 mg kg−1), both of them simultaneously or vehicle alone (saline solution). The animals in (c) were inoculated daily with clonidine (0.1 mg kg−1), rauwolscine (0.5 mg kg−1), both of them simultaneously or vehicle alone (saline solution). The points are the mean+s.e.mean. The significantly different values (as analysed by ANOVA followed by Tukey–Kramer multiple test) are marked in the graph (for clonidine, *P<0.05 and **P<0.01 with respect to control, for rauwolscine, #P<0.05 with respect to control). The experiment was performed twice with similar results.
As the main objective of the present study was to investigate the possibility of using an α2-adrenoceptor antagonist as adjuvant therapy for cancer, two different known antagonists were assessed in vivo. As shown in Figure 3b, yohimbine was able to reverse the significant increase in tumour growth caused by the agonist. However, when yohimbine was given alone, a nonsignificant, but noticeable, increase in this parameter was observed. In Figure 3c, the effect of another antagonist was tested. Rauwolscine not only reversed the agonist effect but also diminished tumour growth in the absence of clonidine, behaving as an inverse agonist.
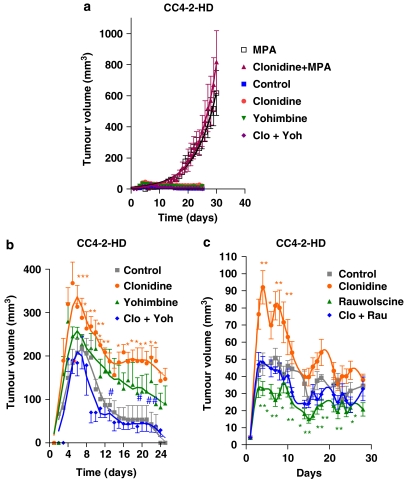
Then, another type of tumour, a slightly less progestin-dependent tumour (CC4-2-HD) was used. Figure 4a depicts the action of clonidine in the presence of MPA. As shown in this figure, in this tumour, the administration of clonidine had no effect in the presence of the progestin. However, we then injected the tumour fragments without the addition of MPA, which might interact with the adrenoceptor agonists/antagonists. The growth curves obtained with this tumour in the absence of MPA show an initial peak of growth followed by a decrease in tumour volume up to a few cells that are not detectable by palpation. However, daily administration of the α2-adrenoceptor agonist clonidine significantly enhanced tumour growth during the peak in which growth is observed. Although the simultaneous injection of the α2-adrenoceptor antagonist yohimbine completely reversed the effect of the agonist (Figure 4b), the group receiving the antagonist alone showed a nonsignificant but noticeable increase in tumour growth. Again, when rauwolscine was tested (Figure 4c), this compound was able to completely reverse the agonist's effect, but when given alone a significant inhibition of tumour growth was observed.
Figure 4.
Effect of different treatments on mouse mammary tumour CC4-2-HD volume. Tumours were inoculated and measured as detailed in Materials and methods. Animals were injected daily with clonidine (0.1 mg kg−1), yohimbine (0.5 mg kg−1), both of them simultaneously or vehicle (saline solution) in (a, b) or clonidine (0.1 mg kg−1), rauwolscine (0.5 mg kg−1), both of them simultaneously or vehicle (saline solution) in (c). A pellet of MPA (depot preparation) was given to two groups in (a). Points represent the mean±s.e.mean. The significantly different points (as analysed by ANOVA followed by Tukey–Kramer multiple test) are marked in the graph (for clonidine, *P<0.05 and **P<0.01 with respect to control, for yohimbine or rauwolscine, #P<0.05, ##P<0.01 and ###P<0.001 with respect to control). The experiment was performed twice with similar results.
