Abstract
No established second-line chemotherapy is available for patients with advanced gastric cancer failing to respond or progressing to first-line chemotherapy. However, 20–40% of these patients commonly receive second-line chemotherapy. We evaluated the influence of clinico-pathologic factors on the survival of 175 advanced gastric cancer patients, who received second-line chemotherapy at three oncology departments. Univariate and multivariate analyses found five factors which were independently associated with poor overall survival: performance status 2 (hazard ratio (HR), 1.79; 95% CI, 1.16–2.77; P=0.008), haemoglobin ⩽11.5 g l−1 (HR, 1.48; 95% CI, 1.06–2.05; P=0.019), CEA level >50 ng ml−1 (HR, 1.86; 95% CI, 1.21–2.88; P=0.004), the presence of greater than or equal to three metastatic sites of disease (HR, 1.72; 95% CI, 1.16–2.53; P=0.006), and time-to-progression under first-line chemotherapy ⩽6 months (HR, 1.97; 95% CI, 1.39–2.80; P<0.0001). A prognostic index was constructed dividing patients into low- (no risk factor), intermediate- (one to two risk factors), or high- (three to five risk factors) risk groups, and median survival times for each group were 12.7 months, 7.1 months, and 3.3 months, respectively (P<0.001). In the absence of data deriving from randomised trials, this analysis suggests that some easily available clinical factors may help to select patients with advanced gastric cancer who could derive more benefit from second-line chemotherapy.
Keywords: second-line chemotherapy, prognostic factor, metastatic gastric cancer
Despite a declining incidence in many developed countries, gastric cancer remains the second most common cause of cancer deaths, and it is responsible for about 12% of all cancer-related deaths worldwide (Parkin et al, 1999). As for most gastrointestinal cancers, the management of gastric cancer is based on the surgical resection of the primary tumour. However, more than two-thirds of patients diagnosed with gastric cancer will have unresectable disease (Parkin et al, 1999; Catalano et al, 2005; Macdonald, 2006), and despite the fact that surgical pathological R0 resection can be curative for many patients, recurrence rates are about 70% and 5-year survival rate is lower than 30% (Catalano et al, 2005). For patients with unresectable disease, or developing recurrent disease after curative resection, evidence supports the use of palliative chemotherapy with the aims of improving symptoms, quality of life, and possibly prolonging survival (Murad et al, 1993; Pyrhonen et al, 1995; Glimelius et al, 1997). Several chemotherapy agents are considered active in advanced gastric cancer. Over the past decades, many anticancer drugs have been investigated, such as 5-fluorouracil (5-FU), cisplatin, anthracyclines, oral fluoropyrimidines, irinotecan, oxaliplatin, and docetaxel (Catalano et al, 2005; Dank et al, 2005; Al-Batran et al, 2006; Kang et al, 2006; Van Cutsem et al, 2006; Cunningham et al, 2008). Combination chemotherapy regimens have been developed in the hopes of improving response rate and overall survival (OS). Unfortunately, the benefits of combination chemotherapy have been modest (Wagner et al, 2006). In general, regimens containing 5-FU and cisplatin are widely accepted as potential standard therapies with a response rate of 25–45% and median OS times of 7–9 months (Ajani 2005; Catalano et al, 2005; Van Cutsem et al, 2006; Cunningham et al, 2008). Although a large proportion of patients with metastatic or recurrent gastric cancer may initially respond to chemotherapy, they ultimately progress. In addition, many patients have primary refractory disease. The median survival at progression after first-line chemotherapy for metastatic gastric cancer is about 2.5 months.
Yet, there is no established second-line chemotherapy for gastric cancer, but it is used in 20–40% of patients (Chau et al, 2004a; Lee et al, 2007). In three phase III randomised trials of 5-FU-based first-line therapy enrolling a total of 1080 patients, 20% of them went on to receive second-line chemotherapy, with a response rate observed as 13.3% (95% CI, 6.8–22.5%), and a median survival from starting second-line therapy of 5.6 months (Chau et al, 2004a).
During the past few decades, many phase II reports of second-line therapy including gastric cancer patients have been published, but no randomised phase III trial investigating second-line chemotherapy compared with best supportive care is available. There is a great heterogeneity within each of phase II trial, partly because of the variability in the responsiveness to first-line chemotherapy, and the nature of previous chemotherapy (cisplatin-based or not). Morevover, available data have limitations because of a publication bias; trials generally include small numbers of participants and they do not support evidence for identifying patients who are more likely to benefit from second-line chemotherapy (Wilson et al, 2005).
