Abstract
Adenosine A2A receptor agonists may be important regulators of inflammation. Such conclusions have come from studies demonstrating that, (i) adenosine A2A agonists exhibit anti-inflammatory properties in vitro and in vivo, (ii) selective A2A antagonists enhance inflammation in vivo and, (iii) knock outs of this receptor aggravate inflammation in a wide variety of in vivo models. Inflammation is a hallmark of asthma and COPD and adenosine has long been suggested to be involved in disease pathology. Two recent publications, however, suggested that an inhaled adenosine A2A receptor agonist (GW328267X) did not affect either the early and late asthmatic response or symptoms associated with allergic rhinitis suggesting that the rationale for treating inflammation with an adenosine A2A receptor agonist may be incorrect. A barrier to fully investigating the role of adenosine A2A receptor agonists as anti-inflammatory agents in the lung is the side effect profile due to systemic exposure, even with inhalation. Unless strategies can be evolved to limit the systemic exposure of inhaled adenosine A2A receptor agonists, the promise of treating lung inflammation with such agents may never be fully explored. Using strategies similar to that devised to improve the therapeutic index of inhaled corticosteroids, UK371,104 was identified as a selective agonist of the adenosine A2A receptor that has a lung focus of pharmacological activity following delivery to the lung in a pre clinical in vivo model of lung function. Lung-focussed agents such as UK371,104 may be suitable for assessing the anti-inflammatory potential of inhaled adenosine A2A receptor agonists.
Keywords: lung inflammation; adenosine receptors; adenosine A2A receptor agonists; inhalation UK371,104
Introduction
Inflammation is a hallmark of diseases such as asthma and chronic obstructive pulmonary disease (COPD). Inhaled corticosteroids and, to a lesser extent, oral leukotriene D4 antagonists have proven efficacious in asthma, although corticosteroids are much less effective in COPD. A continuing focus of research is to discover new and effective non-steroidal anti-inflammatory agents for asthma and COPD (see Barnes, 2006, 2007). This review article will be focussed on discussing the potential broad-spectrum anti-inflammatory potential of inhaled adenosine A2A receptor agonists for asthma and COPD and will not cover other adenosine receptors. For a broader view on the potential role of adenosine and adenosine receptors in the pathology of asthma and COPD, the reader is referred to the following recent reviews (see, van den Berge et al., 2007; Brown et al., 2008).
Adenosine produces a wide range of biological effects by interacting with four cell surface receptors termed A1, A2A, A2B and A3. These receptors have been identified by both molecular and pharmacological techniques (Fredholm et al., 2001). Of the four receptors, the adenosine A2A receptor has been strongly linked to of control inflammation (see below). The receptor is very widely distributed and agonism of this receptor produces a wide range of physiological responses such as hypotension, inhibition of platelet aggregation and regulation of neurotransmitter release (Fredholm et al., 2001). Indeed A2A receptor antagonists have been proposed to have a potential therapeutic benefit in CNS diseases such as Parkinsons (Schwarzschild, 2007). This suggests that inhalation of adenosine A2A receptor agonists would need to be the preferred route of administration, to achieve efficacy in the lung with an acceptable therapeutic index over systemic side effects.
The article will begin by discussing pre-clinical evidence supporting the anti-inflammatory role of the adenosine A2A receptor in general and in the lung. Within this, mechanisms that mediate the anti-inflammatory effects in vitro and in vivo will be discussed. Subsequently this article will then discuss two recent clinical trials where an inhaled adenosine A2A agonist had no obvious benefit in allergic rhinitis and asthma. The article will conclude with a discussion on the potential reasons for the lack of efficacy in these clinical trials and suggest that a more ‘lung-focussed' adenosine A2A receptor agonist is needed to fully explore the concept. The in vitro and in vivo biology of UK371,104 will be presented as an example of an adenosine A2A agonist with a lung focus of pharmacological activity following intra-tracheal administration in a pre-clinical model of lung function.
The anti-inflammatory potential of adenosine A2A receptor agonists
In vitro evidence
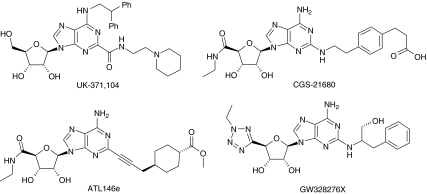
The adenosine A2A receptor is expressed on virtually all cells that are implicated in the inflammatory process such as neutrophils, monocytes, eosinophils, epithelium, endothelium, lymphocytes and NK cells (see Gessi et al., 2000) suggesting that this receptor may play a role in the inflammatory process. Initial evidence promoting the role of the adenosine A2A receptor came from agonists selective for activating this receptor in particular, CGS21680 the first reported agonist which selectively activates this receptor (Hutchinson et al., 1989) and more latterly, other selective A2A receptor agonists such as ATL146e (Sullivan et al., 2001). See Figure 1 for structures of CGS21680 and ATL146e. In vitro experiments have provided a wealth of data that support the broad-spectrum anti-inflammatory potential of adenosine A2A receptor agonists in cells implicated in both COPD and asthma. For instance, in neutrophils adenosine A2A agonists have been demonstrated to inhibit several pro-inflammatory functions such as superoxide, elastase, leukotriene B4, TNFα and VLA4 induction (Sullivan et al., 2001, 2004; Pouliot, 2007). In addition adenosine A2A agonists have been shown to inhibit the phosphorylation of ZAP70, a critical step in T cell activation (Sevigny et al., 2007) and suppresses perforin and FASL-mediated cytotoxicity in lymphokine-activated killer cells (Raskovalova et al., 2006). Inhibitory effects have also been noted on eosinophil secretion (Reeves and Butchers, 1999; Bevan et al., 2007), mast cell migration (Duffy et al., 2007) and on monocyte secretion (Link et al., 2000). In addition adenosine A2A receptor activation has been shown to suppress the pro-inflammatory signalling mechanisms of cytokines, toll-like receptor agonists and LPS in several cell types (for example, Link et al., 2000; Sands et al., 2004, 2006). Further work has also noted that adenosine A2A receptor agonists promote wound healing (Allen-Gipson et al., 2006; Cronstein, 2006).
Figure 1.
Structures of adenosine A2A receptor agonists.