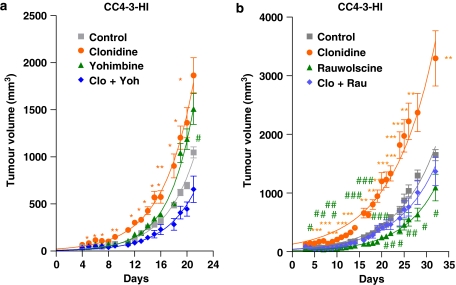
The next step in this investigation was the study of the effect of these antagonists on a progestin-independent tumour (CC4-3-HI), in the absence of MPA. As can be observed in Figure 5a, the administration of clonidine to these animals caused a significant enhancement in tumour growth during all the experiment, whereas the simultaneous administration of the antagonist yohimbine reversed the effect. However, again, yohimbine stimulated tumour growth, although this enhancement was significant only at day 20, the pattern was found throughout the experiment. This tumour growth precludes the utilization of this antagonist as adjuvant therapy. When the effect of rauwolscine was analysed (Figure 5b), this compound completely reversed the agonist's effect. Moreover, the administration of the antagonist alone significantly diminished tumour growth throughout the time tested. This antagonist behaved as a reverse agonist in every tumour tested, making it a suitable candidate compound for adjuvant therapy.
Figure 5.
Effect of the α2-adrenoceptor agonist clonidine and the α2-adrenoceptor antagonist yohimbine (a) or rauwolscine (b) on mouse mammary CC4-3-HI tumour volume. Tumours were inoculated and measured as detailed in Materials and methods. Animals were injected daily with (a) clonidine (0.1 mg kg−1), yohimbine (0.5 mg kg−1), both of them simultaneously or vehicle (saline solution); (b) clonidine (0.1 mg kg−1), rauwolscine (0.5 mg kg−1), both of them simultaneously or vehicle (saline solution). Points represent the mean±s.e.mean. The significantly different points (as analysed by ANOVA followed by Tukey–Kramer multiple test) are marked in the graph (for clonidine, *P<0.05, **P<0.01 and ***P<0.001 with respect to control, for yohimbine or rauwolscine, #P<0.05, ##P<0.01 and ###P<0.001 with respect to control). The experiment was performed twice with similar results.
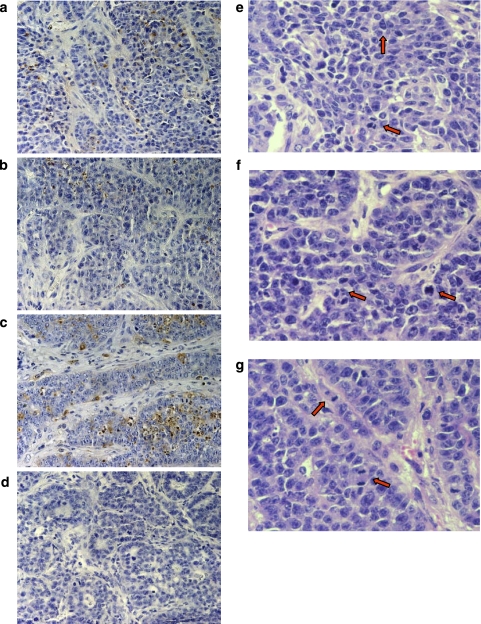
To investigate the mechanism of action of clonidine and rauwolscine in tumour growth, CC4-3-HI tumours were analysed by TUNEL to quantify the percentage of apoptotic cells. Figure 6 depicts a complete field for tumours from control (panel a), clonidine- (panel b) and rauwolscine-treated (panel c) animals and analysed by TUNEL, as well as the negative control (panel d). Table 1 shows the quantification of stained cells showing morphological features of apoptosis. As can be seen in Table 1, clonidine caused a marked though not significant inhibition in apoptosis index, whereas rauwolscine alone caused a nonsignificant but noticeable increase in this parameter. On the other hand, the assessment of mitotic index showed a parallel highly significant enhancement of this parameter in the tumours from clonidine-treated animals (panel e) and a significant decrease in tumours from animals treated with rauwolscine alone (panel f) with respect to control animals (panel g). These results suggest that the agonist (clonidine) exerts its enhancement on tumour growth by enhancing cell proliferation and lowering cell apoptosis, whereas the inverse agonist rauwolscine exerts its protective action both by enhancing apoptosis and reducing cell proliferation.
Figure 6.
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP digoxigenin nick-end labelling (TUNEL) immunohistochemistry for apoptosis (a–d) and hematoxilin and eosine for mitosis (e–g) of CC4-3-HI tumours from control, clonidine- and rauwolscine-treated mice. Tumours were inoculated and animals were treated as stated in Materials and methods. Animals were injected daily with clonidine (b, f; 0.1 mg kg−1), rauwolscine (c, g; 0.5 mg kg−1) or vehicle (a, e; saline solution). A negative control in the absence of TdT was included (d). For mitosis index, haematoxylin and eosin-stained samples were analysed. The red arrows indicate examples of mitosis.