A decision whether starting or not second-line chemotherapy for an individual patient may be a common clinical scenario. Several factors should be considered: the potential cumulative toxicity, especially for those patients with low performance status (PS), the extent of disease, the need of active drugs, the lack of cross-resistance to drugs previously used. But second-line therapy could not be appropriate for all patients, and prediction of treatment outcome may allow for the identification of patients who would derive very little benefit from second-line chemotherapy. The aim of this analysis was to explore clinico-pathologic factors that may be associated with survival of patients treated with second-line chemotherapy. In the absence of definitive phase III trials, consideration of some of these factors, if any, may assist in the selection of patients for further treatment. However, as this analysis was performed on patients receiving cytotoxic drugs, it is not possible to extrapolate results for patients treated with novel biological agents and within clinical trials.
Patients and methods
From January 1995 to August 2006, 625 patients received first-line chemotherapy at three oncology departments (San Salvatore Hospital, Pesaro; Hospital of Urbino; Campus Biomedico, Rome). Of them, 175 (28%) consecutive patients received second-line chemotherapy for locally advanced or metastatic gastric cancer. No patient receiving second-line chemotherapy was excluded from this analysis. The criteria for case inclusion were as follows: (i) histologically confirmed diagnosis of gastric cancer; (ii) previous treatment with first-line chemotherapy, given until disease progression, unacceptable toxicity, or patient's refusal; (iii) presence of measurable disease; (iv) progressing disease after the first-line chemotherapy; (v) availability of clinico-pathological data at the beginning of second-line chemotherapy.
Approval of the study was obtained from the local research and ethics committee.
The clinical tumour response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) (Therasse et al, 2000).
Factors included in the univariate analyses were as follows: age, sex, PS, loss of weight >5%; haemoglobin, serum albumin, CEA levels; previous gastrectomy, Lauren classification, tumour grade, tumour location, metastasis to liver, peritoneum, number of metastatic sites; time-to-progression (TTP), objective response to first-line chemotherapy, regimen used as first-line chemotherapy (oxaliplatin or cisplatin plus 5-FU-based vs 5-FU-based). Laboratory variables were initially recorded as continuous variables and later dichotomised according to the median value of each variable. Performance status was evaluated according to the Eastern Cooperative Oncology Group (ECOG) criteria. Time-to-progression under first-line chemotherapy was measured from the date of the beginning of the treatment to the date of progression. To refine its possible association with survival to second-line chemotherapy, TTP under first-line chemotherapy was studied dichotomising the study population according to disease progression less than or equal to the median TTP vs disease progression greater than the median TTP.
Statistical analysis
This is a multicentric, retrospective study. The primary end point of the study was OS. Overall survival was measured from the date of the first cycle of second-line chemotherapy to the date of death or the last follow-up visit. Survival data were analysed using the Kaplan–Meier product-limit method (Kaplan and Meier, 1958). Comparison of survival curves were performed using log-rank test (Peto and Peto, 1972). A prognostic model was established by searching all variables that significantly influenced OS at a level of P-values <0.05 in the univariate analysis. Multivariate analysis was carried out using stepwise Cox proportional hazards regression modelling. P-values <0.05 were considered statistically significant and all P-values correspond to two-sided significance tests.
Results
The characteristics of patients included in this analysis are shown in Table 1. The median age was 64 years with a range of 38–83 years. Sixty-six percent of patients were male, and 23.4% had ECOG PS 2. Only four patients had locally advanced disease, and 77.7% of patients received previous gastrectomy.
Table 1. Patient characteristics at baseline (n=175).