The adenosine A2A receptor is a Gs-linked receptor elevating the intracellular levels of cyclic AMP and by interacting with several cyclic AMP-binding proteins (such as protein kinase A, PKA and exchange proteins directly activated by cyclic AMP-EPAC, see Sands and Palmer, 2008) elicit the broad-spectrum anti-inflammatory effects noted above. In leukocytes, PDE4 is the major phosphodiesterase-controlling cyclic AMP levels in these cells (Houslay et al., 2005). In support of this many publications have documented that the anti-inflammatory effects (for example, inhibition of superoxide release) can be blocked by selective inhibitors of PKA such as H-89 (for example, Sullivan et al., 2001; Raskovalova et al., 2006; Sevigny et al., 2007). In recent years, however, the molecular mechanisms by which adenosine A2A agonists mediate their effects have expanded considerably. For instance adenosine A2A agonists have been shown to inhibit JAK (Janus Kinase)-STAT (signal transducer and activator of transcription) pathway activation by cytokines that utilize gp130 in their signalling mechanism through a PKA-independent mechanism because the effects are not blocked by H-89 but are inhibited with EPAC1 knockdown using siRNA (Sands et al., 2006). In this scheme, cyclic AMP activates EPAC 1 resulting in induction of SOC3 (suppressor of cytokine signalling) with subsequent inhibition of cytokine receptor signalling. Another major mechanism whereby adenosine A2A receptor activation mediates an anti-inflammatory effect is to inhibit the nuclear factor-kappa B pathway (NF-κB) activated by pro-inflammatory agents such as cytokines and LPS independent of parallel pathways such as p38 (Minguet et al., 2005; Sands et al., 2006). The mechanism of A2A-mediated effects on NF-κB differed according to the cell type studies. In glial cells the mechanism involved blockade of IKK-mediated IκBα phosphorylation that triggers degradation. In contrast, in HUVECs suppression of the NF-κB pathway it did not appear to involve IκBκ degradation (Sands et al., 2004). The adenosine A2A receptor can also influence mitogen-activated protein kinases, which have been implicated in a wide variety of cellular responses involved in the inflammation process including apoptosis and several publications have documented ERK1/2 activation in a wide variety of cells. In CHO, cells induction of ERK expression by adenosine A2A receptor activation is inhibited by H-89 but in HEK 293 cells induction appears to be independent of cyclic AMP (see Schulte and Fredholm, 2003). Inflammatory conditions are associated with hypoxia and this is associated with activation of hypoxia-inducible factor 1 (HIF1), inactivation of which leads to an impaired inflammatory response (Cramer et al., 2003). Adenosine A2A receptor activation has been shown to increase HIF levels in macrophages suggesting another potential mechanism whereby adenosine A2A receptors can control leukocyte function in hypoxic conditions (Lukashev et al., 2004a; de Ponti et al., 2007). In addition, pro-inflammatory mediators such as LPS, TNFα and IL1β have been shown to increase the expression of adenosine A2A receptors in endothelium, macrophages and monocytes (Khoa et al., 2001; Nguyen et al., 2003; Murphree et al., 2005).
Two additional features of the adenosine A2A receptor appear important for the broad-spectrum anti-inflammatory properties noted above. First, in contrast to other adenosine receptors, the adenosine A2A receptor has a very long intracellular C-terminal and this has been suggested to be important in controlling the varied intracellular mechanisms of adenosine A2A receptor activation. In addition, compared with other GPCRs, the adenosine A2A receptor is quite resistant to agonist-induced desensitization and thus may aid the pro-inflammatory signalling in vivo (see Zezula and Freissmuth, 2008).
To conclude, the above in vitro evidence clearly identifies that the adenosine A2A receptor has the potential of having an important and broad-spectrum of function in controlling inflammation. Key properties include the wide spectrum of intracellular signalling mechanisms that mediate the broad-spectrum anti-inflammatory actions demonstrated. In addition the upregulation of the receptor by pro-inflammatory mediators and the resistance to agonist-induced desensitization suggest a positive-feedback mechanism to maintain receptor function in the control of inflammation. For more information on this topic the reader is referred to the following reviews (Sullivan and Linden, 1998; Sitkovsky et al., 2004; Sands and Palmer, 2005; Fredholm et al., 2007; Palmer and Trevethick, 2008).
In vivo evidence
Three lines of in vivo experimental evidence targeting the A2A receptor directly in animal models of inflammation–tissue damage support the in vitro observations by demonstrating: (1) application of selective agonists reduces inflammation and tissue damage, (2) administration of an A2A receptor-selective antagonist enhances inflammation following induction of an inflammatory response and (3) inflammation is exaggerated in animals with targeted deletion of the adenosine A2A receptor. Two groups have provided much of the evidence to be reviewed in this section and the reader is referred to publications from Joel Linden (University of Virginia) and Michail Sitkovsky (North Eastern University) and the following reviews (Sitkovsky, 2003; Lappas et al., 2005; Linden, 2005, 2006; Sitkovsky and Lukashev, 2005; Sitkovsky and Ohta, 2005).
Administration of adenosine A2A receptor agonists have been shown to inhibit inflammation and tissue damage in a wide variety of in vivo models studying organs such as gut, heart, lung, liver, kidney, joints and the CNS (see Hasko and Pacher, 2008). Although supportive of the anti-inflammatory properties, use of receptor ligands in vivo has the potential to mislead as the receptor selectivity of these ligands for the corresponding animal adenosine receptors is not presented, making unequivocal conclusion of an A2A receptor mechanism or the target cells for the anti-inflammatory action difficult. Some publications have confirmed the A2A mechanism by demonstrating that either an adenosine A2A antagonist or use of A2A-deficient animals inhibits the protective action of selective adenosine A2A agonists.
Exacerbation of tissue damage and inflammation with A2A receptor antagonists has been shown in a variety of inflammatory models. For instance, treatment of mice with the selective A2A receptor antagonist ZM241385 enhanced liver injury and inflammation in response to concanavalin A, Pseudomonas aeruginosa and carbon tetrachloride (Ohta and Sitkovsky, 2001; Chan et al., 2006). Similarly, ZM241385 prevented both the anti-inflammatory effects and the increased survival rates induced by low-dose ketamine administration, which promotes adenosine accumulation, in mice where sepsis was induced by LPS or Escherichia coli (Mazar et al., 2005). In the mouse lung, treatment with ZM241385 enhanced lung neutrophilia in response to intra-tracheal administration of LPS (Thiel et al., 2005). However, in other models, ZM241385 did not enhance inflammation/tissue damage in response to an inflammatory stimulus (see, for example, Peirce et al., 2001; Fozard et al., 2002). This may reflect either varying amounts of endogenous adenosine in the different models or, alternatively, administration of a near-maximal dose of inflammatory stimulus, which would make it difficult to observe any further enhancement with an A2A receptor-selective antagonist.
In recent years, great impetus to the field of the anti-inflammatory effects of the adenosine A2A receptor has come from the work of Sitkovsky and coworkers using A2A receptor knockout mice. For instance, mice deficient in the A2A receptor displayed an exaggerated inflammatory response and cytokine release in response to a wide variety of inflammatory insults, such as concanavalin A and carbon tetrachloride-induced liver damage and LPS-induced inflammation in an air pouch model (Ohta and Sitkovsky, 2001; Chan et al., 2006). In addition, adoptive transfer studies employing inflammatory cells from mice lacking the A2A receptor have also supported the concept that this receptor functions as a physiological anti-inflammatory mechanism (Yang et al., 2006).
To conclude, pharmacological and molecular techniques support the concept that in an inflammatory environment the A2A receptor functions as a ‘physiological brake' on inflammatory processes and that agonism of this receptor by selective agonists might represent a novel anti-inflammatory mechanism. A number of reviews have been written, highlighting the concept that the A2A receptor is involved in inflammation and tissue repair (for example, Sitkovsky, 2003; Hasko and Cronstein, 2004; Sitkovsky et al., 2004; Linden, 2005; Fredholm, 2007).
Anti-inflammatory role of the adenosine A2A receptor in the lung: pre clinical studies
Despite the strong evidence supporting a role for A2A agonists in controlling inflammation it is only comparatively recently that publications have appeared documenting the anti-inflammatory role of the adenosine A2A receptor in the lung. Fozard et al. (2002) were the first to report that the adenosine A2A receptor was an important regulator of lung inflammation. In this study, intra-tracheal administration of CGS21680 inhibited lung inflammation in brown Norway rats following sensitization and challenge with ovalbumen. This inhibition was measured as a reduction of eosinophils and neutrophils in the broncho–alveolar fluid (BALF) and this was reversed by the A2A receptor antagonist ZM241385, supporting the A2A mechanism of protection. In mouse models of lung inflammation (ovalbumen sensitization and challenge), Bonneau et al. (2006) reported that intranasal administration of CGS21680 inhibited infiltration of neutrophils, eosinophils, macrophages and lymphocytes into BALF. This suppression of inflammation was not associated with any change in hyper-reactivity of the airways as assessed by the action of nebulized methacholine on lung function (Penh) responses. In contrast, CGS21680 had no effect on leukocyte infiltration into the BALF in mice challenged with LPS or cigarette smoke. These latter models are primarily models of neutrophil infiltration and thus it is surprising given the effects on human leukocytes that no inhibition was observed. CGS21680 did, however, inhibit neutrophil activation as measured by inhibition of elastase release into the BALF. In the LPS model CGS21680 did appear to reduce the levels of pro-inflammatory cytokines (TNFα, KC and MIP-2) in the BALF although this did not reach statistical significance. Reasons for this weak effect in the LPS model are not clear but may reflect that higher doses of CGS21680 were needed to show efficacy in this model.