Table 1.
Mitotic and apoptotic indexes of CC4-3-HI tumours
| Treatment | Apoptotic index (mean±s.e.mean) | Mitotic index (mean±s.e.mean) |
|---|---|---|
| Control | 8.67±1.05 | 0.934±0.091 |
| Clonidine | 6.84±1.84 | 2.14±0.192** |
| Rauwolscine | 10.36±1.50 | 0.650±0.053* |
*P<0.05.
**P<0.01 by transformation to logarithms and ANOVA followed by Dunnett's test against control values.
Tumours from control, clonidine- and rauwolscine-treated animals were processed as stated in Materials and methods. The apoptotic index was expressed as the percentage of stained cells (by terminal deoxynucleotidyl transferase-mediated dUTP digoxigenin nick-end labelling (TUNEL) with morphological features for apoptosis with respect to total cells per field. Mitotic index was expressed likewise in haematoxylin and eosin-stained samples.
Discussion and conclusions
Immunocytochemistry, immunofluorescence and RT-PCR of α2-adrenoceptors
The first step in the investigation of the α2-adrenoceptor effect was to assess whether α2-adrenoceptors were effectively expressed in this model. The immunocytochemistry and immunofluorescence can give a preliminary indication whether the receptors are expressed. These studies indicated that α2-adrenoceptors were expressed in the mouse mammary MC4-L5 cell line. α2-Adrenoceptor subtypes have been described to differ in their trafficking itineraries in cells, with α2A- and α2B-adrenoceptors mainly targeted to the plasma membrane and α2C-adrenoceptors primarily localized in an intracellular membrane compartment, from where they can translocate to the cell surface (Philipp et al., 2002). In the present study, the α2A- and α2B-adrenoceptors were found in the cell membrane and throughout the cytoplasm, whereas the α2C-adrenoceptors were extensively distributed throughout the cytoplasm. However, the G-protein-coupled receptors are synthesized in the endoplasmic reticulum, transported to the Golgi apparatus for post-translational modification (for example, glycosylation), and then moved on to their functional destinations in the plasma membrane (Zhou et al., 2006). If there is some kind of cross-reaction between receptor subtypes it would seem that the α2C-adrenoceptor antibody does not cross-react significantly with the other subtypes, because its cellular location is different as assessed by confocal immunofluorescence. As the antibody subtype specificity is not absolute for those receptors, α2-adrenoceptor expression was confirmed by RT-PCR. This technique gave identical indication of expression of all three subtypes of receptor, although the α2A-subtype seems to have a higher expression in this semiquantitative measurement.
Tumour growth experiments
The effect of two different α2-adrenoceptor agonists was assessed in this cell line to analyse whether the effect previously found in human tumour and non-tumour breast cell lines (Vazquez et al., 2006) was a special feature of these cells or was a more general action. The α2-adrenoceptor agonists clonidine and dexmedetomidine significantly stimulated [3H]thymidine incorporation into MC4-L5 cell nuclei. These cells exhibit high sensitivity to these compounds, similar to the one described previously for human cells. It should be noticed that these experiments were performed with specific agonists. With the natural adrenaline and noradrenaline, human cell lines were less sensitive (Vazquez et al., 1999). In this investigation, the natural compounds were not tested because of their binding to β-adrenoceptors. The expression of these receptors has been described both in human and rat mammary tumours (Marchetti et al., 1990; Vandewalle et al., 1990; Badino et al., 1996; Slotkin et al., 2000). The cell proliferation induced by the α2-adrenoceptor agonists could be related to the results recently reported for a putative pathway by which α2-adrenoceptors activate MAPK and Akt in PC12 cells (Karkoulias et al., 2006). Moreover, in these cells, the α2-adrenoceptor subtypes cause phosphorylation and activation of CREB through a pathway involving stimulation of PLC, AA release, generation of epoxygenase derivative and increase of PKA activity (Karkoulias et al., 2007). α2A-Adrenoreceptors activate ERK and Akt in intestinal cells by a common pathway that depends on PI3 kinase activation and results from EGF receptor transactivation (Buffin-Meyer et al., 2007).