| Characteristics | No. of patients |
|---|---|
| Age (median age, range) | 64 (38–83) |
| Male/female | 116/59 |
| Previous gastrectomy | |
| Performance status (ECOG) | |
| 0/1/2 | 53/76/41 |
| Disease status | |
| Locally advanced/metastatic | 4/171 |
| Site of primary tumour | |
| GEJ/cardia | 28 |
| Body | 66 |
| Antrum | 63 |
| Anastomosis | 9 |
| Multiple sites | 9 |
| Previous gastrectomy | |
| Yes/no | 136/49 |
| Histology | |
| Adenocarcinoma | 139 |
| Signet ring cell carcinoma | 27 |
| Undifferentiated | 9 |
| Grade | |
| Well/moderately/poorly differentiated | 5/41/102 |
| Not known | 27 |
| Lauren classification | |
| Intestinal/diffuse | 105/70 |
| Haemoglobin, g l−1 (median, range) | 11.5 (7.3–15.6) |
| Albumin, g per 100 ml (median, range) | 3.5 (1.8–4.9) |
| CEA, ng ml−1(median, range) | 50 (1 to >500) |
| Sites of disease | |
| Stomach/local relapse | 77 |
| Liver | 66 |
| Peritoneum | 80 |
| Lymph node | 69 |
| Number of metastatic sites | |
| 1–2 | 125 |
| 3–4 | 50 |
ECOG=Eastern Cooperative Oncology Group; GEJ=gastroesophageal junction.
Treatment
First-line chemotherapy contained 5-FU (n=13), 5-FU and cisplatin (n=141), or 5-FU and oxaliplatin (n=21) (Table 2). Twelve patients (6.8%) experienced a complete response, and 71 patients (40.6%) a partial response for an overall response rate of 47.4% (95% CI, 40.0–54.8). Median TTP under first-line chemotherapy was 6 months (range, 1–68 months).
Table 2. Results of first-line and second-line chemotherapy (n=175).
| No. of patients | |
|---|---|
| First-line chemotherapy | |
| Treatment | |
| 5-FU-based | 13 |
| 5FU/cisplatin-based | 141 |
| 5-FU/oxaliplatin-based | 21 |
| Response rate | |
| CR | 12 |
| PR | 71 |
| ORR | 47.4% (95% CI, 40.0–54.8) |
| SD | 48 |
| PD | 44 |
| TTP, median (range) | 6 months (1–68) |
| Second-line chemotherapy | |
| Treatment | |
| 5-FU-based | 47 |
| 5-FU/CDDP-based | 21 |
| 5-FU/oxaliplatin-based | 31 |
| 5-FU/irinotecan-based | 51 |
| 5-FU/taxane | 25 |
| Response rate | |
| CR | 3 |
| PR | 25 |
| ORR | 16.0% (95% CI, 10.6–21.4) |
| SD | 59 |
| PD | 88 |
5-FU=5-fluorouracil; CI=confidence interval; CR=complete response; ORR=overall response rate (according to RECIST); PD=progressive disease; PR=partial response; SD=stable disease; Taxane=docetaxel, paclitaxel; TTP=time-to-progression.
All the patients received 5-FU as second-line chemotherapy, given as bolus i.v. or continuous infusion and combined with other drugs. Second-line chemotherapy consisted of regimens containing 5-FU and cisplatin (n=21), 5-FU and oxaliplatin (n=31), 5-FU and irinotecan (n=51), 5-FU and paclitaxel or docetaxel (n=25), and 5-FU-based (n=47). In any case, no combination of drugs received as first-line treatment was subsequently given at disease progression. A complete response to second-line chemotherapy was achieved in 3 patients and a partial response in 25 patients, for an overall response rate of 16.0% (95% CI, 10.6–21.4).
Univariate and multivariate analysis
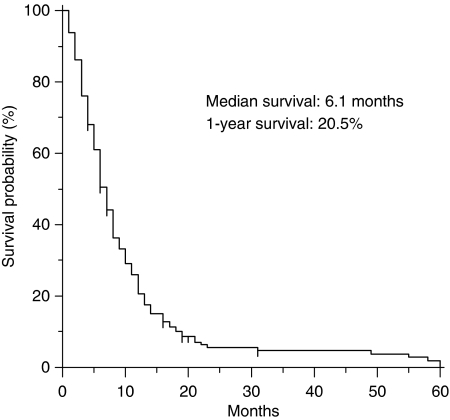
At the time of analysis, 163 (93.1%) patients had died from tumour progression. Median survival for the whole group was 6.1 months, and 1-year OS was 20.5% (95% CI, 14.4–26.6) (Figure 1).
Figure 1.
Overall survival curve for the whole group (n=175).
At univariate analysis (Table 3), eight variables were significantly associated with poor survival time: ECOG PS 2, loss of weight >5%, haemoglobin ⩽11.5 g l−1, CEA >50 ng ml−1, the presence of peritoneal carcinomatosis, a number of metastatic sites 3–4, TTP under first-line chemotherapy ⩽6 months, and previous chemotherapy with regimens containing 5-FU alone. No statistically significative difference was found between each regimen used as second-line chemotherapy.