Further support has come from animals deficient in the adenosine A2A receptor (A2A knockouts). In wild-type mice, Reutershan et al. (2007) demonstrated that the selective A2A receptor agonist ATL202 did inhibit LPS-induced neutrophil infiltration into the BALF. This contrasts with the findings of Bonneau et al. (2006) but may reflect methodology differences such as mice used (C57BL/6 versus BALBc) and route of administration of A2A agonists (inhalation versus intranasal). In contrast, the protective effect of ATL202 was not evident in mice deficient in the A2A receptor (Reutershan et al., 2007). Nadeem et al. (2007) demonstrated enhanced airway reactivity and lung inflammation in ragweed-sensitized mice lacking the adenosine A2A receptor. This study demonstrated enhanced infiltration of lymphocytes, eosinophils and neutrophils in BALF of ragweed-challenged sensitized mice lacking the A2A receptor together with hyper-reactive response to inhaled methacholine challenge as a measure by Penh lung function measurements.
Adenosine deaminase-deficient mice have enhanced levels of adenosine and in the lung this leads to a phenotype characterized by acute and chronic inflammation, mucin overproduction and angiogenesis (see Blackburn, 2003) due, in part, to activation of the low affinity adenosine A2B receptor as evidenced by the protective effects of adenosine A2B antagonists in this model (Sun et al., 2006). A counterpart to this should be that activation of the anti-inflammatory adenosine A2A receptor should also lead to inhibition of lung inflammation in these mice. Adenosine deaminase-deficient mice which had genetic deletion of the adenosine A2A receptor exhibited biology consistent with a key role for the adenosine A2A receptor as a modulator of inflammation (Mohsenin et al., 2007). Adenosine deaminase/adenosine A2A (ADA−/− A2A−/−) deficient animals died on average 3 days earlier than animals with normal levels of the A2A receptor (ADA−/− A2A+/+) suggesting that this receptor plays a tissue protective role in this disease pathology in mice. Histology revealed that early death in ADA−/− A2A−/− animals was associated with enhanced pulmonary inflammation (mainly macrophages) and mucin production, together with thickening of bronchial smooth muscle and hypertrophy of bronchial epithelium. The expression of chemokines CXCL1 and MCP1 were elevated in ADA−/− A2A−/−. These agents have been associated with neutrophil and macrophage recruitment respectively and in other in vivo systems are downregulated by A2A receptor agonists (for example, Harada N et al., 2000). Not all pro-inflammatory molecules were elevated in ADA−/− A2A−/− mice, for instance there was no elevation over ADA−/− A2A+/+ in TNFα and IL6 whereas transcripts for CCL11 and CCL17 were lower in ADA−/− A2A−/−. This might suggest that other adenosine receptors might be involved in this ‘adenosine-driven' model of lung inflammation such as the A2B receptor (Sun et al., 2006). An important observation in this study was the effects on angiogenesis. In ADA−/− A2A−/− there was a significant increase in tracheal angiogenesis and in the chemokine CXCL1. The chemokine CXCL1 stimulates angiogenesis and has been suggested to play a role in chronic lung disease such as bronchiolitis obliterans (Belperio et al., 2002).
To conclude, three lines of evidence support the concept that the adenosine A2A receptor can control inflammation in the lung. First is the inhibition of inflammation provided by treatment with selective agonists of the adenosine A2A receptor, second are the observations that lung inflammation is enhanced in animals deficient in the A2A receptor and third are observations that anti-inflammatory effects of the selective A2A agonists CGS21680 and ATL202 are inhibited in A2A receptor knockout animals or by pre-treatment with an A2A receptor antagonist.
Anti-inflammatory role of the adenosine A2A receptor in the lung: potential mechanisms of the anti-inflammatory effect
Four mechanisms have been proposed to explain the anti-inflammatory effects of adenosine A2A agonists demonstrated from in vivo studies although, at present, few of these have been specifically studied in the lung. These are increases in oxygen supply/demand ratio, ischaemic pre-conditioning and post-conditioning, anti-inflammatory responses and angiogenesis (see Linden, 2005).
The anti-inflammatory mechanism can be discussed under two broad categories—first, are the biochemical mechanisms of the anti-inflammatory actions identified in vitro demonstrable in the lung? and second, what are the target cells/mediators, which might be pivotal for the anti-inflammatory actions for agonists of the adenosine A2A receptor?
Few studies in the lung have investigated the signalling mechanisms underlying the anti-inflammatory effects of adenosine A2A receptor agonists. Regulation of NF-κB activity involves nuclear translocation following release from the inhibitor IκBα, which is achieved by phosphorylation and degradation. Mice deficient in the adenosine A2A receptor demonstrated increased phosphorylation of IκBα consistent with removal of the adenosine ‘A2A brake' on nuclear translocation of NF-κB (Nadeem et al., 2007). The increase in lung NF-κB was associated with increased gene expression in the lung of iNOS and NO production together with increase in lipid peroxidation. In support of this, Lukashev et al. (2004b) demonstrated that splenocytes from A2A-deficient mice had increased levels of mRNA for a wide variety of cytokines following in vivo challenge with LPS and this was associated with an increase in NF-κB. In addition, in animals with normal levels of the adenosine A2A receptor, the selective agonist CGS21680 was shown to inhibit an increase in LPS-induced mRNA cytokine expression. These observations in the lung thus support in vitro studies documenting the interaction of the adenosine A2A receptor with the NF-κB pathway.
Mechanistically, however, the anti-inflammatory effect of the adenosine A2A receptor in the lung is monitored through changes in leukocyte and cytokine levels (decreased through A2A agonists and increased in A2A-deficient animals). Two key questions thus arise—what target cells and cytokines are pivotal in the anti-inflammatory mechanism of the adenosine A2A receptor? First though it is worth noting a clear gap in the literature on the anti-inflammatory effects of adenosine A2A agonists on human versus animal leukocytes. Although many publications have clearly documented the anti-inflammatory properties of adenosine A2A agonists on human isolated leukocytes, apart from equine neutrophils and monocytes (Sun et al., 2007, 2008), there are no publications documenting the anti-inflammatory effects of adenosine A2A receptor agonists on isolated neutrophils from common laboratory animals. Indeed our own studies have failed to find any inhibitory effects of adenosine A2A receptor agonists on mediator release from neutrophils isolated from rat, guinea pig, pig and dog stimulated with agents such as fMLP and LPS (results not shown). One publication has shown that CGS21680 produced a very small inhibition of H2O2 release from fMLP-stimulated granulocytes from lungs of mice with lung inflammation but not in neutrophils from non-inflamed environments (Thiel et al., 2005). There are several explanations for this anomaly: (a) SAR for activation of the leukocytes A2A receptor is different to that of human, (b) studies have not measured the correct response in animal neutrophils to assess the effects of A2A agonism or, (c) the receptor has to be induced to be expressed/activated. This latter hypothesis is supported by data from Thiel et al. (2005) although the data is far from conclusive. Thus in animal models of lung inflammation the neutrophil may not be a target cell underlying the anti-inflammatory action of adenosine A2A receptor agonists and other cells must be involved such as monocyte/macrophages where there is evidence of anti-inflammatory effects of adenosine A2A receptor agonists (for example, Kreckler et al., 2006).