MPA-induced tumours were chosen as experimental model because they allow the evaluation of in vivo actions of the α2-adrenoceptors on tumour growth. The C4-HDs are MPA-dependent tumours, which produced the cell line previously used and they regress in the absence of progestins. When the C4-HD tumour growth was analysed with daily injections of the agonists in the presence of MPA depot, a significant enhancement of this parameter was found throughout the experiment, paralleling the in vitro effect. The sensitivity to the agonist clonidine was evaluated in primary culture of tumours from animals receiving different treatments to assess whether the tumours remained sensitive after the treatment. The different groups showed diverse EC50 showing an ex vivo regulation of sensitivity, but remaining responsive to the agonist.
Since the 1980s, clonidine has been used as an adjuvant to local anaesthetics in various regional techniques to extend the duration of the block (Duma et al., 2005). It has been suggested as an alternative to hormone replacement therapy in the treatment of hot flushes (Angelopoulos et al., 2004). Following the publication of the Women's Health Initiative study (Rossouw et al., 2002), use of clonidine increased substantially among elderly women, at least in Ontario, Canada, whereas similar trends were not observed in the use of other antihypertensive medication (Austin et al., 2004). Clonidine is also a recommended therapy for the treatment of attention deficit hyperactive disorder in children (Spiller et al., 2005). Dexmedetomidine is an α2-adrenoceptor agonist that, in spite of its potent sedative, amnesic and analgesic properties, has a minimal respiratory depressant effect (Ho et al., 2005). Dexmedetomidine is also indicated for sedation of patients receiving mechanical ventilation in the intensive care unit. It has additional off-label uses for co-administration with local, regional and general anaesthesia (Rich, 2005). Dexmedetomidine has been described to show subtype specificity for α2B-adrenoceptor internalization (Olli-Lahdesmaki et al., 2004), but displaying in another experimental model, a 5- and 11-fold higher affinity for the α2A-adrenoceptor over the α2B- and α2C-adrenoceptor subtypes (Ma et al., 2004). The results shown here, even if they involve a murine experimental model, draw attention to the extensive use of these α2-adrenoceptor agonists at least in breast cancer patients.
The possibility of inhibition of tumour growth by α2-adrenoceptor antagonist was then tested with three different tumours: the C4-HD (highly progestin dependent), CC4-2-HD (less progestin dependent) and the CC4-3-HI (a progestin-independent tumour). The administration of clonidine caused a significant enhancement in tumour growth throughout the experiment in every case. This enhancement was effectively reversed by the specific α2-adrenoceptor antagonist yohimbine. However, in the absence of an agonist, this antagonist caused a constant although not significant enhancement in growth. This enhancement made the use of this compound dangerous for the possibility of an adjuvant therapy for breast cancer patients. Another specific antagonist, rauwolscine, was tested for its action on tumour growth. This antagonist significantly reversed the effect in every tumour tested. Moreover, this antagonist, in the absence of an agonist, significantly reduced tumour growth, behaving as an inverse agonist. Inverse agonists are thought to reduce the functional activity of the receptors below the baseline activity observed in the absence of any ligand (Murrin et al., 2000). In a model of rat brain tissue sections, rauwolscine was not found to exert inverse agonist actions (Murrin et al., 2000). However, in stable Chinese hamster ovary cell lines expressing the porcine α2A-adrenoceptor, rauwolscine behaved as the best inverse agonist (Wade et al., 2001). A similar inverse agonism was described for this compound in membranes from PC-12 cells, which stably expressed the cloned α2A-adrenoceptor (Tian et al., 1994, 2000). However, as a weak activity of an agonist at the serotonin autoreceptor of the rabbit brain cortex (Limberger et al., 1986; Molderings et al., 2006), the interaction of rauwolscine with these receptors or others cannot be excluded. To our knowledge, this is the first report of rauwolscine acting as an inverse agonist, in vivo.