Table 3. Univariate analysis (n=175).
| Variable | MST (months) | 1-year survival (%) | P-value |
|---|---|---|---|
| Age | |||
| ⩽64 | 6.0 | 23.2 | 0.396 |
| >64 | 6.0 | 18.2 | |
| Sex | |||
| Male | 6.3 | 18.7 | 0.967 |
| Female | 5.8 | 24.6 | |
| Performance status (ECOG) | |||
| 0–1 | 7.5 | 25.3 | <0.001 |
| 2 | 2.5 | 8.1 | |
| Loss of weight >5% | |||
| Yes | 5.0 | 14.7 | 0.003 |
| No | 7.5 | 24.3 | |
| Haemoglobin | |||
| ⩽11.5 g l−1 | 5.0 | 14.2 | 0.002 |
| >11.5 g l−1 | 7.8 | 28.8 | |
| Albumin | |||
| ⩽3.5 g per 100 ml | 5.5 | 19.5 | 0.08 |
| >3.5 g per 100 ml | 6.8 | 26.7 | |
| CEA | |||
| ⩽50 ng ml−1 | 7.0 | 25.2 | 0.002 |
| >50 ng ml−1 | 5.0 | 3.8 | |
| Previous gastrectomy | |||
| Yes | 6.6 | 21.7 | 0.23 |
| No | 4.1 | 16.6 | |
| Lauren classification | |||
| Intestinal | 6.5 | 19.4 | 0.791 |
| Diffuse | 5.5 | 22.2 | |
| Grade | |||
| Well to moderate | 6.5 | 18.4 | 0.447 |
| Poor | 6.2 | 22.2 | |
| Site of primary | |||
| GEJ to cardia | 6.5 | 25.4 | 0.365 |
| Body to antrum | 6.0 | 19.7 | |
| Liver metastasis | |||
| Yes | 5.5 | 13.9 | 0.194 |
| No | 6.6 | 24.8 | |
| Peritoneal metastasis | |||
| Yes | 5.0 | 14.6 | 0.005 |
| No | 7.3 | 25.5 | |
| Number of metastatic sites | |||
| 1–2 | 7.2 | 26.2 | <0.001 |
| 3–4 | 3.8 | 6.2 | |
| First-line chemotherapy | |||
| 5-FU-based | 4.5 | 0.0 | 0.021 |
| 5FU/platinum-based | 6.1 | 19.1 | |
| Response to first-line chemotherapy | |||
| Yes | 7.0 | 24.3 | 0.297 |
| No | 5.5 | 16.6 | |
| TTP of first-line chemotherapy⩽6 mos | |||
| Yes | 4.8 | 9.1 | <0.001 |
| No | 11.0 | 36.6 | |
| Year | |||
| 1995–2000 | 5.5 | 3.8 | 0.128 |
| 2001–2006 | 7.2 | 4.9 | |
5-FU=5-fluorouracil; ECOG=Eastern Cooperative Oncology Group; GEJ=gastroesophageal junction; mos=months; MST=median survival time; Platinum=cisplatin, ovaliplatin; TTP=time-to-progression.
Multivariate regression analysis (Table 4) included the eight variables that were found to have prognostic significance in univariate analysis. These features were available for all 175 patients. Five factors, PS, haemoglobin, CEA, the number of metastatic sites, and TTP under first-line chemotherapy, showed independent prognostic role.
Table 4. Multivariate analysis (n=175).
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| ECOG performance status 2 | 1.79 | 1.16–2.77 | 0.008 |
| Loss of weight >5% | 1.05 | 0.73–1.53 | 0.77 |
| Haemoglobin ⩽11.5 g l−1 | 1.48 | 1.06–2.05 | 0.019 |
| CEA>50 ng ml−1 | 1.86 | 1.21–2.88 | 0.004 |
| Peritoneal metastasis | 1.16 | 0.80–1.68 | 0.41 |
| Number of metastatic sites 3–4 | 1.72 | 1.16–2.53 | 0.006 |
| 5-FU-based first-line chemotherapy | 1.97 | 0.86–3.74 | 0.0733 |
| TTP of the first-line chemotherapy ⩽6 months | 1.97 | 1.39–2.80 | <0.0001 |
5-FU=5-fluorouracil; CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; HR=hazard ratio; TTP=time-to-progression.
The multivariate analysis was also performed by stratificating for the second-line chemotherapy received. However, we found no interaction between any second-line treatment and each clinico-pathologic variable (data not shown).