Further understanding of the potential cell mediators involved in the anti-inflammatory action of the adenosine A2A receptor agonists has come from studies assessing inflammatory response in other organs following ischaemia-reperfusion. Ischaemia-reperfusion injury (IRI) is associated with tissue damage during ischaemia and further injury following reperfusion. During reperfusion, tissue damage is associated with the generation of free radicals, cytokines, induction of adhesion molecules and infiltration of leukocytes into the tissue. Many studies have shown that the inhibitory action of adenosine A2A agonists on IRI is because of an action of CD4+ T cells in orchestrating the inflammatory process such as neutrophil infiltration. For instance, infarct size in response to IR is smaller in Rag1 knockout mice, which lack mature lymphocytes, and is increased by adoptive transfer of CD4+ T cells from control animals and those lacking the A2AAR but not in those T cells lacking the ability to secrete IFNγ. Importantly, agonism of the A2A receptor, reduced both infarct size and inflammation in Rag1 knockout mice reconstituted with mature T cells but not in those reconstituted with CD4+ T cells lacking the A2AAR (Yang et al., 2006). Likewise, in the kidney the protective effect of the selective adenosine A2A agonist, ATL-146e was inhibited in mice whose bone marrow had been ablated and reconstituted with bone marrow from A2A receptor-deficient animals. Using adoptive transfer techniques, the mechanism of protection of ATL-146e was shown to be because of an action of A2A receptor agonists on CD4+ T cells and involved an inhibition of IFNγ release (Day et al., 2003, 2006). Further mechanistic insights have come from studies of IRI in the liver. Lappas et al. (2006) noted that the rapid induction of reperfusion injury was not consistent with the known time course for activation of conventional CD4+ T cell responses and suggested that injury must therefore be mediated by a rapidly activated T cell subset. Their studies demonstrated that CD1d-dependent NKT type 1 cells were key to initiating IRI. NKT type 1 cells resemble CD4+ Th2 cells and rapidly synthesize and release large quantities of cytokines but have an invariant T cell receptor that recognises glycolipids (see Meyer et al., 2008). Evidence to support this included (a) Depletion of NKT cells or administration of a CD1d-blocking antibody resulted in inhibition of IRI, (b) Transfer of NKT cells to NKT-depleted animals enhanced IRI and (c) neutrophil and IL13 elevations following IRI were significantly reduced in NKT-depleted animals. In this model A2A receptor agonism inhibited IRI but this was prevented in NKT-deficient animals or in animals where NKT cells lacked the adenosine A2A receptor. This was supported by in vitro observations documenting the inhibitory effect of A2A receptor agonism on IL13 production form NKT cells. NKT cells account for a high percentage of liver T cells and thus an action of a tissue-localized cell (NKT type 1) appears to be pivotal for initiating the anti-inflammatory effects of A2A agonists in this model.
CD1d is expressed in the epithelium of airways as well as inflammatory cells suggesting that this mechanism might be involved in the anti-inflammatory effects of adenosine A2A receptor agonists in animal models of lung inflammation. Studies in animals suggest that these cells may be important for airway hyper-responsiveness (AHR) to methacholine as CD1d knockout animals or animals treated with CD1d-blocking antibody inhibited AHR and reduced (but not abolished) the inflammatory response (see Meyer et al., 2008 for a review). In asthma, lung inflammation is dominated by the presence of eosinophils and CD+ T cells and NKT cells have been found in the lung and some, but not all studies, show elevated levels of NKT cells in asthmatics compared with controls (see Ho, 2007; Meyer et al., 2008). Thus NKT cells may play an important role in lung inflammation.
Within the lung, activation of non-myelinated bronchopulmonary C fibres by various stimuli (such as capsaicin) in animal models can induce many of the symptoms of clinical asthma such as bronchoconstriction, apnea, hyper-reactivity, cough and local inflammation (Coleridge and Coleridge, 1984). Likewise, inhalation of capsaicin induces bronchoconstriction and enhances hyper-reactivity in asthmatics. The mechanism is suggested to be through activation of the transient receptor potential (TRP) ion channel, which is expressed in human lung bronchpulmonary C fibres (see Jia and Lu-Yuan, 2007). In anaesthetized guinea pigs, Morimoto et al. (1993) demonstrated that capsaicin-induced increases in bronchoconstriction, substance P release and tracheal plasma extravasation could be inhibited by adenosine A2 agonists such as NECA and CGS21680. This suggests the involvement of the A2A receptor in mediating this inhibitory response. Recent evidence has also shown that the A2A agonist CGS21680 inhibits capsaicin-induced activation of TRPV1 stably expressed in HEK cells (Puntambekar et al., 2004). These data thus suggest that A2A receptor agonism can suppress C fibre activation through inhibition of TRPV1.
There have been several publications documenting the wound-repairing properties of adenosine A2A agonists in many organs including the lung (Allen-Gipson et al., 2006; Cronstein, 2006). Angiogenesis (growth of new blood vessels from pre existing vasculature) is an important mechanism in this process but little work has been published directly examining the lung. Nevertheless, angiogenesis is evident in the tracheas and lungs of patients with lung inflammation (Tanaka et al., 2003) and it has been suggested that this may contribute to airway hyper-reactivity/obstruction and may serve as new routes for inflammatory cell entry and tissue remodeling (Charan et al., 1997). The role of adenosine A2A receptors in controlling lung angiogenesis is not fully elucidated but certainly angiogenesis is increased in adenosine deaminase-deficient mice, which lack the A2A receptor (Mohsenin et al., 2007). In other tissues A2A agonism stimulates angiogenesis and increases the rate at which wounds close through regulation of the angiogenic factor VEGF (for example, Montesinos et al., 2004) but other factors can also be involved such as downregulation of antiangiogenic factors such as thronmbospondin 1 in human umbilical vein endothelium (Desai et al., 2005). In addition, macrophages are converted from a pro-inflammatory phenotype to a pro-angiogenic phenotype that secretes VEGF (Olah and Caldwell, 2003) by the adenosine A2A receptor and this may involve signalling through MyD88 to effect this switch (Macedo et al., 2007). Thus overall there is an emerging evidence about the role of adenosine and the A2A receptor in promoting angiogenesis but more work is necessary to define the effects that this will have in the lung.
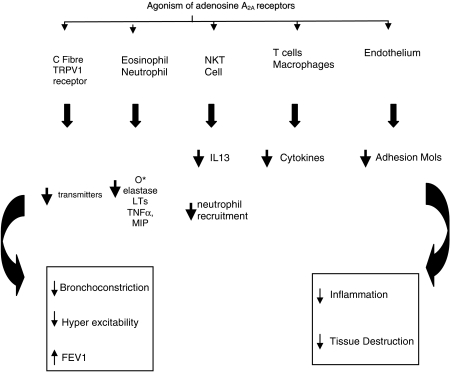
To conclude, by combining data from human isolated leukocytes with in vivo studies in animal models of inflammation, the mechanisms by which adenosine A2A agonists inhibit the inflammatory process is beginning to clarify. Two major mechanisms appear to be involved. First is a direct inhibition of the pro-inflammatory properties of all the major leukocyte groups and recent data from animal models suggest that a tissue-localized cell (such as NKT cells) may be responsible for an early initiation step of the inflammatory process and subsequent recruitment of inflammatory cells into the tissue. Thus in an inflamed lung activation of the adenosine A2A receptor would inhibit the pro-inflammatory effects of leukocytes and also inhibit a major initiation pathway (through NKT cells). In addition, the adenosine A2A receptor has been shown to inhibit the activity of the TRPV1 receptor and subsequent effects on bronchoconstriction and inflammation. This, coupled with reports that adenosine A2A agonist can promote wound healing, suggests that treatment with an adenosine A2A agonist would inhibit inflammatory processes by at least three potential mechanisms: (a) inhibition of leukocyte pro-inflammatory properties, (b) through TRP inhibition, reduction in C fibre activity with potential effects on reducing bronchial hyper-reactivity and, (c) aid in the repair process. If these mechanisms do indeed occur in the human lung then this should be manifested as improvements in lung function (see Figure 4). Only two studies have measured lung function in animal models of inflammation. In A2A-deficient animals (Nadeem et al., 2007) lung inflammation is exaggerated and this is coupled with a greater bronchoconstrictive response to methacholine compared with normal animals. On the contrary, the inhibitory effects of CGS21680 on inflammation in rat lung were not accompanied by inhibition of hyper-reactivity to methacholine (Fozard et al., 2002). This lack of effect on lung function may reflect the action of CGS21680 at non-A2A receptors (see Bonneau et al., 2006).