To have an indication of the mechanism of action of the inverse agonist rauwolscine, the CC4-3-HI tumours were analysed for the apoptotic and mitotic indexes. The last technique probably underestimates the quantity of cells in mitosis because only cells with visible chromosomes were counted. The results shown here indicate that the α2-adrenoceptor agonist clonidine caused a very significant increase in cell proliferation while inhibiting apoptosis (although this effect was not significant), whereas the inverse agonist rauwolscine acted by significantly inhibiting cell proliferation and causing a marked although nonsignificant enhancement of apoptosis. Although other mechanisms, such as necrosis, cannot be ruled out with these experiments, it can be concluded that at least these two mechanisms act in concert inhibiting tumour growth.
Women who are diagnosed with breast cancer are at high risk of experiencing significant emotional distress, with a subgroup manifesting symptoms of anxiety and depression that meet the criteria for psychiatric disorder for up to several years after the diagnosis. We have found an increase in thymidine incorporation by adrenaline and noradrenaline into MCF-7 breast cancer cells (Vazquez et al., 1999) and by the specific α2-adrenoceptor agonist clonidine in several tumour and non-tumour human breast cancer cell lines (Vazquez et al., 2006). We demonstrate here a significant enhancement of mouse mammary tumour growth by the specific α2-adrenoceptor agonist clonidine. The possibility of blocking this α2-adrenoceptor-mediated tumour enhancement by hormones released during stress could be an interesting adjuvant therapy for breast cancer patients. The growth-inhibiting action of the specific α2-adrenoceptor antagonist rauwolscine in this experimental model makes it an excellent candidate for adjuvant therapy of this kind.
Acknowledgments
This study was supported by the ‘Agencia Nacional de Promoción Científica y Tecnológica' and ‘Consejo Nacional de Investigaciones Científicas y Técnicas' (CONICET), Argentina. AB, CPP, LFC and PR (postdoctoral fellow) are CONICET fellows; MGS is a fellow from the ‘Agencia Nacional de Promoción Científica y Tecnológica'. CL and IAL are members of the Research Career, CONICET, Argentina. We gratefully acknowledge the technical help of Ms Julieta Bolado and the discussion and suggestions from Drs Carlos Davio, Juan Pablo Cerliani and Mariela Bilotas.
Abbreviations
- DMEM:F12
Dulbecco's modified Eagle's medium:Ham's F12 medium
- FCS
foetal calf serum
- MPA
medroxyprogesterone acetate
- PBS
phosphate-buffered saline
- RT
reverse transcription
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelopoulos N, Barbounis V, Livadas S, Kaltsas D, Tolis G. Effects of estrogen deprivation due to breast cancer treatment. Endocr Relat Cancer. 2004;11:523–535. doi: 10.1677/erc.1.00783. [DOI] [PubMed] [Google Scholar]
- Austin PC, Mamdani MM, Tu K. The impact of the Women's Health Initiative study on incident clonidine use in Ontario, Canada. Can J Clin Pharmacol. 2004;11:e191–e194. [PubMed] [Google Scholar]
- Badino GR, Novelli A, Girardi C, Di Carlo F. Evidence for functional beta-adrenoceptor subtypes in CG-5 breast cancer cell. Pharmacol Res. 1996;33:255–260. doi: 10.1006/phrs.1996.0036. [DOI] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffin-Meyer B, Crassous PA, Delage C, Denis C, Schaak S, Paris H. EGF receptor transactivation and PI3-kinase mediate stimulation of ERK by alpha(2A)-adrenoreceptor in intestinal epithelial cells: a role in wound healing. Eur J Pharmacol. 2007;574:85–93. doi: 10.1016/j.ejphar.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Čikoš S, Rehak P, Czikkova S, Vesela J, Koppel J. Expression of adrenergic receptors in mouse preimplantation embryos and ovulated oocytes. Reproduction. 2007;133:1139–1147. doi: 10.1530/REP-07-0006. [DOI] [PubMed] [Google Scholar]
- Dowdy S, Wearden S. Statistics for Research. John Wiley & Sons Inc: New York; 1983. [Google Scholar]