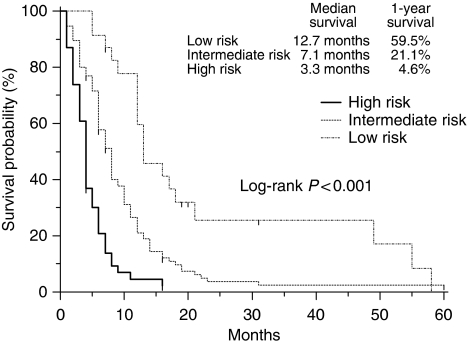
Then, a multivariate prognostic model was constructed by incorporating all the five adverse prognostic factors. The prognostic grouping was carried out according to the following criteria: low-risk group, patients with no prognostic factor (n=25); intermediate-risk group, patients with one or two negative prognostic factors (n=101); high-risk group, patients with three to five negative prognostic factors (n=49). The survival curves according to the prognostic model are shown in Figure 2. Median survival times for low-, intermediate-, and high-risk groups were 12.7, 7.1, and 3.3 months, respectively. One-year survival rates for each group were 59.5% (95% CI, 39.0–79.9), 21.1% (95% CI, 12.7–29.5), and 4.6% (95% CI, 0–10.8), respectively. There were marked significant survival differences among the three risk groups (P<0.001).
Figure 2.
Survival curves according to the risk groups. Marks on survival curves denote censored observations.
When compared to the low-risk group, the intermediate-risk group had a two-fold (HR, 2.18; 95% CI, 1.38–3.30), and the high-risk group had a 3.5-fold (HR, 3.61; 95% CI, 3.4–11.67) increased risk of death.
Discussion
Despite new schedules and association of drugs, patients with advanced gastric cancer treated with first-line chemotherapy have median OS rarely approaching 11 months (Catalano et al, 2005; Dank et al, 2005; Al-Batran et al, 2006; Kang et al 2006; Van Cutsem et al, 2006; Cunningham et al, 2008). No significant steps ahead have been moved since the past decade. Many patients did not respond to first-line chemotherapy or have progression of their disease within some months from the end of treatment.
To improve survival of advanced gastric cancer patients, more effective first-line regimens are necessary. No established second-line therapy is available and decision whether to treat or not such patients with second-line chemotherapy is crucial.
This analysis based on the individual data of 175 consecutive patients treated with second-line chemotherapy identifies five independent prognostic factors: PS, haemoglobin level, CEA level, number of metastatic sites, TTP under first-line chemotherapy. To our knowledge, this is the first report, which assess pre-treatment clinical prognostic factors for patients receiving second-line therapy for advanced gastric cancer. Conversely, three different studies (Yoshida et al, 2004; Chau et al, 2004b; Lee et al, 2007) aimed to identify clinical prognostic factors for metastatic gastric cancer patients undergoing first-line chemotherapy. Chau et al (2004b) assessed prognostic factors on 1080 patients with locally advanced and metastatic oesophago-gastric cancer. Four independent poor prognostic factors were identified by multivariate analysis: PS ⩾2, liver metastases, peritoneal metastases, and alkaline phosphatase ⩾100 U l−1. A prognostic index was developed dividing patients into good (no risk factor), moderate (one to two risk factors) or poor (three to four risk factors) risk groups. One-year survival for good, moderate, and poor risk groups were 48.5, 25.7, and 11%, respectively, with a highly significant difference among the three groups (P<0.0001). In the second study (Yoshida et al, 2004), the Japan Clinical Oncology Group (JCOG) showed that better PS, a limited number of metastatic sites, and macroscopically scirrhous type tumours were significantly associated with poor survival in metastatic gastric cancer patients.
Recently, Lee et al (2007) published a multivariate analysis on 1445 gastric cancer patients undergoing first-line chemotherapy. No previous gastrectomy, albumin <3.6 g per 100 ml, alkaline phosphatase >85 U l−1, PS⩾2, bone metastases, and ascites were the main clinical parameters associated with poor survival. The authors used these factors to develop a prognostic model to predict survival by categorising patients into three risk groups: low, intermediate, and high, with corresponding median survival times of 12.5, 7, 2.7 months, respectively (P<0.001).