Anti-inflammatory role of the adenosine A2A receptor: clinical studies in allergic rhinitis and asthma
Two clinical studies have recently been reported on the effects of the A2A agonist GW328267X (Bevan et al., 2007) on allergic rhinitis (Rimmer et al., 2007) and on allergen-induced asthmatic response (Luijk et al., 2008). See Figure 1 for structure of GW328267X. These studies do not support the impressive pre-clinical studies documenting the anti-inflammatory profile outlined above. In allergic rhinitis, the effect of intranasal administration of GW328267X (50 μg two times daily) for 7 days was compared with fluticasone (200 μg two times daily). The A2A agonist-induced a very small improvement in nasal blockage but had no effect on peak nasal inspiratory flow, rhinorrhoea or sneezes. In contrast, fluticasone significantly improved nasal blockage, peak nasal inspiratory flow and rhinorrhoea but had no effect on sneezes. GW328267X did produce a small but significant inhibition of tryptase and ECP whereas fluticasone inhibited ECP, IL5 and IL8. A similar lack of efficacy of GW328267X was also shown on the early and late asthmatic response in response to antigen (Luijk et al., 2008). In this study, inhalation of GW328267X (25 μg two times daily for 7 days) was compared with inhalation of fluticasone (250 μg bid). The A2A agonist had no effect on the early or late asthmatic response or on any marker of inflammation measured. On the contrary, fluticasone totally inhibited both the early and late asthmatic response and some of the inflammatory markers measured such NO, eosinophils and ECP.
These studies cast a doubt on the concept that agonists of the adenosine A2A receptor have clinical benefit despite the impressive benefits demonstrated in animal models of inflammation and lung function.
Is the adenosine A2A receptor agonist concept flawed or is the idea still to be fully evaluated?
Assuming that adenosine A2A agonists will provide clinical benefit, then two explanations could be advanced to explain the negative effects in the above studies. First is the lack of receptor selectivity of the agonist used and second, the lack of a sufficient therapeutic index. The receptor selectivity of agonists is often undertaken in human recombinant receptors and levels of receptor expression can radically alter the potential receptor selectivity identified. In consequence, therefore, actions at other adenosine receptors might have offset clinical benefits. For instance activation of the A1 receptor has been shown to be pro-inflammatory in human isolated leukocytes, cause constriction of human isolated bronchi and enhance bronchoconstriction in anaesthetized guinea pigs through activity on sensory nerves (see Brown et al., 2008). In addition, there is some evidence to suggest that activation of the A3 receptor can also cause bronchoconstriction and may also be anti-inflammatory but other work demonstrates opposite effects and much more work is needed to elucidate the function of A3 receptors in inflammation (see Gessi et al., 2008). Finally there is also evidence to support the concept that antagonism of the A2B receptor might also produce an anti-inflammatory effect (see Sun et al., 2006). In ligand-binding studies GW328267X was shown to bind with quite high affinity to A2A and A3 but was very much weaker at A1 and A2B. Follow up functional studies demonstrated A2A agonism and A3 antagonism. Given the known functional pharmacology of GW328267X it is likely therefore that the major pharmacological drive to exert an anti-inflammatory effect is A2A receptor agonism.
It is known that the adenosine A2A receptor is widely distributed and that activation can lead to a wide variety of responses such as hypotension (see Fredholm et al., 2001). Work by Fozard et al. (2002) was the first demonstration that an A2A receptor agonist could inhibit lung inflammation when administered directly to the lung of the rat. This publication also documented that, at the doses inhibiting lung inflammation, there were falls in blood pressure and the authors suggested that a selective action of an A2A agonist in the lung would be mandatory to avoid the systemic side effects associated with absorption of A2A agonists into the periphery. In the clinical studies discussed above both stated that it might have been possible to achieve efficacy if higher amounts of compound could have been administered but this was not possible on account of the narrow therapeutic index for GW328267X, which manifests as tachycardia and hypotension. Thus, in these two clinical studies, it is unclear whether a pharmacologically active dose of the compound was administered.
If, therefore, adenosine A2A receptor agonists can indeed have clinical benefits in lung diseases such as asthma and COPD, strategies need to be developed to produce an adenosine A2A agonist with a much superior therapeutic index.
UK371,104: an inhaled adenosine A2A receptor agonist with a lung focus of pharmacological activity
Given the prophetic warning by Fozard et al. (2002) and the subsequent speculation on lack of efficacy of GW328267X in clinical trials, our strategy has been to design adenosine A2A receptor agonists which have a focus of pharmacological activity in the lung in pre clinical models. Work with inhaled corticosteroids has suggested properties which can improve the therapeutic index for inhaled agents such as prolonged effect in the lung coupled with low oral bioavailability, rapid clearance and high plasma protein binding (Derendorf, 2007).
UK371,104 is a potent and selective agonist of the human adenosine A2A receptor (Barnard et al., 2007; Mantell et al., 2008), which also inhibits mediator release from human isolated neutrophils (Table 1). In this regard, the overall in vitro anti-inflammatory profile of UK371,104 is very similar to CGS21680 (Table 1). See Figure 1 for structure of UK371,104.
Table 1.
Comparison of inhibitory effects of UK371,104 and CGS21680 on release of pro-inflammatory mediators from human isolated neutrophils
| Stimulus and assay | UK371,104 | CGS21680 |
|---|---|---|
| IC50 nM | IC50 nM | |
| fMLP | ||
| Superoxide | 55 (40–75) | 44 (30–65) |
| Elastase | 126 (86–186) | 67 (33–148) |
| Leukotriene B4 | 41 (16–107) | 19 (13–34) |
| C5a | ||
| Superoxide | 30 (12–72) | 25 (10, 62) |
| Elastase | 28 (16–49) | 23 (11–46) |
| LPS | ||
| MIP1β | 54 (20–146) | 207 (108–396) |
| TNFα | 150 (12–181) | 115 (45–299) |
Human neutrophils were obtained from healthy volunteers and isolated by density gradient centrifugation. All assays were carried out in 96-well format in HBSS buffer pH7.4. Compounds were pre-incubated with isolated neutrophils for 10 min, followed by stimulation with fMLP (30 nM–1 μM), C5a (10 nM) or LPS (10–100 ng ml−1). Elastase and superoxide release were measured over 3 min post stimulation by chromogenic substrate cleavage and cytochrome c reduction, respectively. Leukotriene B4 (LTB4), TNFα and MIP-1β biosynthesis was measured post-stimulation by ELISA. IC50 is the concentration required to inhibit mediator release by 50%. UK-371,104 and CGS-21,680 produced near maximal inhibition of release of all mediators with similar potencies (P>0.05 unpaired t-test). Data are geometric mean with 95% CI with n>6 (fMLP) and >4 C5a and LPS studies.