- Duma A, Urbanek B, Sitzwohl C, Kreiger A, Zimpfer M, Kapral S. Clonidine as an adjuvant to local anaesthetic axillary brachial plexus block: a randomized, controlled study. Br J Anaesth. 2005;94:112–116. doi: 10.1093/bja/aei009. [DOI] [PubMed] [Google Scholar]
- Ho AM, Chen S, Karmakar MK. Central apnoea after balanced general anaesthesia that included dexmedetomidine. Br J Anaesth. 2005;95:773–775. doi: 10.1093/bja/aei263. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources Commission on Life Sciences, National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press: Washington, DC; 1996. [Google Scholar]
- James GD, Berge-Landry HH, Valdimarsdottir HB, Montgomery GH, Bovbjerg DH. Urinary catecholamine levels in daily life are elevated in women at familial risk of breast cancer. Psychoneuroendocrinology. 2004;29:831–838. doi: 10.1016/S0306-4530(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Karkoulias G, Mastrogianni O, Lymperopoulos A, Paris H, Flordellis C. Alpha(2)-adrenergic receptors activate MAPK and Akt through a pathway involving arachidonic acid metabolism by cytochrome P450-dependent epoxygenase, matrix metalloproteinase activation and subtype-specific transactivation of EGFR. Cell Signal. 2006;18:729–739. doi: 10.1016/j.cellsig.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Karkoulias G, Mastrogianni O, Papathanasopoulos P, Paris H, Flordellis C. Alpha(2)-adrenergic receptors activate cyclic AMP-response element-binding protein through arachidonic acid metabolism and protein kinase A in a subtype-specific manner. J Neurochem. 2007;103:882–895. doi: 10.1111/j.1471-4159.2007.04852.x. [DOI] [PubMed] [Google Scholar]
- Knaus AE, Muthig V, Schickinger S, Moura E, Beetz N, Gilsbach R, et al. Alpha(2)-adrenoceptor subtypes––unexpected functions for receptors and ligands derived from gene-targeted mouse models. Neurochem Int. 2007;51:277–281. doi: 10.1016/j.neuint.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Lamb C, Simian M, Molinolo A, Pazos P, Lanari C. Regulation of cell growth of a progestin-dependent murine mammary carcinoma in vitro: progesterone receptor involvement in serum or growth factor-induced cell proliferation. J Steroid Biochem Mol Biol. 1999;70:133–142. doi: 10.1016/s0960-0760(99)00108-9. [DOI] [PubMed] [Google Scholar]
- Lanari C, Kordon E, Molinolo A, Pasqualini CD, Charreau EH. Mammary adenocarcinomas induced by medroxyprogesterone acetate: hormone dependence and EGF receptors of BALB/c in vivo sublines. Int J Cancer. 1989;43:845–850. doi: 10.1002/ijc.2910430518. [DOI] [PubMed] [Google Scholar]
- Lanari C, Luthy I, Lamb CA, Fabris V, Pagano E, Helguero LA, et al. Five novel hormone-responsive cell lines derived from murine mammary ductal carcinomas: in vivo and in vitro effects of estrogens and progestins. Cancer Res. 2001;61:293–302. [PubMed] [Google Scholar]
- Lanari C, Molinolo AA. Progesterone receptors––animal models and cell signalling in breast cancer. Diverse activation pathways for the progesterone receptor: possible implications for breast biology and cancer. Breast Cancer Res. 2002;4:240–243. doi: 10.1186/bcr539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanari C, Molinolo AA, Pasqualini CD. Induction of mammary adenocarcinomas by medroxyprogesterone acetate in BALB/c female mice. Cancer Lett. 1986;33:215–223. doi: 10.1016/0304-3835(86)90027-3. [DOI] [PubMed] [Google Scholar]
- Limberger N, Bonanno G, Spath L, Starke K. Autoreceptors and alpha 2-adrenoceptors at the serotonergic axons of rabbit brain cortex. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:324–331. doi: 10.1007/BF00500082. [DOI] [PubMed] [Google Scholar]
- Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Fortier MA, Poyet P, Follea N, Pelletier G, Labrie F. Beta-adrenergic receptors in the rat mammary gland during pregnancy and lactation: characterization, distribution, and coupling to adenylate cyclase. Endocrinology. 1990;126:565–574. doi: 10.1210/endo-126-1-565. [DOI] [PubMed] [Google Scholar]
- Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril. 2000;74:760–766. doi: 10.1016/s0015-0282(00)01522-3. [DOI] [PubMed] [Google Scholar]
- Molderings GJ, Bruss M, Gothert M. Functional and molecular identification of 5-hydroxytryptamine receptors in rabbit pulmonary artery: involvement in complex regulation of noradrenaline release. Pharmacol Rep. 2006;58:188–199. [PubMed] [Google Scholar]
- Murrin LC, Gerety ME, Happe HK, Bylund DB. Inverse agonism at alpha(2)-adrenoceptors in native tissue. Eur J Pharmacol. 2000;398:185–191. doi: 10.1016/s0014-2999(00)00317-4. [DOI] [PubMed] [Google Scholar]
- Olli-Lahdesmaki T, Tiger M, Vainio M, Scheinin M, Kallio J. Ligand-induced alpha2-adrenoceptor endocytosis: relationship to Gi protein activation. Biochem Biophys Res Commun. 2004;321:226–233. doi: 10.1016/j.bbrc.2004.06.131. [DOI] [PubMed] [Google Scholar]
- Parkin DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–R295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- Philipp M, Hein L. Adrenergic receptor knockout mice: distinct functions of 9 receptor subtypes. Pharmacol Ther. 2004;101:65–74. doi: 10.1016/j.pharmthera.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Rich JM. Dexmedetomidine as a sole sedating agent with local anesthesia in a high-risk patient for axillofemoral bypass graft: a case report. AANA J. 2005;73:357–360. [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Zhang J, Dancel R, Garcia SJ, Willis C, Seidler FJ. Beta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2000;60:153–166. doi: 10.1023/a:1006338232150. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Klein-Schwartz W, Colvin JM, Villalobos D, Johnson PB, Anderson DL. Toxic clonidine ingestion in children. J Pediatr. 2005;146:263–266. doi: 10.1016/j.jpeds.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Tian WN, Duzic E, Lanier SM, Deth RC. Determinants of alpha 2-adrenergic receptor activation of G proteins: evidence for a precoupled receptor/G protein state. Mol Pharmacol. 1994;45:524–531. [PubMed] [Google Scholar]
- Tian WN, Miller DD, Deth RC. Bidirectional allosteric effects of agonists and GTP at alpha(2A/D)-adrenoceptors. J Pharmacol Exp Ther. 2000;292:664–671. [PubMed] [Google Scholar]
- Vandewalle B, Revillion F, Lefebvre J. Functional beta-adrenergic receptors in breast cancer cells. J Cancer Res Clin Oncol. 1990;116:303–306. doi: 10.1007/BF01612908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzulli S, Efeyan A, Benavides F, Helguero LA, Peters G, Shen J, et al. p21, p27 and p53 in estrogen and antiprogestin-induced tumor regression of experimental mouse mammary ductal carcinomas. Carcinogenesis. 2002;23:749–758. doi: 10.1093/carcin/23.5.749. [DOI] [PubMed] [Google Scholar]
- Vazquez SM, Mladovan AG, Perez C, Bruzzone A, Baldi A, Luthy IA. Human breast cell lines exhibit functional alpha(2)-adrenoceptors. Cancer Chemother Pharmacol. 2006;58:50–61. doi: 10.1007/s00280-005-0130-4. [DOI] [PubMed] [Google Scholar]
- Vazquez SM, Pignataro O, Luthy IA. Alpha2-adrenergic effect on human breast cancer MCF-7 cells. Breast Cancer Res Treat. 1999;55:41–49. doi: 10.1023/a:1006196308001. [DOI] [PubMed] [Google Scholar]
- Wade SM, Lan K, Moore DJ, Neubig RR. Inverse agonist activity at the alpha(2A)-adrenergic receptor. Mol Pharmacol. 2001;59:532–542. doi: 10.1124/mol.59.3.532. [DOI] [PubMed] [Google Scholar]
- Wendel V, Vazquez SM, Durante PC, Lemoine AP, Segura ET, Calandra RS, et al. Prazosin and stress effect on tumoral growth of 7,12-dimethylbenz[A]anthracene-induced rat mammary tumors. Acta Physiol Pharmacol Ther Latinoam. 1996;46:277–285. [PubMed] [Google Scholar]
- Zhou F, Filipeanu CM, Duvernay MT, Wu G. Cell-surface targeting of alpha(2)-adrenergic receptors––inhibition by a transport deficient mutant through dimerization. Cell Signal. 2006;18:318–327. doi: 10.1016/j.cellsig.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]