Even if no effective second-line chemotherapy has been established yet, in this setting several phase II trials were published during the last decades (Schöffski 2002; Wilson et al, 2005; Sastre et al, 2006). These studies suggest response rates similar to those achieved in other cancers, such as colorectal, lung, ovarian cancers (Schöffski 2002; Colombo et al, 2006; Goldberg et al, 2007; Vansteenkiste, 2007). However, we still lack of phase III trials comparing second-line chemotherapy vs best supportive care.
In this report, we used the independent prognostic factors to define three different risk groups of patients, low-risk group, patients with no prognostic factor; intermediate-risk group, patients with one or two negative prognostic factors; high-risk group, patients with three to five negative prognostic factors, with median survival time of 12.7, 7.1, and 3.3 months, respectively. The benefit of each risk group is quite similar to that reported in the other series including patients treated with first-line chemotherapy (Chau et al, 2004b; Lee et al, 2007). Therefore, it is plausible that patients with no or a limited number of poor prognostic factors may derive the same benefit from second-line chemotherapy as patients treated with first-line chemotherapy.
This analysis confirms the importance of PS when receiving second-line treatment (Yoshida et al, 2004; Chau et al, 2004b; Lee et al, 2007). As general conditions of patients with advanced gastric cancer may rapidly deteriorate after first-line chemotherapy, second-line therapy should be limited to patients with good PS. Moreover, consideration of good PS is of high importance not only as it is associated with OS, but also when we consider tolerability of chemotherapy.
Park et al (2006) suggested that low baseline haemoglobin level (<10 g l−1) is a strong and independent prognostic factor for the outcomes of advanced gastric cancer patients receiving 5-FU-based first-line chemotherapy. Similarly, in our analysis, haemoglobin level was found of prognostic value after uni- and multivariate analysis, while conflicting results were emphasised in other reports considering the presence of anaemia at onset of first-line chemotherapy (Chau et al, 2004b; Lee et al, 2007).
Serum CEA level is a common preoperative and follow-up marker of gastrointestinal tumours (Holyoke et al, 1975; Korenaga et al, 1995). Serum CEA is a useful indicator of potential curability in patients who undergo gastrectomy and it provides predictive value in determining tumour stage and prognostic information for patients with potentially resectable gastric cancer (Ikeda et al, 1995; Tachibana et al, 1998; Kochi et al, 2000). Increased CEA was found to be univariate adverse prognostic factor for survival in locally advanced or metastatic gastric cancer patients treated with first-line chemotherapy (Louvet et al, 2003). In our series, CEA >50 ng ml−1 was a negative prognostic factor and this may reflect the metastatic potential of malignant cells (Hostetter et al, 1990).
The presence of peritoneal metastases or ascites are poor independent prognostic factors for gastric cancer patients receiving first-line chemotherapy (Chau et al, 2004b; Lee et al, 2007). In the present analysis, peritoneal metastasis had some prognostic value at the univariate analysis, but the multivariate analysis failed to show any independent prognostic significance. We cannot rule out a possible interaction of peritoneal disease with other factors, such as PS, number of metastatic sites, or TTP under first-line chemotherapy. Patients with clinically or radiologically gross peritoneal metastases also more frequently have lower PS, poor tumour response to chemotherapy and survival than patients with small amount of peritoneal disease or diagnosed at explorative laparotomy (Sadeghi et al, 2000; Chau et al, 2004b). Number of metastatic sites of disease was significantly associated with OS, and this confirms what was previously reported in gastrointestinal cancers (Mitry et al, 2004; Yoshida et al, 2004).
In a small report, TTP under first-line treatment has been suggested as useful selection criteria for second-line chemotherapy (Stahl et al, 2005). It was a predictor for patients (n=27) who did benefit from the second-line chemotherapy with a median survival from the start of second-line therapy of 10.6 months, compared to 5.1 months for the overall group. For this reason, in our analysis we explored the possible prognostic role of TTP under first-line chemotherapy. Patients with TTP under first-line chemotherapy ⩽6 months and treated with second-line chemotherapy had a median survival time since the starting date of the second-line therapy of 11 months, which represents the best median OS of active first-line triple-agents regimens for metastatic gastric cancer (Wagner et al, 2006). Then, patients with rapidly progressing disease are an unfavourable setting of patients more unlikely to take advantage from second-line chemotherapy.