A key property was to assess pharmacological activity in the lung and simultaneously assess the therapeutic index over the cardiovascular properties associated with activation of peripheral activation of adenosine A2A receptors such as hypotension. We chose not to investigate these properties in animal models of lung inflammation for two reasons: (a) there is overwhelming evidence that adenosine A2A agonists are anti-inflammatory in the lung and other organs and (b) standard in vivo assays of lung inflammation study the effects of an agent on inflammatory indices at a single time point and assessment of a therapeutic index (such as effects on blood pressure) is difficult in these models. Thus to simultaneously assess the effect of an A2A agonist in the lung and cardiovascular system we chose to use a model originally described by Morimoto et al. (1993) in the anaesthetized guinea pig. In this model, adenosine analogues were demonstrated to inhibit capsaicin-induced bronchoconstriction by inhibiting substance P release. The mechanism is through inhibition of TRPV1 activity and inhibition of this receptor has been suggested as a route to control hyper-reactivity and some inflammatory responses within the lung (see Jia and Lu-Yuan, 2007).
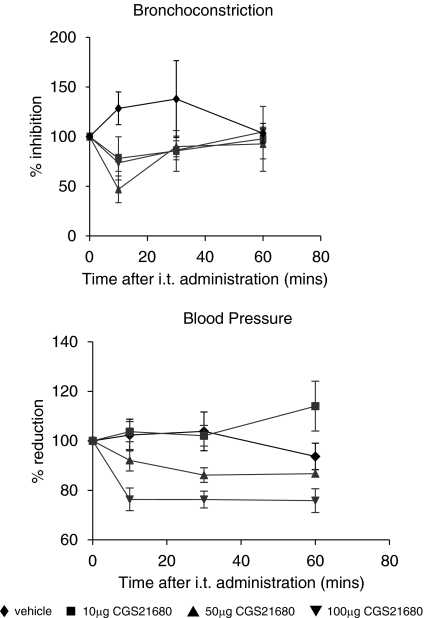
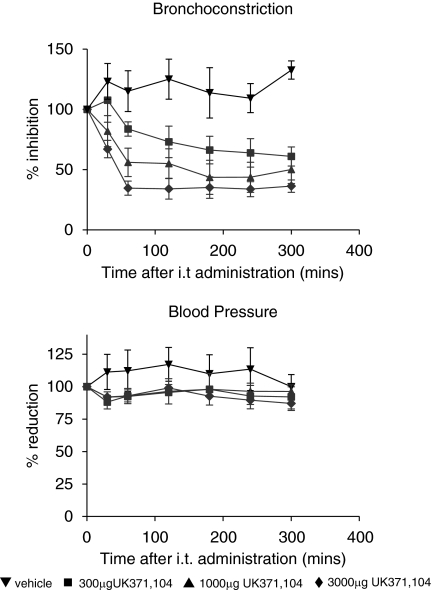
Thus we investigated the potency, efficacy and duration of action of intra-tracheal administration of UK371,104 and CGS21680 on changes in heart rate, blood pressure and on capsaicin-induced bronchoconstriction in the anaesthetized guinea pig. Results with CGS21680 agreed with the observations of Fozard et al. (2002) in that CGS21680 inhibition of the capsaicin response in the lung was associated with falls in blood pressure. In contrast, UK371,104 inhibited the effects of capsaicin on lung resistance but had no effect on blood pressure (see Figures 2 and 3). In addition the duration of action of UK371,104 in the lung was considerably longer than that of CGS21680. This suggests that UK371,104 has a focus of pharmacological activity (and a long duration of action) in the lung following intra-tracheal administration (Figure 4).
Figure 2.
CGS 21680 inhibits capsaicin-induced bronchoconstriction and lowers blood pressure in the anaesthetized guinea pig. CGS21680 or solvent was administered in solution into the trachea (it) of anaesthetized guinea pigs. At the time points in the graphs above capsaicin was administered i.v. and the effects on lung resistance measured. Blood pressure was recorded just before administration of capsaicin. Values are mean with s.e.m. of at least four individual experiments. CGS21680 produced a small but significant inhibition of lung resistance at 15 min only at 50 and 100 μg intra-tracheal dose. Both of these doses caused a fall in blood pressure, which was significant at all time points with 100 μg and at 30 min only for 50 μg (P<0.05 unpaired t-test).
Figure 3.
UK371,104 inhibits capsaicin-induced bronchoconstriction and has no effect on blood pressure in the anaesthetized guinea pig. UK371,104 or solvent was administered in solution into the trachea (it) of anaesthetized guinea pigs. At the time points in the graphs above capsaicin was administered i.v. and the effects on lung resistance measured. Blood pressure was recorded just before administration of capsaicin. Values are mean with s.e.m of at least four individual experiments. UK371,104 produced significant inhibition of lung resistance at all time 1–5 h post-administration (P<0.05 unpaired t-test) there was no effect on blood pressure at any dose (P>0.05 unpaired t-test).
Figure 4.
Potential mechanisms whereby adenosine A2A receptor agonism can inhibit inflammatory processes in the lung. At least three mechanisms can be proposed: (1) inhibition of leukocyte proinflammatory properties of NKT cells, other leukocytes and endothelium, (2) reduction in C fibre activity by TRPV1 inhibition and (3) aid in the repair process. This should manifest as decreases in AHR (airway hyper reactivity also known as airway hyper excitability), improvements in lung function accompanied by less tissue destruction and potential to aid in the repair process. FEV1, forced expiratory volume in 1 second; O*, superoxide; TNFα, tumour necrosis factor α; MIP, macrophage inflammatory protein; NKT, natural killer T cell; LTs, leukotrienes; TRPV1, transient receptor potential vanilloid 1.
The cardiovascular side effects following intra-tracheal administration of adenosine A2A agonists are likely to reflect the maximum free plasma concentration (Cmax). Thus this suggests that the free Cmax of UK371,104 was much lower than CGS21680. This was assessed in parallel studies in the rat (conscious male rats, 250 g) by comparing the normalized Cmax (free Cmax/dose) of both compounds. At a normalized intra-tracheal dose of 1 mg/kg, the free systemic Cmax of CGS21680 was 271 nM whereas that for UK371,104 was 20 nM. This concentration of CGS21680 is much higher than that required to activate the human A2A receptor, in contrast, levels of UK371,104 are lower than those required to activate this receptor. Properties of UK 371,104 aiding this desirable low free Cmax compared with CGS21680 are increased lipophilicity, molecular weight, clearance in vivo and increased plasma protein binding (Mantell et al., 2008). Preliminary studies in our laboratory have also shown that at a normalized intra-tracheal dose of 1 mg kg−1 the free systemic Cmax of GW328267X was significantly in excess of that required to activate the human A2A receptor (results not shown).
Summary and perspectives on treating lung inflammation with inhaled agonists of the adenosine A2A receptor
The adenosine A2A receptor has an impressive pedigree as a potential anti-inflammatory agent. Adenosine A2A agonists exhibit broad-spectrum anti-inflammatory effect in vitro on virtually all human cells involved in the inflammatory process. This is strongly supported by the anti-inflammatory actions of adenosine A2A receptor agonists in a wide variety of in vivo models including the lung. The very wide spread distribution of the adenosine A2A receptor suggests that, as far as the lung is concerned, inhalation would be the preferred route of administration to reduce the systemic side effects associated with activating this receptor. The lack of efficacy of inhaled GX328267X in clinical trials may be on account of the fact that the inhaled dose was too low and that the noted side effect profile (hypotension, tachycardia) precluded investigating higher inhaled doses. Using strategies similar to that devised to improve the therapeutic index of inhaled corticosteroids, the adenosine A2A agonist, UK371,104 demonstrated efficacy in the lung without any obvious effects on blood pressure. Such ‘lung-focussed' compounds may be valuable to study the anti-inflammatory potential of inhaled adenosine A2A agonists in clinical trials. UK432,097 is an analogue of UK371,104 with a similar lung focus of pharmacological activity in the anaesthetized guinea pig model discussed above. UK432,097 is currently in phase II trials for COPD.