The present analysis has been performed in patients receiving second-line chemotherapy and it cannot account for novel biologic agents. Therefore, any conclusion cannot be generalised for the treatments including cytotoxic drugs and biologic agents. Clinico-pathologic and tumour biologic factors may help to find optimal candidates for additional therapy with targeted agents and cytotoxic drugs, also in the context of clinical trials. This strategy may be crucial to develop new approaches for this lethal disease. Moreover, patients with high-risk factors should be refrained from fruitless cytotoxic therapy, and this group of patients may be the one that should be candidate for non-cytotoxic treatments.
In conclusion, in the absence of a standard regimen, this analysis suggests that some clinical factors, which are easily identifiable, may define some groups of patients with gastric cancer more likely to benefit from a second-line chemotherapy. Phase III randomised trials comparing second-line chemotherapy vs best supportive care in patients with advanced gastric cancer are highly warranted. However, we also need to evaluate new agents including the targeted agents for this population incorporating prognostic and predictive markers as a component of trial design.
References
- Ajani JA (2005) Evolving chemotherapy for advanced gastric cancer. Oncologist 10(Suppl 3): 49–58 [DOI] [PubMed] [Google Scholar]
- Al-Batran S, Hartmann J, Probst S, Hofheinz R, Stoehlmacher H, Schmalenberg H, Hollerbach S, Schuch G, Homann N, Jäger E (2006) A randomized phase III trial in patients with advanced adenocarcinoma of the stomach receiving first-line chemotherapy with fluorouracil, leucovorin and oxaliplatin (FLO) versus fluorouracil, leucovorin and cisplatin (FLP). J Clin Oncol 24(Suppl 18): LBA4016a [Google Scholar]
- Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E (2005) Gastric cancer. Crit Rev Oncol Hematol 54: 209–241 [DOI] [PubMed] [Google Scholar]
- Chau I, Norman AR, Ross PJ, Waters JS, Oates J, Cunningham D (2004a) Multivariate prognostic factor analysis and second-line treatment in locally advanced and metastatic oesophago-gastric cancer – pooled analysis of 1080 patients from three multicentre randomised controlled trials using individual patient data. ASCO Gastrointestinal Cancers Symposium; abstract 5 [DOI] [PubMed]
- Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ (2004b) Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 22: 2395–2403 [DOI] [PubMed] [Google Scholar]
- Colombo N, Van Gorp T, Parma G, Amant F, Gatta G, Sessa C, Vergote I (2006) Ovarian cancer. Crit Rev Oncol Hematol 60: 159–179 [DOI] [PubMed] [Google Scholar]
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 58: 36–46 [DOI] [PubMed] [Google Scholar]
- Dank M, Zaluski J, Barone C, Valvere V, Peschel C, Wenczl M, Goker E, Risse M, Awad L, Bugat R (2005) Randomized phase 3 trial of irinotecan (CPT-11)+5FU/folinic acid (FA) vs CDDP+5FU in 1st-line advanced gastric cancer patients. J Clin Oncol 23(Suppl 16): 4003a15767649 [Google Scholar]
- Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H, Heuman R (1997) Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 8: 163–168 [DOI] [PubMed] [Google Scholar]
- Goldberg RM, Rothenberg ML, Van Cutsem E, Benson III AB, Blanke CD, Diasio RB, Grothey A, Lenz HJ, Meropol NJ, Ramanathan RK, Becerra CH, Wickham R, Armstrong D, Viele C (2007) The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist 12: 38–50 [DOI] [PubMed] [Google Scholar]
- Holyoke ED, Chu TM, Murphy GP (1975) CEA as a monitor of gastrointestinal malignancy. Cancer 35: 830–836 [DOI] [PubMed] [Google Scholar]
- Hostetter RJ, Augustus LB, Mankarious R, Chi KF, Fan D, Toth C, Thomas P, Jessup JM (1990) Carcinoembryonic antigen as a selective enhancer of colorectal cancer metastasis. J Natl Cancer Inst 82: 380–385 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Oomori H, Koyanagi N, Mori M, Kamakura T, Minagawa S, Tateishi H, Sugimachi K (1995) Prognostic value of combination assays for CEA and CA 19-9 in gastric cancer. Oncology 52: 483–486 [DOI] [PubMed] [Google Scholar]
- Kang Y, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Salas MP, Suarez T, Santamaria J (2006) Randomized phase III trial of capecitabine/cisplatin (XP) vs. continuous infusion of 5-FU/cisplatin (FP) as first-line therapy in patients (pts) with advanced gastric cancer (AGC): efficacy and safety results. J Clin Oncol 24(Suppl 18): LBA4018a [Google Scholar]
- Kaplan EL, Meier P (1958) Non parametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481 [Google Scholar]
- Kochi M, Fujii M, Kanamori N, Kaiga T, Kawakami T, Aizaki K, Kasahara M, Mochizuki F, Kasakura Y, Yamagata M (2000) Evaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer 3: 177–186 [DOI] [PubMed] [Google Scholar]
- Korenaga D, Funahashi S, Yano K, Maekawa S, Ikeda T, Sugimachi K (1995) Relationship between peritoneal collagen type IV concentrations and the presence of disseminated metastases in gastric cancer. Arch Surg 130: 769–773 [DOI] [PubMed] [Google Scholar]
- Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, Park JO, Park YS, Lim HY, Sohn TS, Noh JH, Heo JS, Park CK, Kim S, Kang WK (2007) Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol 18: 886–891 [DOI] [PubMed] [Google Scholar]
- Louvet C, Carrat F, Mal F, Mabro M, Beerblock K, Vaillant JC, Cady J, André T, Gamelin E, de Gramont A (2003) Prognostic factor analysis in advanced gastric cancer patients treated with hydroxyurea, leucovorin, 5-fluorouracil, and cisplatin (HLFP regimen). Cancer Invest 21: 14–20 [DOI] [PubMed] [Google Scholar]
- Macdonald JS (2006) Gastric cancer—new therapeutic options. N Engl J Med 355: 76–77 [DOI] [PubMed] [Google Scholar]
- Mitry E, Douillard JY, Van Cutsem E, Cunningham D, Magherini E, Mery-Mignard D, Awad L, Rougier P (2004) Predictive factors of survival in patients with advanced colorectal cancer: an individual data analysis of 602 patients included in irinotecan phase III trials. Ann Oncol 15: 1013–1017 [DOI] [PubMed] [Google Scholar]
- Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M (1993) Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 72: 37–41 [DOI] [PubMed] [Google Scholar]
- Park SH, Lee J, Lee SH, Park JO, Kim K, Kim WS, Jung CW, Park YS, Kang WK, Park K, Kim S, Bang SM, Cho EK, Shin DB, Lee JH (2006) Anemia is the strongest prognostic factor for outcomes of 5-fluorouracil-based first-line chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol 57: 91–96 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Pisani P, Ferlay J (1999) Global Cancer Statistics. CA Cancer J Clin 49: 33–64 [DOI] [PubMed] [Google Scholar]
- Peto R, Peto J (1972) Asymptotically efficient invariant procedures. JR Stat Soc A 135: 185–206 [Google Scholar]
- Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M (1995) Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 71: 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, Gilly FN (2000) Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE-1 multicentric prospective study. Cancer 88: 358–363 [DOI] [PubMed] [Google Scholar]
- Sastre J, Garcia-Saenz JA, Diaz-Rubio E (2006) Chemotherapy for gastric cancer. World J Gastroenterol 12: 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffski P (2002) New drugs for treatment of gastric cancer. Ann Oncol 13(Suppl 4): 13–22 [DOI] [PubMed] [Google Scholar]
- Stahl M, Müller C, Köster W, Wilke H (2005) Second-line chemotherapy of advanced disseminated gastric cancer after cisplatin, infusional 5-fluorouracil, folinic acid (PLF): benefit dependent on progression-free interval after first-line therapy. Onkologie 28: 499–502 [DOI] [PubMed] [Google Scholar]
- Tachibana M, Takemoto Y, Nakashima Y, Kinugasa S, Kotoh T, Dhar DK, Kohno H, Nagasue N (1998) Serum carcinoembryonic antigen as a prognostic factor in resectable gastric cancer. J Am Coll Surg 187: 64–68 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J Clin Oncol 24: 4991–4997 [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J (2007) Improving patient management in metastatic non-small cell lung cancer. Lung Cancer 57(Suppl 2): 12–17 [DOI] [PubMed] [Google Scholar]
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24: 2903–2909 [DOI] [PubMed] [Google Scholar]
- Wilson D, Hiller L, Geh JI (2005) Review of second-line chemotherapy for advanced gastric adenocarcinoma. Clin Oncol 17: 81–90 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Ohtsu A, Boku N, Miyata Y, Shirao K, Shimada Y, Hyodo I, Koizumi W, Kurihara M, Yoshida S, Yamamoto S (2004) Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol 34: 654–659 [DOI] [PubMed] [Google Scholar]