Acknowledgments
We thank Sandy Monaghan and Peter Stephenson for help in chemical design and synthesis.
Abbreviations
- ATL146e
4-{3-[6-amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]-prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester
- CGS21680
2-[4-(2-carboxyethyl)phenethylamino]-5′-ethylcarboxamidoadenosine
- GW328267
(2R,3R,4S,5R)-2-{6-amino-2-[(1-benzyl-2-hydroxyethyl)amino]-9H-purin-9-yl}-5-(2-ethyl-2H-tetrazol-5-yl)tetrahydrofuran-3,4-diol
- UK371,104
N-(2,2-diphenylethyl)-2-{[(2-piperidin-1-ylethyl)amino]carbonyl}adenosine
Conflict of interest
All authors are employees of Pfizer Inc. Pfizer do not sell any of the drugs mentioned in the article (Figure 1). UK432,097 is currently in phase II trials for COPD.
References
- Allen-Gipson DS, Wong J, Spurzem JR, Sisson JH, Wyatt TA. Adenosine A2A receptors promote adenosine-stimulated wound healing in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L849–L855. doi: 10.1152/ajplung.00373.2005. [DOI] [PubMed] [Google Scholar]
- Barnard A, Keir R, Salmon G, Stuart E, Trevethick M, Yeadon M. UK-371,104, a novel adenosine A2A receptor agonist, inhibits acute mediator release in the human neutrophil: comparison with CGS-21,680 and the phosphodiesterase 4 inhibitor, cilomilast. 2007.
- Barnes PJ. New therapies for asthma. Trends Mol Med. 2006;12:515–520. doi: 10.1016/j.molmed.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New molecular targets for the treatment of neutrophilic diseases. J Allergy Clin Immunol. 2007;119:1055–1062. doi: 10.1016/j.jaci.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Xue YY, Li K, et al. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- Bevan N, Butchers PR, Cousins R, Coates J, Edgar EV, Morrison V, et al. Pharmacological characterisation and inhibitory effects of (2R,3R,4S,5R)-2-(6-amino-2-{[(1S)-2-hydroxy-1-(phenylmethyl)ethyl]amino}-9H-purin-9-yl)-5-(2-ethyl-2H-tetrazol-5-yl)tetrahydro-3,4-furandiol, a novel ligand that demonstrates both adenosine A2A receptor agonist and adenosine A3 receptor antagonist activity. Eur J Pharmacol. 2007;564:219–525. doi: 10.1016/j.ejphar.2007.01.094. [DOI] [PubMed] [Google Scholar]
- Blackburn MR. Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci. 2003;24:66–70. doi: 10.1016/S0165-6147(02)00045-7. [DOI] [PubMed] [Google Scholar]
- Bonneau O, Wyss D, Ferretti S, Blaydon C, Stevenson CS, Trifilieff A. Effect of adenosine A2A receptor activation in murine models of respiratory disorders. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1036–L1043. doi: 10.1152/ajplung.00422.2005. [DOI] [PubMed] [Google Scholar]
- Brown RA, Spina D, Page CP. Adenosine receptors and asthma. Br J Pharmacol. 2008;153:S446–S456. doi: 10.1038/bjp.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, et al. Adenosine A2A receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan NB, Baile EM, Paré PD. Bronchial vascular congestion and angiogenesis. Eur Respir J. 1997;10:1173–1180. doi: 10.1183/09031936.97.10051173. [DOI] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lung and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN. Adenosine receptors and wound healing, revised. Scientific World J. 2006;6:984–991. doi: 10.1100/tsw.2006.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine A2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol. 2006;176:3108–3114. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- De Ponti C, Carini R, Alchera E, Nitti MP, Locati M, Albano E, et al. Adenosine A2a receptor-mediated, normoxic induction of HIF-1 through PKC and PI-3K-dependent pathways in macrophages. J Leukoc Biol. 2007;82:392–402. doi: 10.1189/jlb.0107060. [DOI] [PubMed] [Google Scholar]
- Derendorf H. Pharmacokinetic and pharmacodynamic properties of inhaled ciclesonide. J Clin Pharmacol. 2007;47:782–789. doi: 10.1177/0091270007299763. [DOI] [PubMed] [Google Scholar]
- Desai A, Victor-Vega C, Gadangi S, Montesinos MC, Chu CC, Cronstein BN. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- Duffy SM, Cruse G, Brightling CE, Bradding P. Adenosine closes the K+ channel KCa3.1 in human lung mast cells and inhibits their migration via the adenosine A2A receptor. Eur J Immunol. 2007;37:1653–1662. doi: 10.1002/eji.200637024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard JR, Ellis KM, Villela Dantas MF, Tigani B, Mazzoni L. Effects of CGS 21680, a selective adenosine A2A receptor agonist, on allergic airways inflammation in the rat. Eur J Pharmacol. 2002;438:183–188. doi: 10.1016/s0014-2999(02)01305-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signalling. Prog Neurobiol. 2007;83:263–276. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gessi S, Varani K, Merighi S, Ongini E, Borea PA. A2A adenosine receptors in human peripheral blood cells. Br J Pharmacol. 2000;129:2–11. doi: 10.1038/sj.bjp.0703045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Okajima K, Murakami K, Usune S, Sato C, Ohshima K, et al. Adenosine and selective A(2A) receptor agonists reduce ischemia/reperfusion injury of rat liver mainly by inhibiting leukocyte activation. J Pharmacol Exp Ther. 2000;294:1034–1042. [PubMed] [Google Scholar]
- Hasko G, Cronstein BM. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hasko G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J Leukoc Biol. 2008;83:447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L-P. Natural killer T cells in asthma—toward increased understanding. N Engl J Med. 2007;356:1466–1468. doi: 10.1056/NEJMe078014. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KYJ. Phosphodiesterase 4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Hutchinson AJ, Webb RL, Oei HH, Ghai GR, Zimmerman MB, Williams M. CGS21680, an A2-selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther. 1989;251:47–55. [PubMed] [Google Scholar]
- Jia Y, Lu-Yuan L. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta. 2007;1772:915–927. doi: 10.1016/j.bbadis.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas CM, Sullivan GW, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Investig Drugs. 2005;14:797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- Linden J. Cell biology. Purinergic chemotaxis. Science. 2006;314:1689–1690. doi: 10.1126/science.1137190. [DOI] [PubMed] [Google Scholar]
- Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, et al. Ligand activation of the adenosine A2A receptor inhibits IL12 production by human monocytes. J Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- Luijk B, van den Berge M, Kerstjens HA, Postma DS, Cass L, Sabin A, et al. Effect of an inhaled adenosine A2A agonist on the allergen-induced late asthmatic response. Allergy. 2008;63:75–80. doi: 10.1111/j.1398-9995.2007.01557.x. [DOI] [PubMed] [Google Scholar]
- Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: physiologic attenuation of pro-inflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004a;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- Lukashev D, Ohta A, Sitkovsky M. Targeting hypoxia-A2A adenosine receptor mediated mechansims of tissue protection. Drug Discov Today. 2004b;9:403–409. doi: 10.1016/S1359-6446(04)03044-2. [DOI] [PubMed] [Google Scholar]
- Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol. 2007;171:1774–1788. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantell SJ, Stephenson PT, Monaghan SM, Maw GN, Trevethick MA, Yeadon M, et al. Inhaled adenosine A2A receptor agonists for the treatment of chronic obstructive pulmonary disease. Bioorg Med Chem Lett. 2008;18:1284–1287. doi: 10.1016/j.bmcl.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Mazar J, Rogachev B, Shaked G, Ziv NY, Czeiger D, Chaimovitz C, et al. Involvement of adenosine in the anti-inflammatory action of ketamine. Anesthesiology. 2005;102:1174–1181. doi: 10.1097/00000542-200506000-00017. [DOI] [PubMed] [Google Scholar]
- Meyer EH, DeKruyff RH, Umetsu DT. T cells and NKT cells in the pathogenesis of asthma. Annu Rev Med. 2008;59:281–292. doi: 10.1146/annurev.med.59.061506.154139. [DOI] [PubMed] [Google Scholar]
- Minguet S, Huber M, Rosenkranz L, Schamel WW, Reth M, Brummer T. Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. Eur J Immunol. 2005;35:31–41. doi: 10.1002/eji.200425524. [DOI] [PubMed] [Google Scholar]
- Mohsenin A, Mi T, Xia Y, Kellems RE, Chen JF, Blackburn MR. Genetic removal of the A2A adenosine receptor enhances pulmonary inflammation, mucin production, and angiogenesis in adenosine deaminase-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L753–L761. doi: 10.1152/ajplung.00187.2007. [DOI] [PubMed] [Google Scholar]
- Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto H, Yamashita M, Imazumi K, Matsuda A, Ochi T, Seki N, et al. Effects of adenosine A2 receptor agonists on the excitation of capsaicin-sensitive afferent sensory nerves in airway tissues. Eur J Pharmacol. 1993;240:121–126. doi: 10.1016/0014-2999(93)90889-p. [DOI] [PubMed] [Google Scholar]
- Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A, Fan M, Ansari HR, Ledent C, Mustafa SJ. Enhanced airway reactivity and inflammation in A2A adenosine receptor-deficient allergic mice. Am J Physiol Lung Cell Mol Physiol. 2007;2:L1335–L1344. doi: 10.1152/ajplung.00416.2006. [DOI] [PubMed] [Google Scholar]
- Nguyen DK, Montesinos MC, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signalling elements in human microvascular endothelial cells. J Immunol. 2003;15:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in down regulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Olah ME, Caldwell CC. Adenosine receptors and mammalian toll-like receptors: synergism in macrophages. Mol Interv. 2003;3:370–374. doi: 10.1124/mi.3.7.370. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Trevethick M. New insights into the anti-inflammatory effects of A2A adenosine receptor agonist: an introduction. Br J Pharmacol. 2008;153:S27–S34. doi: 10.1038/sj.bjp.0707524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce SM, Skalak TC, Rieger JM, Macdonald TL, Linden J. Selective A2A adenosine receptor activation reduces skin pressure ulcer formation and inflammation. Am J Physiol Heart Circ Physiol. 2001;281:H67–H74. doi: 10.1152/ajpheart.2001.281.1.H67. [DOI] [PubMed] [Google Scholar]
- Pouliot M.Adenosine and neutrophil function Adenosine Receptors: Therapeutic Aspects for Inflammatory and Immune Diseases 2007Taylor and Francis Group, LLC: London UK; 89–101.In: Hasko G, Cronstein B, Szabo C (eds). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntambekar P, Van Buren J, Raisinghani M, Premkumar LS, Ramkumar V. Direct interaction of adenosine with the TRPV1 channel protein. J Neurosci. 2004;7:3663–3671. doi: 10.1523/JNEUROSCI.4773-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme I (PKA I) Immunol Res. 2006;36:91–99. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]
- Reeves JJ, Butchers PR. Human eosinophils and neutrophils express inhibitory adenosine A2A receptors. Eur Resp J. 1999;14:P1553. [Google Scholar]
- Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2A stimulation in lipopolysaccharide-induced lung injury. J Immunol. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- Rimmer J, Peake HL, Santos CM, Lean M, Bardin P, Robson R, et al. Targeting adenosine receptors in the treatment of allergic rhinitis: a randomized, double-blind, placebo-controlled study. Clin Exp Allergy. 2007;37:8–14. doi: 10.1111/j.1365-2222.2006.02546.x. [DOI] [PubMed] [Google Scholar]
- Sands WA, Martin AF, Strong EW, Palmer TM. Specific inhibition of nuclear factor-kappaB-dependent inflammatory responses by cell type-specific mechanisms upon A2A adenosine receptor gene transfer. Mol Pharmacol. 2004;66:1147–1159. doi: 10.1124/mol.104.001107. [DOI] [PubMed] [Google Scholar]
- Sands WA, Palmer TM. Adenosine receptors and the control of endothelial cell function in inflammatory disease. Immunol Lett. 2005;101:1–11. doi: 10.1016/j.imlet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20:460–466. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol. 2006;26:6333–6346. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15:813–827. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA. Adenosine A2A antagonists as neurotherapeutics: crossing the bridge. Prog Neurobiol. 2007;83:261–262. doi: 10.1016/j.pneurobio.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny CP, Li L, Awad AS, Huang L, McDuffie M, Linden J, et al. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol. 2007;178:4240–4249. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]
- Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV. Use of the A2A adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem Pharmacol. 2003;65:493–501. doi: 10.1016/s0006-2952(02)01548-4. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Ohta A. The ‘danger' sensors that STOP the immune response: the A2 adenosine receptors. Trends Immuno. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Lee DD, Ross WG, DiVietro JA, Lappas CM, Lawrence MB, et al. Activation of A2A adenosine receptors inhibits expression of α4/β1 integrin (very late antigen-4) on stimulated human neutrophils. J Leukoc Biol. 2004;75:127–134. doi: 10.1189/jlb.0603300. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Linden J. Role of A2A adenosine receptors in inflammation. Drug Dev Res. 1998;45:103–112. [Google Scholar]
- Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyladenosine A2A receptor agonists. Br J Pharmacol. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, et al. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WC, Moore JN, Hurley DJ, Vandenplas ML, Linden J, Cao Z, et al. Adenosine A2A receptor agonists inhibit lipopolysaccharide-induced production of tumor necrosis factor-alpha by equine monocytes. Vet Immunol Immunopathol. 2008;121:91–100. doi: 10.1016/j.vetimm.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Sun WC, Moore JN, Hurley DJ, Vandenplas ML, Murray TF. Effects of stimulation of adenosine A2A receptors on lipopolysaccharide-induced production of reactive oxygen species by equine neutrophils. Am J Vet Res. 2007;68:649–656. doi: 10.2460/ajvr.68.6.649. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yamada G, Saikai T, Hashimoto M, Tanaka S, Suzuki K, et al. Increased airway vascularity in newly diagnosed asthma using a high-magnification bronchovideoscope. Am J Respir Crit Care Med. 2003;168:1495–1499. doi: 10.1164/rccm.200306-727OC. [DOI] [PubMed] [Google Scholar]
- Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;6:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berge M, Hylkema MN, Versluis M, Postma DS. Role of adenosine receptors in the treatment of asthma and chronic obstructive pulmonary disease: recent developments. Drugs RD. 2007;8:13–23. doi: 10.2165/00126839-200708010-00002. [DOI] [PubMed] [Google Scholar]
- Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, et al. Myocardial infarct-sparing effect of A2AAR activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- Zezula J, Freissmuth M. The A(2A)-adenosine receptor: a GPCR with unique features. Br J Pharmacol. 2008;153 Suppl 1:S184–S190. doi: 10.1038/sj.bjp.0707674. [DOI] [PMC free article] [PubMed] [Google Scholar]